Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Proteome Subtraction
2.2. Physiochemical Properties
2.3. Profiling of T Cell and B Cell Epitopes and Features
2.3.1. CTL Binding Epitope Screening and Profiling
2.3.2. HTL Binding Epitope Screening and Profiling
2.3.3. LBL Binding Epitope Screening and Profiling
2.4. Epitope Conservancy Analysis
2.5. mRNA-Based Vaccine Construction, Its Structure Prediction and Characterization
2.6. Secondary and Tertiary Structure Prediction, Refinement, and Verification
2.7. Prediction of Continuous and Discontinuous B Cell Epitopes
2.8. Molecular Docking
2.9. Normal Mode Analysis
2.10. Molecular Dynamic Simulation
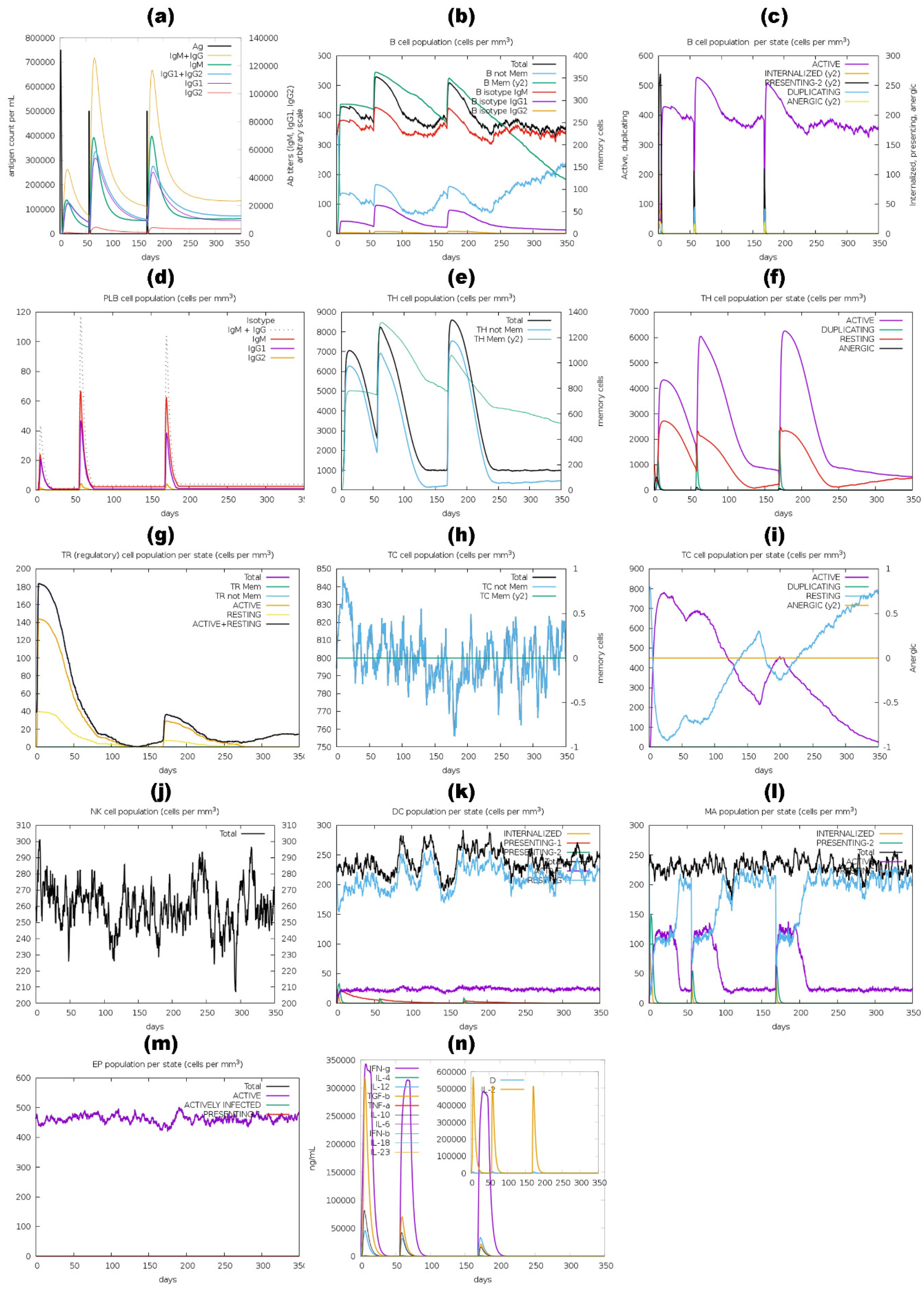
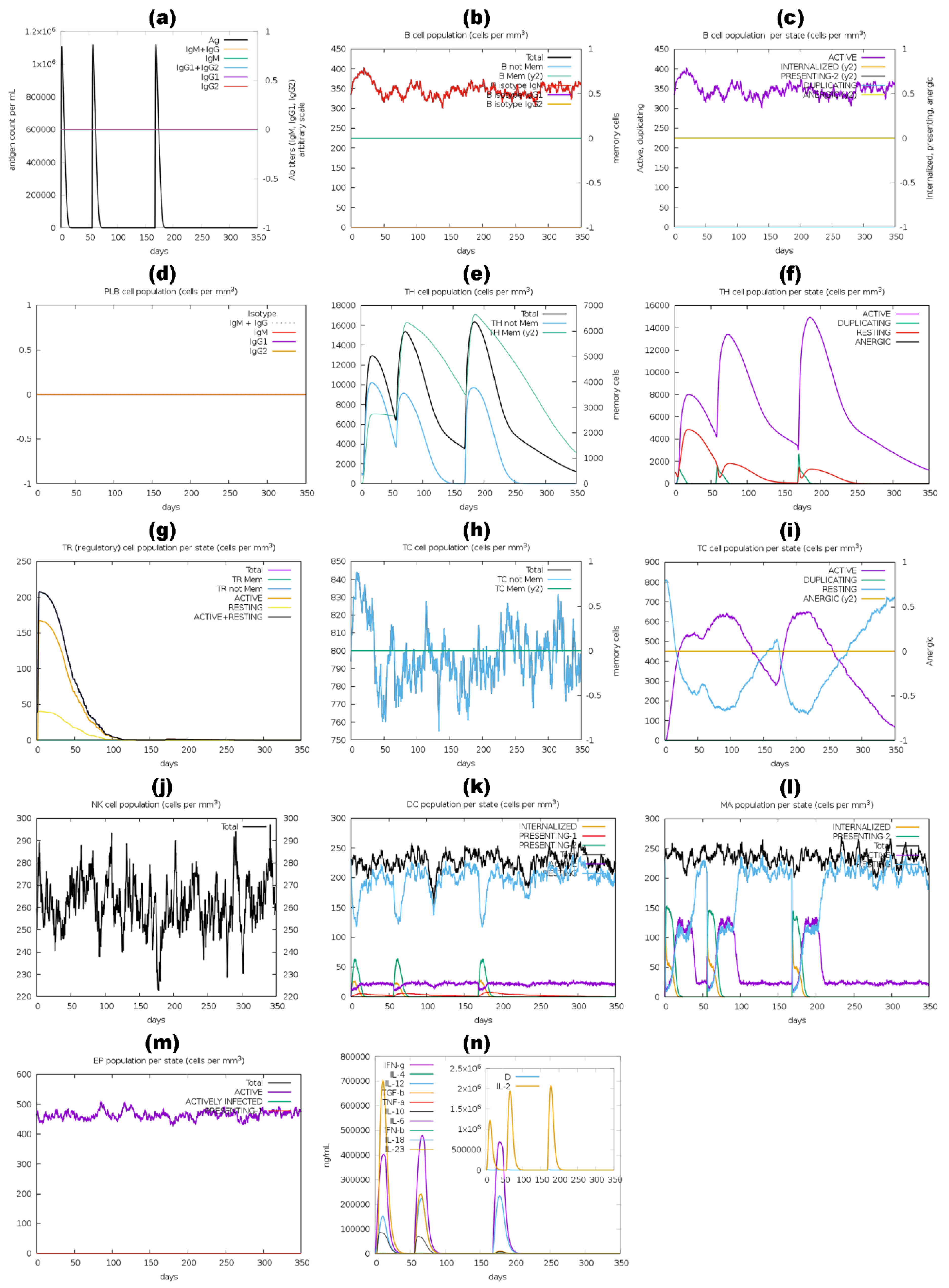
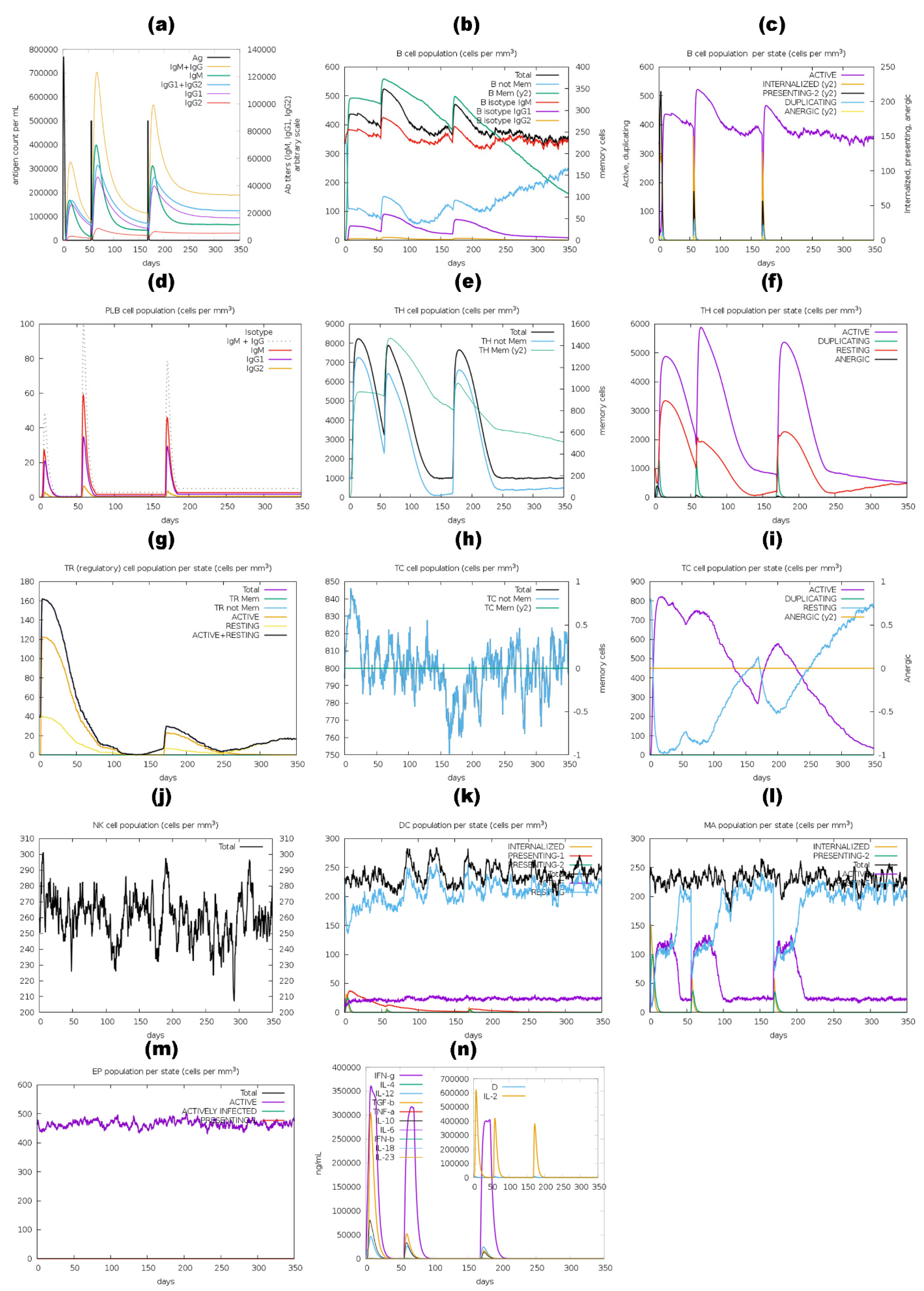
2.11. Immune Simulation of Vaccine Constructs
3. Results
3.1. Protein Selection
3.2. Physiochemical Properties
3.3. T Cell and B Cell Epitope and Feature Profiling
3.3.1. CTL Binding Epitope Prediction
3.3.2. HTL Binding Epitope Prediction
3.3.3. LBL Binding Epitope Prediction
3.4. Epitope Conservancy Analysis
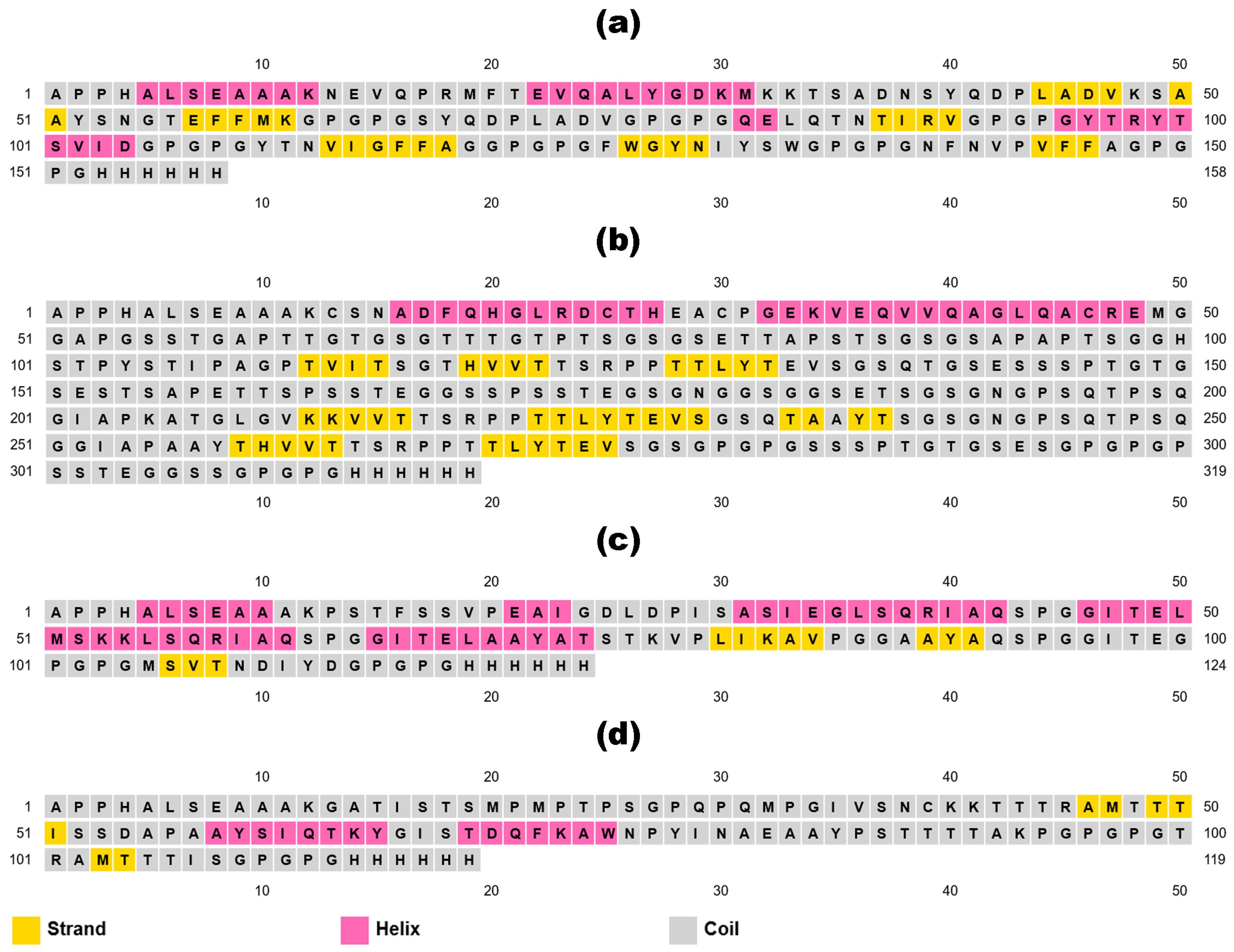
3.5. mRNA-Based Vaccine Construction and Characterization
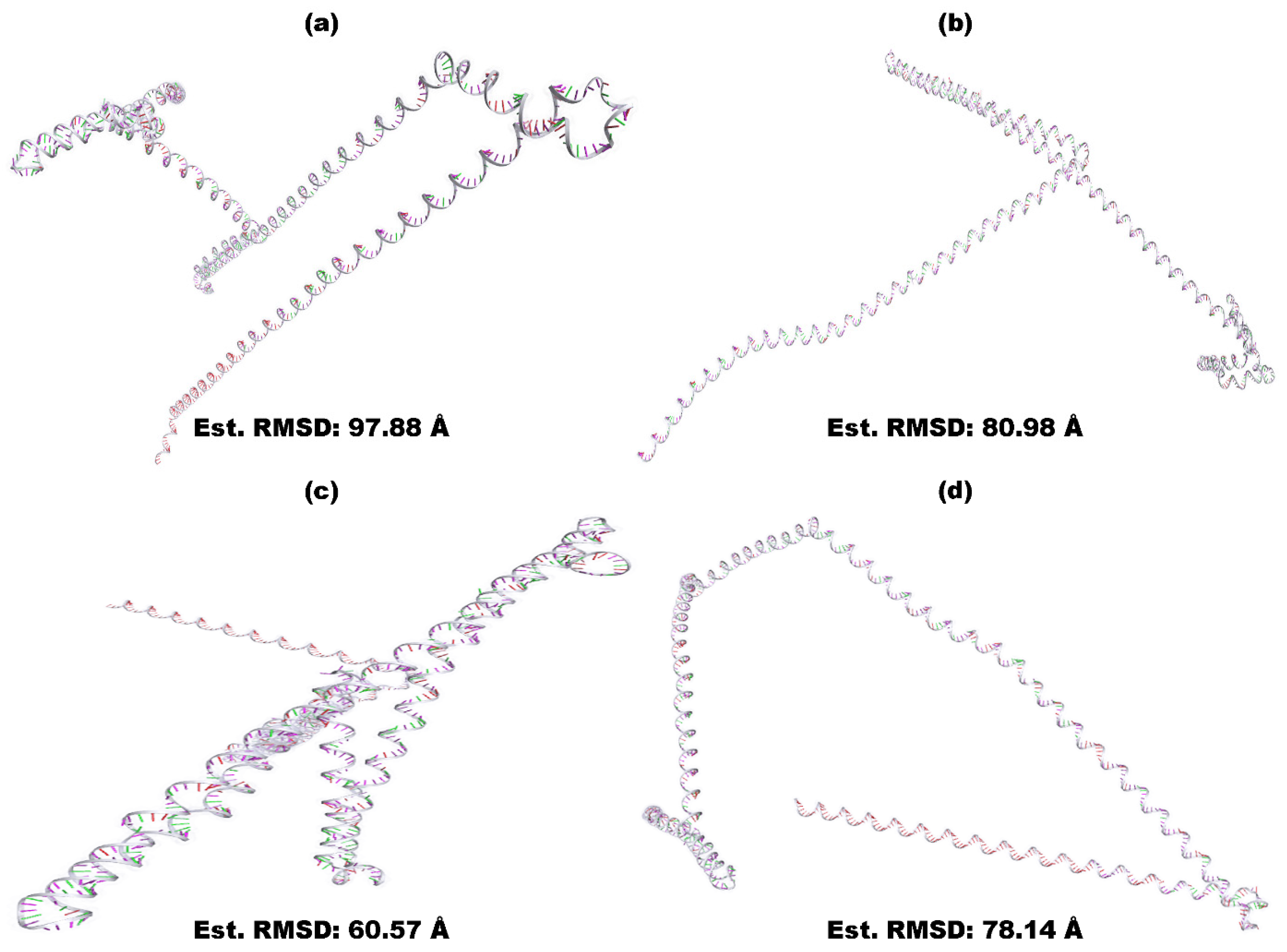
3.6. Secondary and Tertiary Structure Modeling, Refinement, and Verification
3.7. Discontinuous and Continuous B Cell Epitope Prediction
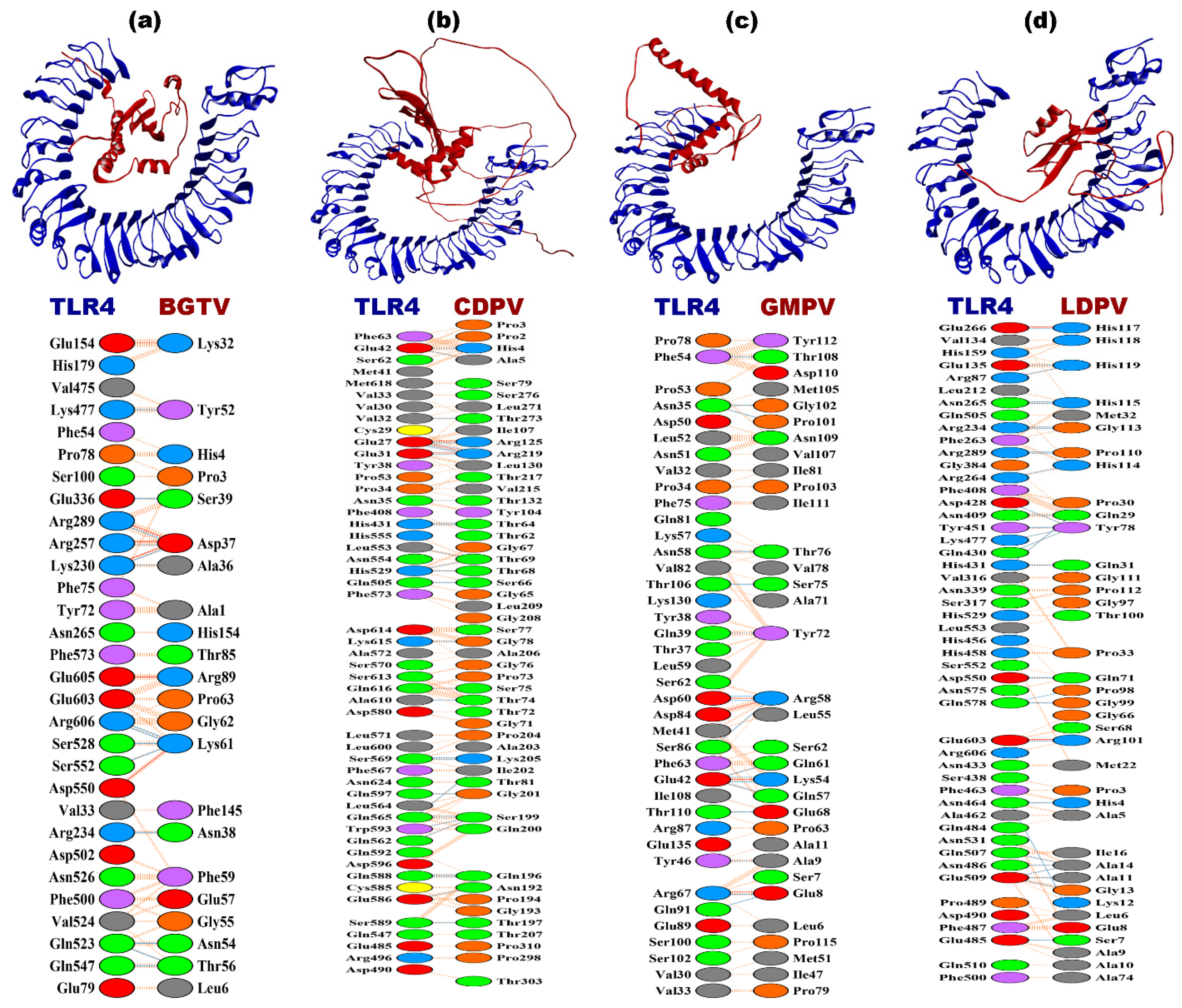
3.8. Molecular Docking
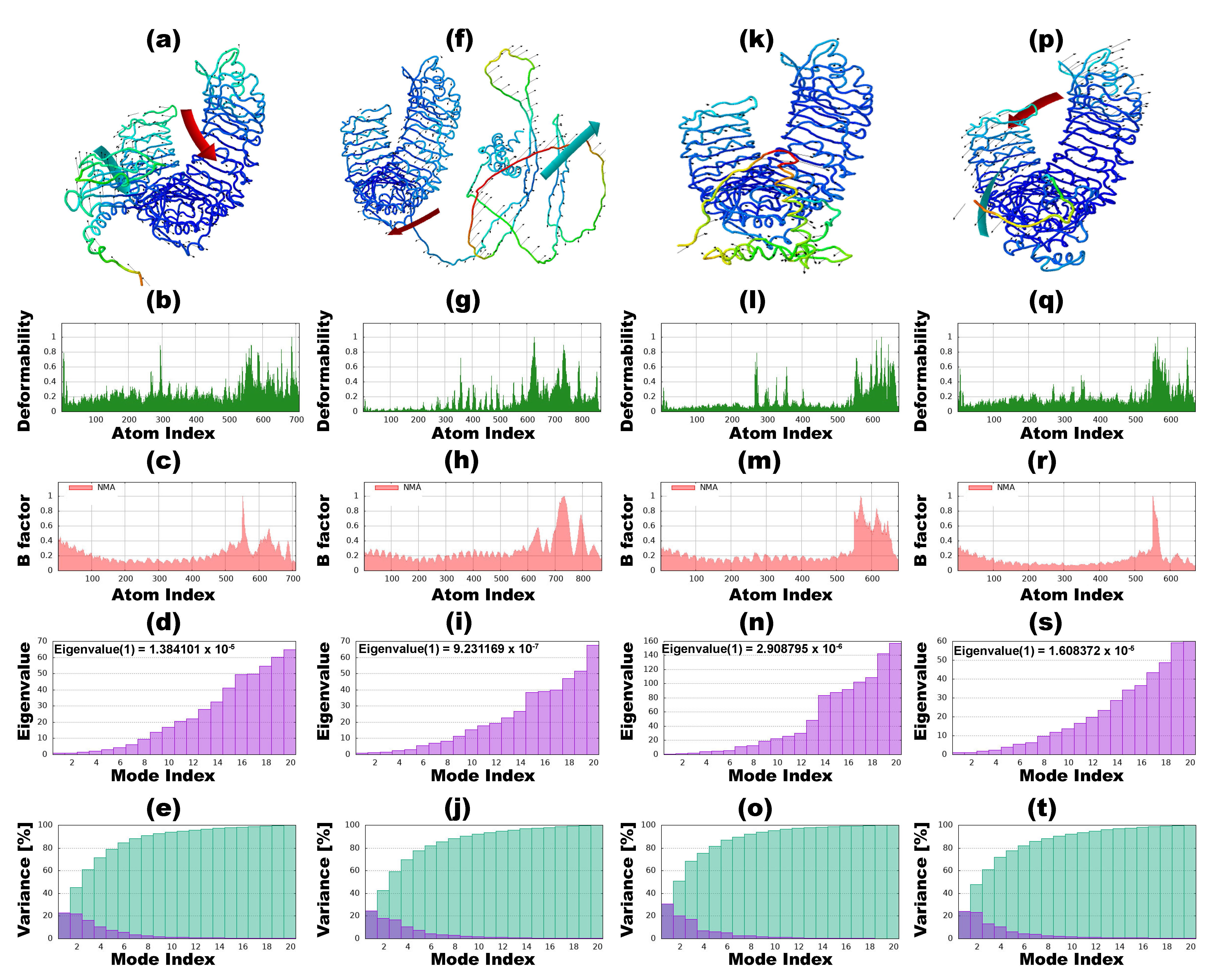
3.9. Normal Mode Analysis
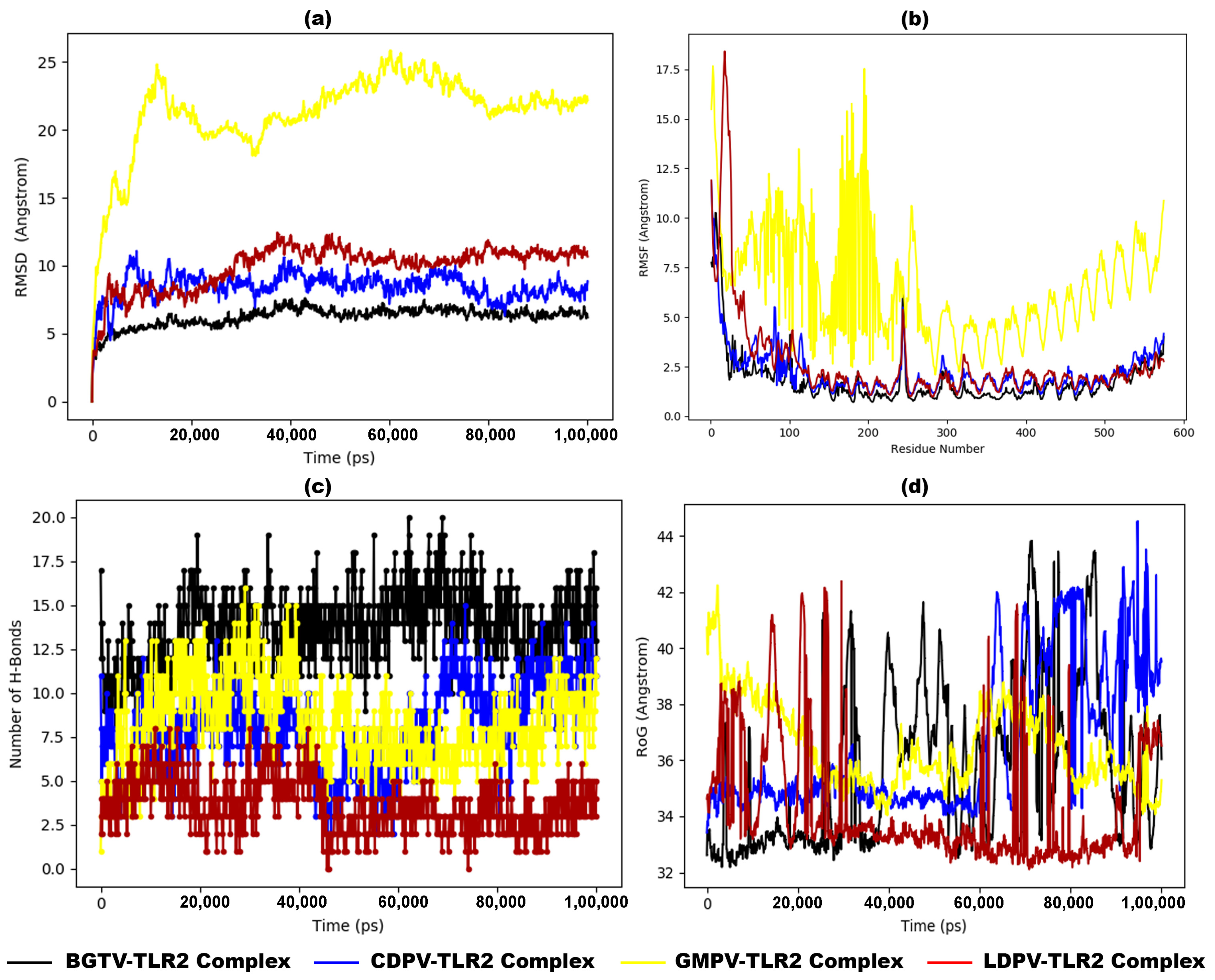
3.10. Molecular Dynamic Simulation
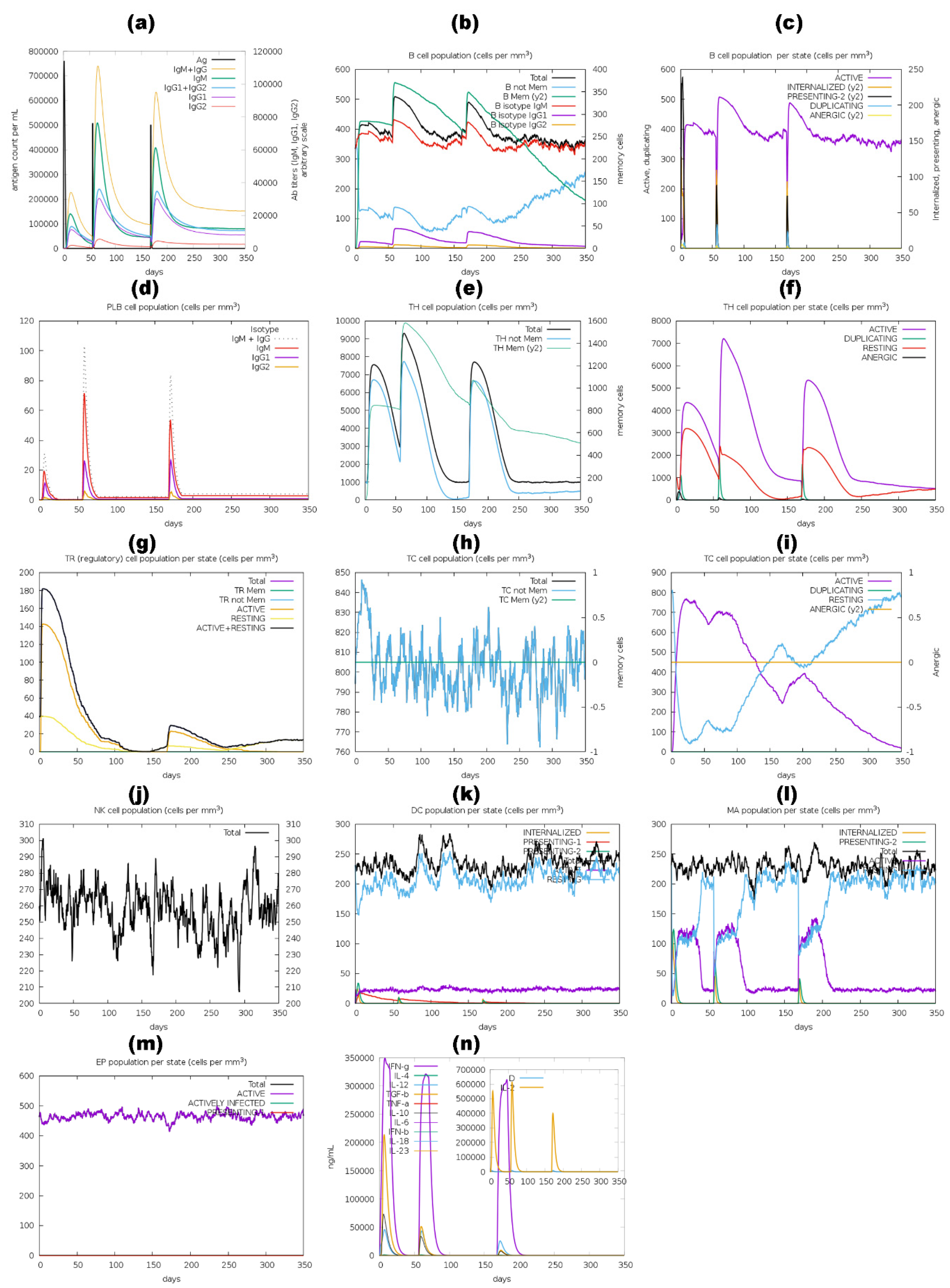
3.11. Immune Simulation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal Resistance in Dermatophytes: Recent Trends and Therapeutic Implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef]
- Sardana, K.; Kaur, R.; Arora, P.; Goyal, R.; Ghunawat, S. Is Antifungal Resistance a Cause for Treatment Failure in Dermatophytosis: A Study Focused on Tinea Corporis and Cruris from a Tertiary Centre? Indian Dermatol. Online J. 2018, 9, 90. [Google Scholar] [CrossRef]
- Bishnoi, A.; Mahajan, R. Tinea Cruris. In Diagnostics to Pathogenomics of Sexually Transmitted Infections; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 329–340. [Google Scholar] [CrossRef]
- Ismail, F.; Ghani, A.; Akbar, S. Emergence of Antifungal Azole Resistance in the Fungal Strains of Tinea Corporis, Tinea Capitis, Tinea Cruris and Tinea Pedis from the Locality of Southern Punjab, Pakistan. RADS J. Biol. Res. Appl. Sci. 2021, 12, 24–38. [Google Scholar] [CrossRef]
- Kashif, S.; Uddin, F.; Nasir, F.; Zafar, S.; Shahjabee; Kumar, S. Prevalence of Dermatophytes in Superficial Skin Infections in a Tertiary Care Hospital. J. Pak. Assoc. Dermatol. 2021, 31, 484–488. [Google Scholar]
- Ahmed, L.T.; Darweesh, Z.A.; Hussain, W.M. Prevalence of Dermatophytes Fungal Infection among Different Gender. Indian J. Forensic Med. Toxicol. 2020, 14, 1717–1722. [Google Scholar] [CrossRef]
- Khurana, A.; Agarwal, A.; Agrawal, D.; Panesar, S.; Ghadlinge, M.; Sardana, K.; Sethia, K.; Malhotra, S.; Chauhan, A.; Mehta, N. Effect of Different Itraconazole Dosing Regimens on Cure Rates, Treatment Duration, Safety, and Relapse Rates in Adult Patients with Tinea Corporis/Cruris: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 1269–1278. [Google Scholar] [CrossRef]
- Ameen, M. Epidemiology of Superficial Fungal Infections. Clin. Dermatol. 2010, 28, 197–201. [Google Scholar] [CrossRef]
- Sahoo, A.; Mahajan, R. Management of Tinea Corporis, Tinea Cruris, and Tinea Pedis: A Comprehensive Review. Indian Dermatol. Online J. 2016, 7, 77. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New Antifungal Agents and New Formulations Against Dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Y.; Hong, N.; Yang, Y.; Lei, W.; Du, L.; Zhao, J.; Lei, X.; Xiong, L.; Cai, L.; et al. Epidemiology of Fungal Infections in China. Front. Med. 2018, 12, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Girish, V.N.; Veerabhadra Goud, G.K.; Sudha, P.; Jagadevi. Prevalence of Tinea Corporis and Tinea Cruris in Chitradurga Rural Population. IP Indian J. Clin. Exp. Dermatol. 2020, 4, 221–225. [Google Scholar] [CrossRef]
- Patel, G.A.; Wiederkehr, M.; Schwartz, R.A. Tinea Cruris in Children. Cutis 2009, 84, 133–137. [Google Scholar]
- Keshwania, P.; Kaur, N.; Chauhan, J.; Sharma, G.; Afzal, O.; Alfawaz Altamimi, A.S.; Almalki, W.H. Superficial Dermatophytosis across the World’s Populations: Potential Benefits from Nanocarrier-Based Therapies and Rising Challenges. ACS Omega 2023, 8, 31575–31599. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Fedorowicz, Z.; El-Gohary, M. Evidence-Based Topical Treatments for Tinea Cruris and Tinea Corporis: A Summary of a Cochrane Systematic Review. Br. J. Dermatol. 2015, 172, 616–641. [Google Scholar] [CrossRef]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Emerging Trends in the Use of Topical Antifungal-Corticosteroid Combinations. J. Fungi 2022, 8, 812. [Google Scholar] [CrossRef]
- Hay, R. Therapy of Skin, Hair and Nail Fungal Infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef]
- Paiva, J.A.; Pereira, J.M. New Antifungal Antibiotics. Curr. Opin. Infect. Dis. 2013, 26, 168–174. [Google Scholar] [CrossRef]
- Sahni, K.; Singh, S.; Dogra, S. Newer Topical Treatments in Skin and Nail Dermatophyte Infections. Indian Dermatol. Online J. 2018, 9, 149. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Rajendran, P.; Ali, E.M. Potential Treatment of Dermatophyte Trichophyton rubrum in Rat Model Using Topical Green Biosynthesized Silver Nanoparticles with Achillea Santolina Extract. Molecules 2023, 28, 1536. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, V. Application of Anti-Fungal Vaccines as a Tool against Emerging Anti-Fungal Resistance. Front. Fungal Biol. 2023, 4, 1241539. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Harvey, S.; Armstrong-James, D. Immunotherapeutic Approaches for Fungal Infections. Curr. Opin. Microbiol. 2020, 58, 130–137. [Google Scholar] [CrossRef]
- Nanjappa, S.G.; Klein, B.S. Vaccine Immunity against Fungal Infections. Curr. Opin. Immunol. 2014, 28, 27–33. [Google Scholar] [CrossRef]
- Lionakis, M.S. Exploiting Antifungal Immunity in the Clinical Context. Semin. Immunol. 2023, 67, 101752. [Google Scholar] [CrossRef]
- Inácio, M.M.; Moreira, A.L.E.; Cruz-Leite, V.R.M.; Mattos, K.; Silva, L.O.S.; Venturini, J.; Ruiz, O.H.; Ribeiro-Dias, F.; Weber, S.S.; Soares, C.M.d.A.; et al. Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics. J. Fungi 2023, 9, 633. [Google Scholar] [CrossRef]
- The Last of Us Raises the Question: Why Don’t We Have Vaccines for Fungal Infections?—Vox. Available online: https://www.vox.com/science-and-health/2023/2/11/23592955/last-of-us-fungal-vaccines-infections-cordyceps (accessed on 12 April 2024).
- Edwards, J.E., Jr.; Schwartz, M.M.; Schmidt, C.S.; Sobel, J.D.; Nyirjesy, P.; Schodel, F.; Marchus, E.; Lizakowski, M.; DeMontigny, E.A.; Hoeg, J.; et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2018, 66, 1928–1936. [Google Scholar] [CrossRef]
- Da Silva, L.B.R.; Taborda, C.P.; Nosanchuk, J.D. Advances in Fungal Peptide Vaccines. J. Fungi 2020, 6, 119. [Google Scholar] [CrossRef]
- Rachini, A.; Pietrella, D.; Lupo, P.; Torosantucci, A.; Chiani, P.; Bromuro, C.; Proietti, C.; Bistoni, F.; Cassone, A.; Vecchiarelli, A. An Anti-β-Glucan Monoclonal Antibody Inhibits Growth and Capsule Formation of Cryptococcus neoformans In Vitro and Exerts Therapeutic, Anticryptococcal Activity In Vivo. Infect. Immun. 2007, 75, 5085–5094. [Google Scholar] [CrossRef]
- Cassone, A.; Casadevall, A. Recent Progress in Vaccines against Fungal Diseases. Curr. Opin. Microbiol. 2012, 15, 427–433. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for Human Fungal Diseases: Close but Still a Long Way to Go. npj Vaccines 2021, 6, 33. [Google Scholar] [CrossRef]
- Posch, W.; Steger, M.; Wilflingseder, D.; Lass-Flörl, C. Promising Immunotherapy against Fungal Diseases. Expert Opin. Biol. Ther. 2017, 17, 861–870. [Google Scholar] [CrossRef]
- Ba, A.; Umitzhanov, M.; Umitzhanov, M.; Aa, I.; Gk, O.; Nz, B. Characteristics of the Trichophyton Mentagrophytes F-01 Strain Used for the Manufacture of Biologics against Bovine Trichophyton OPEN ACCESS Citation. World J. Surg. Surg. Res. 2022, 5, 1397. [Google Scholar]
- Abo-Elyazeed, H.; Soliman, R.; Hassan, H.; El-Seedy, F.R.; Aboul-Ella, H. Development, Preparation, and Evaluation of a Novel Non-Adjuvanted Polyvalent Dermatophytes Vaccine. Sci. Rep. 2023, 13, 157. [Google Scholar] [CrossRef]
- Rayens, E.; Rabacal, W.; Willems, H.M.E.; Kirton, G.M.; Barber, J.P.; Mousa, J.J.; Celia-Sanchez, B.N.; Momany, M.; Norris, K.A. Immunogenicity and Protective Efficacy of a Pan-Fungal Vaccine in Preclinical Models of Aspergillosis, Candidiasis, and Pneumocystosis. PNAS Nexus 2022, 1, pgac248. [Google Scholar] [CrossRef]
- Hataway, L. New Vaccine Targets Life-Threatening Fungal Infections. Available online: https://news.uga.edu/new-vaccine-targets-fungal-infections/ (accessed on 13 April 2024).
- Rodríguez-Cerdeira, C.; Molares-Vila, A.; Sánchez-Cárdenas, C.D.; Velásquez-Bámaca, J.S.; Martínez-Herrera, E. Bioinformatics Approaches Applied to the Discovery of Antifungal Peptides. Antibiotics 2023, 12, 566. [Google Scholar] [CrossRef]
- Avina, S. Breakthroughs and Challenges in Fungal Vaccine Development. Available online: https://asm.org/articles/2023/november/breakthroughs-and-challenges-in-fungal-vaccine-dev (accessed on 12 April 2024).
- Basu, A. In Silico Epitope-Based Vaccine Prediction against Fungal Infection Aspergillosis. Challenges 2022, 13, 29. [Google Scholar] [CrossRef]
- Bidmos, F.A.; Siris, S.; Gladstone, C.A.; Langford, P.R. Bacterial Vaccine Antigen Discovery in the Reverse Vaccinology 2.0 Era: Progress and Challenges. Front. Immunol. 2018, 9, 2315. [Google Scholar] [CrossRef]
- Delany, I.; Rappuoli, R.; Seib, K.L. Vaccines, Reverse Vaccinology, and Bacterial Pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a012476. [Google Scholar] [CrossRef]
- Elalouf, A.; Yaniv-Rosenfeld, A. Immunoinformatic-Guided Designing and Evaluating Protein and MRNA-Based Vaccines against Cryptococcus neoformans for Immunocompromised Patients. J. Genet. Eng. Biotechnol. 2023, 21, 108. [Google Scholar] [CrossRef]
- Zhang, L. Multi-Epitope Vaccines: A Promising Strategy against Tumors and Viral Infections. Cell. Mol. Immunol. 2018, 15, 182–184. [Google Scholar] [CrossRef]
- Nosrati, M.; Behbahani, M.; Mohabatkar, H. Towards the First Multi-Epitope Recombinant Vaccine against Crimean-Congo Hemorrhagic Fever Virus: A Computer-Aided Vaccine Design Approach. J. Biomed. Inform. 2019, 93, 103160. [Google Scholar] [CrossRef]
- Hajighahramani, N.; Nezafat, N.; Eslami, M.; Negahdaripour, M.; Rahmatabadi, S.S.; Ghasemi, Y. Immunoinformatics Analysis and In Silico Designing of a Novel Multi-Epitope Peptide Vaccine against Staphylococcus aureus. Infect. Genet. Evol. 2017, 48, 83–94. [Google Scholar] [CrossRef]
- Sette, A.; Livingston, B.; McKinney, D.; Appella, E.; Fikes, J.; Sidney, J.; Newman, M.; Chesnut, R. The Development of Multi-Epitope Vaccines: Epitope Identification, Vaccine Design and Clinical Evaluation. Biologicals 2001, 29, 271–276. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Nezafat, N.; Barzegar, A.; Negahdaripour, M.; Nikanfar, A.R.; Zarghami, N.; Ghasemi, Y. Harnessing Bioinformatics for Designing a Novel Multiepitope Peptide Vaccine Against Breast Cancer. Curr. Pharm. Biotechnol. 2016, 17, 1100–1114. [Google Scholar] [CrossRef]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in MRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of MRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research Progress on the Quality Control of MRNA Vaccines. Expert Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. MRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10. [Google Scholar] [CrossRef]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 MRNA Vaccines: Platforms and Current Developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- Latge, J.P.; Debeaupuis, J.P.; Sarfati, J.; Diaquin, M.; Paris, S. Cell Wall Antigens in Aspergillus fumigatus. Arch. Med. Res. 1993, 24, 269–274. [Google Scholar]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 492056. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 28513415. [Google Scholar] [CrossRef]
- López-Ribot, J.L.; Casanova, M.; Murgui, A.; Martínez, J.P. Antibody Response to Candida albicans Cell Wall Antigens. FEMS Immunol. Med. Microbiol. 2004, 41, 187–196. [Google Scholar] [CrossRef]
- Kaur, G.; Chawla, S.; Kumar, P.; Singh, R. Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers. Vaccines 2023, 11, 1658. [Google Scholar] [CrossRef]
- Gu, Y.; Duan, J.; Yang, N.; Yang, Y.; Zhao, X. MRNA Vaccines in the Prevention and Treatment of Diseases. MedComm 2022, 3, e167. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A Server for Prediction of Protective Antigens, Tumour Antigens and Subunit Vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Nielsen, H.; Winther, O. DeepLoc 2.0: Multi-Label Subcellular Localization Prediction Using Protein Language Models. Nucleic Acids Res. 2022, 50, W228–W234. [Google Scholar] [CrossRef]
- Bagheri, S.H.; Asghari, A.; Farhadi, M.; Shamshiri, A.R.; Kabir, A.; Kamrava, S.K.; Jalessi, M.; Mohebbi, A.; Alizadeh, R.; Honarmand, A.A.; et al. Coincidence of COVID-19 Epidemic and Olfactory Dysfunction Outbreak in Iran. Med. J. Islam. Repub. Iran 2020, 34, 62. [Google Scholar] [CrossRef]
- Naveed, M.; Sheraz, M.; Amin, A.; Waseem, M.; Aziz, T.; Khan, A.A.; Ghani, M.; Shahzad, M.; Alruways, M.W.; Dablool, A.S.; et al. Designing a Novel Peptide-Based Multi-Epitope Vaccine to Evoke a Robust Immune Response against Pathogenic Multidrug-Resistant Providencia Heimbachae. Vaccines 2022, 10, 1300. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Walker, J.M. The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Rawal, K.; Sinha, R.; Abbasi, B.A.; Chaudhary, A.; Nath, S.K.; Kumari, P.; Preeti, P.; Saraf, D.; Singh, S.; Mishra, K.; et al. Identification of Vaccine Targets in Pathogens and Design of a Vaccine Using Computational Approaches. Sci. Rep. 2021, 11, 17626. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, W.; Cai, K.; Ma, S.; Wang, Y.; Ma, Y.; Zhao, H. Identification of Vaccine Candidates against Rhodococcus equi by Combining Pangenome Analysis with a Reverse Vaccinology Approach. Heliyon 2023, 9, e18623. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. ISBN 978-1-59259-890-8. [Google Scholar]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef] [PubMed]
- Hon, J.; Marusiak, M.; Martinek, T.; Kunka, A.; Zendulka, J.; Bednar, D.; Damborsky, J. SoluProt: Prediction of Soluble Protein Expression in Escherichia coli. Bioinformatics 2021, 37, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fleri, W.; Paul, S.; Dhanda, S.K.; Mahajan, S.; Xu, X.; Peters, B.; Sette, A. The Immune Epitope Database and Analysis Resource in Epitope Discovery and Synthetic Vaccine Design. Front. Immunol. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved Predictions of MHC Antigen Presentation by Concurrent Motif Deconvolution and Integration of MS MHC Eluted Ligand Data. Nucleic Acids Res. 2021, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Raghava, G.P.S. ProPred1: Prediction of Promiscuous MHC Class-I Binding Sites. Bioinformatics 2003, 19, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Naorem, L.D.; Jain, S.; Raghava, G.P.S. ToxinPred2: An Improved Method for Predicting Toxicity of Proteins. Brief. Bioinform. 2022, 23, bbac174. [Google Scholar] [CrossRef] [PubMed]
- Calis, J.J.A.; Maybeno, M.; Greenbaum, J.A.; Weiskopf, D.; De Silva, A.D.; Sette, A.; Keşmir, C.; Peters, B. Properties of MHC Class I Presented Peptides That Enhance Immunogenicity. PLoS Comput. Biol. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A Server for in Silico Prediction of Allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Singh, H.; Raghava, G.P.S. ProPred: Prediction of HLA-DR Binding Sites. Bioinformatics 2002, 17, 1236–1237. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, G.; Usmani, S.S.; Dhanda, S.K.; Kaur, H.; Singh, S.; Sharma, M.; Raghava, G.P.S. Computer-Aided Designing of Immunosuppressive Peptides Based on IL-10 Inducing Potential. Sci. Rep. 2017, 7, srep42851. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Vir, P.; Raghava, G.P.S. Designing of Interferon-Gamma Inducing MHC Class-II Binders. Biol. Direct 2013, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Gupta, S.; Vir, P.; Raghava, G.P. Prediction of IL4 Inducing Peptides. Clin. Dev. Immunol. 2013, 2013, 263952. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving Sequence-Based B-Cell Epitope Prediction Using Conformational Epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. Prediction Methods for B-Cell Epitopes. Methods Mol. Biol. 2007, 409, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. Prediction of Continuous B-Cell Epitopes in an Antigen Using Recurrent Neural Network. Proteins Struct. Funct. Genet. 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.H.; Sidney, J.; Li, W.; Fusseder, N.; Sette, A. Development of an Epitope Conservancy Analysis Tool to Facilitate the Design of Epitope-Based Diagnostics and Vaccines. BMC Bioinform. 2007, 8, 361. [Google Scholar] [CrossRef]
- Sanches, R.C.O.; Tiwari, S.; Ferreira, L.C.G.; Oliveira, F.M.; Lopes, M.D.; Passos, M.J.F.; Maia, E.H.B.; Taranto, A.G.; Kato, R.; Azevedo, V.A.C.; et al. Immunoinformatics Design of Multi-Epitope Peptide-Based Vaccine Against Schistosoma Mansoni Using Transmembrane Proteins as a Target. Front. Immunol. 2021, 12, 621706. [Google Scholar] [CrossRef]
- Tarrahimofrad, H.; Rahimnahal, S.; Zamani, J.; Jahangirian, E.; Aminzadeh, S. Designing a Multi-Epitope Vaccine to Provoke the Robust Immune Response against Influenza A H7N9. Sci. Rep. 2021, 11, 24485. [Google Scholar] [CrossRef]
- Hasan, M.; Mia, M. Exploratory Algorithm of a Multi-Epitope-Based Subunit Vaccine Candidate against Cryptosporidium hominis: Reverse Vaccinology-Based Immunoinformatic Approach. Int. J. Pept. Res. Ther. 2022, 28, 134. [Google Scholar] [CrossRef]
- Tarang, S.; Kesherwani, V.; LaTendresse, B.; Lindgren, L.; Rocha-Sanchez, S.M.; Weston, M.D. In Silico Design of a Multivalent Vaccine Against Candida albicans. Sci. Rep. 2020, 10, 1066. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Gong, T.; Zhang, Z.; Sun, X. Intranasal Vaccination against HIV-1 with Adenoviral Vector-Based Nanocomplex Using Synthetic TLR-4 Agonist Peptide as Adjuvant. Mol. Pharm. 2016, 13, 885–894. [Google Scholar] [CrossRef]
- Shanmugam, A.; Rajoria, S.; George, A.L.; Mittelman, A.; Suriano, R.; Tiwari, R.K. Synthetic Toll like Receptor-4 (TLR-4) Agonist Peptides as a Novel Class of Adjuvants. PLoS ONE 2012, 7, e30839. [Google Scholar] [CrossRef]
- Rai, D.K.; Segundo, F.D.S.; Schafer, E.; Burrage, T.G.; Rodriguez, L.L.; de los Santos, T.; Hoeprich, P.D.; Rieder, E. Novel 6xHis Tagged Foot-and-Mouth Disease Virus Vaccine Bound to Nanolipoprotein Adjuvant via Metal Ions Provides Antigenic Distinction and Effective Protective Immunity. Virology 2016, 495, 136–147. [Google Scholar] [CrossRef]
- Mukhtar, M.; Wajeeha, A.W.; us Sahar Sadaf Zaidi, N.; Bibi, N. Engineering Modified MRNA-Based Vaccine against Dengue Virus Using Computational and Reverse Vaccinology Approaches. Int. J. Mol. Sci. 2022, 23, 13911. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Nezafat, N.; Negahdaripour, M.; Eskandari, S.; Zamani, M. In Silico Design and Evaluation of a Novel MRNA Vaccine against BK Virus: A Reverse Vaccinology Approach. Immunol. Res. 2022, 1, 422–441. [Google Scholar] [CrossRef]
- Al Tbeishat, H. Novel In Silico MRNA Vaccine Design Exploiting Proteins of M. Tuberculosis That Modulates Host Immune Responses by Inducing Epigenetic Modifications. Sci. Rep. 2022, 12, 4645. [Google Scholar] [CrossRef]
- Imdad, M.J.; Khan, M.N.; Alam, H.S.; Khan, A.B.; Mirani, Z.A.; Khan, A.; Ahmed, F. Design and In Silico Analysis of MRNA Vaccine Construct against Salmonella. J. Biomol. Struct. Dyn. 2023, 41, 7248–7264. [Google Scholar] [CrossRef]
- Grote, A.; Hiller, K.; Scheer, M.; Münch, R.; Nörtemann, B.; Hempel, D.C.; Jahn, D. JCat: A Novel Tool to Adapt Codon Usage of a Target Gene to Its Potential Expression Host. Nucleic Acids Res. 2005, 33, W526–W531. [Google Scholar] [CrossRef]
- Mathews, D.H.; Disney, M.D.; Childs, J.L.; Schroeder, S.J.; Zuker, M.; Turner, D.H. Incorporating Chemical Modification Constraints into a Dynamic Programming Algorithm for Prediction of RNA Secondary Structure. Proc. Natl. Acad. Sci. USA 2004, 101, 7287–7292. [Google Scholar] [CrossRef]
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA Websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Wang, W.; Feng, C.; Han, R.; Wang, Z.; Ye, L.; Du, Z.; Wei, H.; Zhang, F.; Peng, Z.; Yang, J. TrRosettaRNA: Automated Prediction of RNA 3D Structure with Transformer Network. Nat. Commun. 2023, 14, 7266. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of Mrna-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Magnan, C.N.; Zeller, M.; Kayala, M.A.; Vigil, A.; Randall, A.; Felgner, P.L.; Baldi, P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 2010, 26, 2936–2943. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. Sopma: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinform. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED Protein Structure Prediction Server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Ko, J.; Park, H.; Heo, L.; Seok, C. GalaxyWEB Server for Protein Structure Prediction and Refinement. Nucleic Acids Res. 2012, 40, W294–W297. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J.M. PROCHECK: Validation of Protein-Structure Coordinates. In International Tables of Crystallography, Volume F. Crystallography of Biological Macromolecules; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 684–687. [Google Scholar]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A New Structure-Based Tool for the Prediction of Antibody Epitopes. BMC Bioinformatics 2008, 9, 514. [Google Scholar] [CrossRef]
- Levitz, S.M. Interactions of Toll-like Receptors with Fungi. Microbes Infect. 2004, 6, 1351–1355. [Google Scholar] [CrossRef]
- Masure, D.; Vlaminck, J.; Wang, T.; Chiers, K.; Van den Broeck, W.; Vercruysse, J.; Geldhof, P. A Role for Eosinophils in the Intestinal Immunity against Infective Ascaris Suum Larvae. PLoS Neglected Trop. Dis. 2013, 7, e2138. [Google Scholar] [CrossRef]
- Nogueira, D.S.; De Oliveira, L.M.; Amorim, C.C.O.; Gazzinelli-Guimaraes, A.C.; Barbosa, F.S.; Oliveira, F.M.S.; Kraemer, L.; Mattos, M.; Cardoso, M.S.; Resende, N.M.; et al. Eosinophils Mediate SIgA Production Triggered by TLR2 and TLR4 to Control Ascaris Suum Infection in Mice. PLoS Pathog. 2021, 17, e1010067. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro Web Server for Protein-Protein Docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Kozakov, D. New Additions to the ClusPro Server Motivated by CAPRI. Proteins Struct. Funct. Bioinform. 2017, 85, 435–444. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Hameed, S.; Ali, M.; Zaheer, A. In Silico Molecular Docking Analysis of Limonene with The Fat Mass and Obesity-Associated Protein by Using Autodock Vina. Sci. J. Inform. 2021, 8, 154–160. [Google Scholar]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A Web Server for Predicting the Binding Affinity of Protein-Protein Complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Vangone, A.; Bonvin, A.M.J.J. Contacts-Based Prediction of Binding Affinity in Protein–Protein Complexes. Elife 2015, 4, e07454. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Hutchinson, E.G.; Michie, A.D.; Wallace, A.C.; Jones, M.L.; Thornton, J.M. PDBsum: A Web-Based Database of Summaries and Analyses of All PDB Structures. Trends Biochem. Sci. 1997, 22, 488–490. [Google Scholar] [CrossRef] [PubMed]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. IMODS: Internal Coordinates Normal Mode Analysis Server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, SC’06, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Ferreira, L.G.; Oliva, G.; Andricopulo, A.D. Target-Based Molecular Modeling Strategies for Schistosomiasis Drug Discovery. Future Med. Chem. 2015, 7, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z. Computational Analysis of Action Mechanism and Evolutionary Computational Analysis of Action Mechanism and Evolutionary Insight of Chitin Degrading Enzymes in Bacillus cereus. J. Xi’an Shiyou Univ. Nat. Sci. Ed. 2024, 67, 55–101. [Google Scholar]
- Hildebrand, P.W.; Rose, A.S.; Tiemann, J.K.S. Bringing Molecular Dynamics Simulation Data into View. Trends Biochem. Sci. 2019, 44, 902–913. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Iqbal, M.N.; Saddick, S.; Ali, I.; Khan, F.S.; Kanwal, S.; Ahmed, D.; Ibrahim, M.; Afzal, U.; Awais, M. Identification of Lead Compounds against Scm (Fms10) in Enterococcus Faecium Using Computer Aided Drug Designing. Life 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies Using Molecular Dynamics Free Energy Perturbation and the Opls Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2012, 7, 146–157. [Google Scholar] [CrossRef]
- Castiglione, F.; Bernaschi, M. C-Immsim: Playing with the Immune Response. In Proceedings of the 16th International Symposium on Mathematical Theory of Networks and Systems (MTNS2004), Leuven, Belgium, 5–9 July 2004; pp. 1–7. [Google Scholar]
- Elalouf, A.; Kedarya, T.; Elalouf, H.; Rosenfeld, A. Computational Design and Evaluation of MRNA- and Protein-Based Conjugate Vaccines for Influenza A and SARS-CoV-2 Viruses. J. Genet. Eng. Biotechnol. 2023, 21, 120–124. [Google Scholar] [CrossRef]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern Recognition Receptors in Fungal Immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Deng, Z.; Wu, H.; Zhao, Q.; Li, T.; Zhu, W.; Wang, X.; Tang, L.; Wang, C.; Cui, S.Z.; et al. A Small Secreted Protein Triggers a TLR2/4-Dependent Inflammatory Response during Invasive Candida albicans Infection. Nat. Commun. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity; Frontiers Media SA: Lausanne, Switzerland, 2022; Volume 13, p. 812774. [Google Scholar]
- Kardani, K.; Bolhassani, A.; Namvar, A. An Overview of In Silico Vaccine Design against Different Pathogens and Cancer. Expert Rev. Vaccines 2020, 19, 699–726. [Google Scholar] [CrossRef] [PubMed]
- Raoufi, E.; Hemmati, M.; Eftekhari, S.; Khaksaran, K.; Mahmodi, Z.; Farajollahi, M.M.; Mohsenzadegan, M. Epitope Prediction by Novel Immunoinformatics Approach: A State-of-the-Art Review. Int. J. Pept. Res. Ther. 2020, 26, 1155–1163. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal Co-Infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Safavi, A.; Kefayat, A.; Sotoodehnejadnematalahi, F.; Salehi, M.; Modarressi, M.H. Production, Purification, and In Vivo Evaluation of a Novel Multiepitope Peptide Vaccine Consisted of Immunodominant Epitopes of SYCP1 and ACRBP Antigens as a Prophylactic Melanoma Vaccine. Int. Immunopharmacol. 2019, 76, 105872. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, S.; Habibi, M.; Shokrgozar, M.A.; Ahangari Cohan, R.; Ahmadi, K.; Asadi Karam, M.R.; Bouzari, S. In Silico Analysis and In Vivo Assessment of a Novel Epitope-Based Vaccine Candidate against Uropathogenic Escherichia coli. Sci. Rep. 2020, 10, 16258. [Google Scholar] [CrossRef]
- Soltan, M.A.; Magdy, D.; Solyman, S.M.; Hanora, A. Design of Staphylococcus aureus New Vaccine Candidates with B and T Cell Epitope Mapping, Reverse Vaccinology, and Immunoinformatics. OMICS A J. Integr. Biol. 2020, 24, 195–204. [Google Scholar] [CrossRef]
- Pan, X.; Ke, H.; Niu, X.; Li, S.; Lv, J.; Pan, L. Protection against Helicobacter Pylori Infection in BALB/c Mouse Model by Oral Administration of Multivalent Epitope-Based Vaccine of Cholera Toxin B Subunit-HUUC. Front. Immunol. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Holanda, R.A.; Muñoz, J.E.; Dias, L.S.; Silva, L.B.R.; Santos, J.R.A.; Pagliari, S.; Vieira, É.L.M.; Paixão, T.A.; Taborda, C.P.; Santos, D.A.; et al. Recombinant Vaccines of a CD4+T-Cell Epitope Promote Efficient Control of Paracoccidioides Brasiliensis Burden by Restraining Primary Organ Infection. PLoS Negl. Trop. Dis. 2017, 11, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Trailokya, A.A. Difficult to Treat Superficial Fungal Infections: Which Factors One Should Consider in Clinical Practice—An Indian Perspective. IP Indian J. Clin. Exp. Dermatol. 2023, 9, 249–251. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Rao, T.; Saeed, A.; Mutahir, Z.; Hameed, S.; Inayat, S.; Shahzad, H.; Ullah, N.; Abaid-Ullah, M.; Ibrahim, M.; et al. Antifungal Drugs: Mechanism of Action and Resistance. In Biochemistry of Drug Resistance; Springer International Publishing: Cham, Switzerland, 2021; pp. 143–165. [Google Scholar]
- Ahmed, S.; Ahmed, M.Z.; Rafique, S.; Almasoudi, S.E.; Shah, M.; Jalil, N.A.C.; Ojha, S.C. Recent Approaches for Downplaying Antibiotic Resistance: Molecular Mechanisms. Biomed. Res. Int. 2023, 2023, 5250040. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.E.; Somayaji, R.; Haber, R.M.; Parsons, L. Diagnosis and Management of Cutaneous Tinea Infections. Adv. Ski. Wound Care 2019, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mouyna, I.; Hartl, L.; Latgé, J.P. β-1,3-Glucan Modifying Enzymes in Aspergillus fumigatus. Front. Microbiol. 2013, 4, 41198. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; O’Donnell, H.; Routier, F.H.; Tiralongo, J.; Haselhorst, T. Glycobiology of Human Fungal Pathogens: New Avenues for Drug Development. Cells 2019, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zheng, X.; Wang, X.; Yang, J.; Zheng, X.; Zeng, Q.; Li, J.; Zhuge, Q.; Xiong, Q. Systematic Identification and Functional Characterization of the CFEM Proteins in Poplar Fungus Marssonina brunnea. Front. Cell. Infect. Microbiol. 2022, 12, 1045615. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, Y.; Li, H.; Bao, Y.; Duan, Z.; Wang, C.; Powell, C.A.; Wang, K.; Hu, Q.; Chen, B.; et al. Identification of Common Fungal Extracellular Membrane (CFEM) Proteins in Fusarium sacchari That Inhibit Plant Immunity and Contribute to Virulence. Microbiol. Spectr. 2023, 11, e0145223. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Kelkar, H.S.; Dean, R.A. An Eight-Cysteine-Containing CFEM Domain Unique to a Group of Fungal Membrane Proteins. Trends Biochem. Sci. 2003, 28, 118–121. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, W.; Wu, Y.; Zhou, Y.; Peng, F.; Zhang, S.; Chen, P.; Xu, X. BcCFEM1, a CFEM Domain-Containing Protein with Putative GPI-Anchored Site, Is Involved in Pathogenicity, Conidial Production, and Stress Tolerance in Botrytis Cinerea. Front. Microbiol. 2017, 8, 290968. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Hou, J.; Zhang, H.; Lei, J.H.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. Systematic Contributions of CFEM Domain-Containing Proteins to Iron Acquisition Are Essential for Interspecies Interaction of the Filamentous Pathogenic Fungus Beauveria bassiana. Environ. Microbiol. 2022, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Muszewska, A.; Piłsyk, S.; Perlińska-Lenart, U.; Kruszewska, J.S. Diversity of Cell Wall Related Proteins in Human Pathogenic Fungi. J. Fungi 2018, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Schmalhorst, P.S.; Routier, F.H. Biosynthesis of the Fungal Cell Wall Polysaccharide Galactomannan Requires Intraluminal GDP-Mannose. J. Biol. Chem. 2012, 287, 44418–44424. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fiorin, G.L.; Kombrink, A.; Mesters, J.R.; Thomma, B.P.H.J. Fungal Dual-Domain LysM Effectors Undergo Chitin-Induced Intermolecular, and Not Intramolecular, Dimerization. Plant Physiol. 2022, 190, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Muraosa, Y.; Toyotome, T.; Yahiro, M.; Kamei, K. Characterisation of Novel-Cell-Wall LysM-Domain Proteins LdpA and LdpB from the Human Pathogenic Fungus Aspergillus fumigatus. Sci. Rep. 2019, 9, 3345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dong, C.; Sun, X.; Zhang, Y.; Dai, H.; Bai, S. Overexpression of an Apple LysM-Containing Protein Gene, MdCERK1-2, Confers Improved Resistance to the Pathogenic Fungus, Alternaria alternata, in Nicotiana benthamiana. BMC Plant Biol. 2020, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Kombrink, A.; Thomma, B.P.H.J. LysM Effectors: Secreted Proteins Supporting Fungal Life. PLoS Pathog. 2013, 9, e1003769. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Fernandez, M.; Aragon-Perez, A.; Lopez-Llorca, L.V.; Lopez-Moya, F. Putative Lysm Effectors Contribute to Fungal Lifestyle. Int. J. Mol. Sci. 2021, 22, 3147. [Google Scholar] [CrossRef]
- Audain, E.; Ramos, Y.; Hermjakob, H.; Flower, D.R.; Perez-Riverol, Y. Accurate Estimation of Isoelectric Point of Protein and Peptide Based on Amino Acid Sequences. Bioinformatics 2016, 32, 821–827. [Google Scholar] [CrossRef]
- Can, H.; Köseoğlu, A.E.; Erkunt Alak, S.; Güvendi, M.; Döşkaya, M.; Karakavuk, M.; Gürüz, A.Y.; Ün, C. In Silico Discovery of Antigenic Proteins and Epitopes of SARS-CoV-2 for the Development of a Vaccine or a Diagnostic Approach for COVID-19. Sci. Rep. 2020, 10, 22387. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, M.; Pandiyan, G.N.; Paramasivan, R.; Kumar, A.; Gupta, B. Identification and Prioritisation of Potential Vaccine Candidates Using Subtractive Proteomics and Designing of a Multi-Epitope Vaccine against Wuchereria bancrofti. Sci. Rep. 2024, 14, 1970. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Peng, H.; Goher, F.; Islam, M.A.; Xu, S.; Guo, J.; Kang, Z.; Guo, J. A Candidate Effector Protein PstCFEM1 Contributes to Virulence of Stripe Rust Fungus and Impairs Wheat Immunity. Stress Biol. 2022, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Amini-Khoei, H.; Tahmasebian, S.; Ghatrehsamani, M.; Ghatreh Samani, K.; Edalatpanah, Y.; Rostampur, S.; Salehi, M.; Ghasemi-Dehnoo, M.; Azadegan-Dehkordi, F.; et al. Designing a Novel Multi-epitope Vaccine against Ebola Virus Using Reverse Vaccinology Approach. Sci. Rep. 2022, 12, 7757. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.; Zhang, X.; Zhang, L.; Yang, A.; Wang, Y.; Chen, X. Proteomics-Based Vaccine Targets Annotation and Design of Multi-Epitope Vaccine against Antibiotic-Resistant Streptococcus gallolyticus. Sci. Rep. 2024, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.S.; Venkateswarulu, T.C.; Abraham Peele, K.; Srirama, K. Design of a Multi-Epitope-Based Vaccine Targeting M-Protein of SARS-CoV-2: An Immunoinformatics Approach. J. Biomol. Struct. Dyn. 2022, 40, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Selvaraj, G.; Gopalan, V.; Padmanabhan, P.; Ramesh, K.; Govindan, K.; Chandran, A.; Dhandapani, P.; Krishnasamy, K.; Kitambi, S. Epitope Identification and Designing a Potent Multi-Epitope Vaccine Construct against SARS-CoV-2 Including the Emerging Variants. J. Glob. Infect. Dis. 2022, 14, 24–30. [Google Scholar] [CrossRef]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of Defined TLR Ligands as Adjuvants within Human Vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. Tlr Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Do, K.T.H.; Willenzon, S.; Ristenpart, J.; Janssen, A.; Volz, A.; Sutter, G.; Förster, R.; Bošnjak, B. The Effect of Toll-like Receptor Agonists on the Immunogenicity of MVA-SARS-2-S Vaccine after Intranasal Administration in Mice. Front. Cell. Infect. Microbiol. 2023, 13, 1259822. [Google Scholar] [CrossRef]


| Protein ID | Protein Name | Length | Antigenicity | Localizations | Extra Cellularity Score | Human Homolog Identity | Transmembrane Helix Score | Molecular Weight |
|---|---|---|---|---|---|---|---|---|
| F2SF86 | 1,3-beta-glucanosyltransferase | 531 | 0.74 | Extracellular | 0.8228 | 0.00% | 0 | 57.45 kDa |
| F2SCX9 | CFEM domain-containing protein | 263 | 1.13 | Extracellular | 0.8623 | 0.00% | 0 | 24.95 kDa |
| F2SDA6 | Cell wall galactomannoprotein | 177 | 0.67 | Extracellular | 0.9471 | 0.00% | 0 | 18.99 kDa |
| A0A080WV70 | LysM domain-containing protein | 283 | 0.98 | Extracellular | 0.942 | 0.00% | 0 | 31.15 kDa |
| Physiochemical Properties | 1,3-Beta-glucanosyltransferase | CFEM Domain-Containing Protein | Cell Wall Galactomannoprotein | LysM Domain-Containing Protein |
|---|---|---|---|---|
| Number of amino acids | 531 | 263 | 177 | 283 |
| Theoretical pI (ExPASy-ProtParam) | 5.73 | 4.66 | 5.51 | 6.57 |
| Theoretical pI (EMBOSS-PEPSTATS) | 5.63 | 4.37 | 5.32 | 6.96 |
| Negatively charged residues (Asp + Glu) | 57 | 18 | 23 | 18 |
| Positively charged residues (Arg + Lys) | 54 | 7 | 21 | 17 |
| Formula | C2531H3924N658O807S30 | C1029H1666N298O397S12 | C839H1363N225O258S8 | C1375H2135N365O418S21 |
| Total number of atoms | 7950 | 3402 | 2693 | 4314 |
| Ext. coefficient (ExPASy-ProtParam) | 64,595 | 3480 | 3105 | 39,015 |
| Molar ext. coefficients (EMBOSS-PEPSTATS) | 63,720 (reduced), 64,595 (cystine bridges) | 2980 (reduced), 3480 (cystine bridges) | 2980 (reduced), 3105 (cystine bridges) | 38,390 (reduced), 39,015 (cystine bridges) |
| Estimated Half-life (mammalian reticulocytes, in vitro) (hours) | >30 | >30 | >30 | >30 |
| Estimated Half-life (yeast, in vivo) (hours) | >20 | >20 | >20 | >20 |
| Estimated Half-life (Escherichia coli, in vivo) (hours) | >10 | >10 | >10 | >10 |
| Instability index | 25.42 | 47.14 | 50.6 | 26.67 |
| Stability classification | Stable | Unstable | Unstable | Stable |
| Aliphatic index | 65.59 | 53.12 | 90.51 | 72.76 |
| Grand average of hydropathicity (GRAVY) | −0.333 | −0.244 | 0.013 | −0.142 |
| Solubility | 0.475 | 0.148 | 0.306 | 0.271 |
| Improbability of expression in inclusion bodies | 0.816 | 0.97 | 0.571 | 0.773 |
| Proteins | Position | Peptide | Antigenic Score | Toxin | Immunogenicity Score | Allergen |
|---|---|---|---|---|---|---|
| 1,3-beta-glucanosyltransferase | 36–44 | SNGTEFFMK | 0.78 | No | 0.25 | No |
| 63–71 | SYQDPLADV | 1.5 | No | 0.01 | No | |
| 82–90 | QELQTNTIR | 0.94 | No | 0.02 | No | |
| 83–91 | ELQTNTIRV | 1.99 | No | 0.18 | No | |
| 137–145 | YTRYTSVID | 1.37 | No | 0.007 | No | |
| 150–158 | YTNVIGFFA | 1.4 | No | 0.37 | No | |
| 151–159 | TNVIGFFAG | 1.8 | No | 0.41 | No | |
| 194–202 | FWGYNIYSW | 2.2 | No | 0.02 | No | |
| 222–230 | NFNVPVFFA | 1.08 | No | 0.23 | No | |
| CFEM domain-containing protein | 22–30 | THVVTTSRP | 1.08 | No | 0.02 | No |
| 27–35 | TSRPPTTLY | 0.83 | No | 0.05 | No | |
| 28–36 | SRPPTTLYT | 1.75 | No | 0.04 | No | |
| 33–41 | TLYTEVSGS | 1.4 | No | 0.05 | No | |
| 47–55 | SSSPTGTGS | 1.64 | No | 0.05 | No | |
| 48–56 | SSPTGTGSE | 1.96 | No | 0.03 | No | |
| 49–57 | SPTGTGSES | 1.17 | No | 0.03 | No | |
| 66–74 | PSSTEGGSS | 1.7 | No | 0.04 | No | |
| Cell wall galactomannoprotein | 50–58 | AQSPGGITE | 1.98 | No | 0.13 | No |
| 60–68 | MSVTNDIYD | 0.59 | No | 0.17 | No | |
| LysM domain-containing protein | 73–81 | PSTTTTAKP | 0.67 | No | 0.03 | No |
| 101–109 | TRAMTTTIS | 2.33 | No | 0.02 | No |
| Proteins | Position | Peptide | Antigenic Score | Toxin | IFN | IL4 Inducer | IL10 Inducer | Allergen |
|---|---|---|---|---|---|---|---|---|
| 1,3-beta-glucanosyltransferase | 58–72 | TSADNSYQDPLADVK | 0.7 | No | Positive | Yes | Yes | No |
| 59–73 | SADNSYQDPLADVKS | 0.91 | No | Positive | Yes | Yes | No | |
| CFEM domain-containing protein | 24–38 | VVTTSRPPTTLYTEV | 0.7 | No | Positive | No | No | No |
| 25–39 | VTTSRPPTTLYTEVS | 1.07 | No | Positive | No | No | No | |
| 27–41 | TSRPPTTLYTEVSGS | 1.28 | No | Positive | No | No | No | |
| 29–43 | RPPTTLYTEVSGSQT | 1.71 | No | Positive | No | No | No | |
| 88–102 | TSGSGNGPSQTPSQG | 1.0 | No | Positive | No | No | No | |
| 89–103 | SGSGNGPSQTPSQGI | 1.03 | No | Positive | No | No | No | |
| 90–104 | GSGNGPSQTPSQGIA | 1.03 | No | Positive | No | No | No | |
| 91–105 | SGNGPSQTPSQGIAP | 0.57 | No | Positive | No | No | No | |
| Cell wall galactomannoprotein | 45–59 | LSQRIAQSPGGITEL | 1.87 | No | Positive | No | No | No |
| 111–125 | ATSTKVPLIKAVPGG | 1.7 | No | Positive | No | No | No | |
| LysM domain-containing protein | 99–113 | TTTRAMTTTISSDAP | 1.55 | No | Positive | Yes | No | No |
| 138–152 | SIQTKYGISTDQFKA | 2.43 | No | Positive | Yes | No | No | |
| 139–153 | IQTKYGISTDQFKAW | 2.58 | No | Positive | Yes | No | No | |
| 141–155 | TKYGISTDQFKAWNP | 1.94 | No | Positive | Yes | No | No | |
| 142–156 | KYGISTDQFKAWNPY | 1.99 | No | Positive | Yes | No | No | |
| 146–160 | STDQFKAWNPYINAE | 1.81 | No | Positive | Yes | No | No | |
| 143–157 | YGISTDQFKAWNPYI | 2.3 | No | Positive | Yes | No | No |
| Proteins | Position | Peptide | Length | Antigenicity Score | Toxin | Allergen |
|---|---|---|---|---|---|---|
| 1,3-beta-glucanosyltransferase | 235–253 | NEVQPRMFTEVQALYGDKM | 19 | 0.7053 | No | No |
| CFEM domain-containing protein | 53–251 | CSNADFQHGLRDCTHEACPGEKVEQVVQAGLQACREMGGAPGSSTGAPTTGTGSGTTTGTPTSGSGSETTAPSTSGSGSAPAPTSGGHSTPYSTIPAGPTVITSGTHVVTTSRPPTTLYTEVSGSQTGSESSSPTGTGSESTSAPETTSPSSTEGGSSPSSTEGSGNGGSGGSETSGSGNGPSQTPSQGIAPKATGLGV | 199 | 1.152 | No | No |
| Cell wall galactomannoprotein | 22–61 | PSTFSSVPEAIGDLDPISASIEGLSQRIAQSPGGITELMS | 40 | 1.297 | No | No |
| LysM domain-containing protein | 176–202 | GATISTSMPMPTPSGPQPQMPGIVSNC | 27 | 0.5542 | No | No |
| Vaccines | BGTV | CDPV | GMPV | LDPV |
|---|---|---|---|---|
| Targeting Proteins | 1,3-beta-glucanosyltransferase | CFEM domain-containing protein | Cell wall galactomannoprotein | LysM domain-containing protein |
| Vaccine Sequence | APPHALSEAAAKNEVQPRMFTEVQALYGDKMKKTSADNSYQDPLADVKSAAYSNGTEFFMKGPGPGSYQDPLADVGPGPGQELQTNTIRVGPGPGYTRYTSVIDGPGPGYTNVIGFFAGGPGPGFWGYNIYSWGPGPGNFNVPVFFAGPGPGHHHHHH | APPHALSEAAAKCSNADFQHGLRDCTHEACPGEKVEQVVQAGLQACREMGGAPGSSTGAPTTGTGSGTTTGTPTSGSGSETTAPSTSGSGSAPAPTSGGHSTPYSTIPAGPTVITSGTHVVTTSRPPTTLYTEVSGSQTGSESSSPTGTGSESTSAPETTSPSSTEGGSSPSSTEGSGNGGSGGSETSGSGNGPSQTPSQGIAPKATGLGVKKVVTTSRPPTTLYTEVSGSQTAAYTSGSGNGPSQTPSQGGIAPAAYTHVVTTSRPPTTLYTEVSGSGPGPGSSSPTGTGSESGPGPGPSSTEGGSSGPGPGHHHHHH | APPHALSEAAAKPSTFSSVPEAIGDLDPISASIEGLSQRIAQSPGGITELMSKKLSQRIAQSPGGITELAAYATSTKVPLIKAVPGGAAYAQSPGGITEGPGPGMSVTNDIYDGPGPGHHHHHH | APPHALSEAAAKGATISTSMPMPTPSGPQPQMPGIVSNCKKTTTRAMTTTISSDAPAAYSIQTKYGISTDQFKAWNPYINAEAAYPSTTTTAKPGPGPGTRAMTTTISGPGPGHHHHHH |
| Number of Amino Acids | 158 | 319 | 124 | 119 |
| Molecular Weight (Da) | 16,673.4 | 30,329.02 | 12,476.94 | 12,292.77 |
| Theoretical pI (ExPASy-ProtParam) | 6.14 | 5.68 | 6.14 | 9.47 |
| Theoretical pI (EMBOSS-PEPSTATS) | 6.6 | 6.05 | 6.6 | 9.8 |
| Negatively Charged Residues (Asp + Glu) | 12 | 19 | 10 | 4 |
| Positively Charged Residues (Arg + Lys) | 9 | 10 | 7 | 8 |
| Ext. coefficient (ExPASy-ProtParam) | 24,410 | 9190 | 4470 | 11,460 |
| Molar ext. coefficients (EMBOSS-PEPSTATS) | 24,410 (reduced), 24,410 (cystine bridges) | 8940 (reduced), 9190 (cystine bridges) | 4470 (reduced), 4470 (cystine bridges) | 11,460 (reduced), 11,460 (cystine bridges) |
| Estimated Half-life (mammalian reticulocytes, in vitro) | 4.4 h | 4.4 h | 4.4 h | 4.4 h |
| Estimated Half-life (yeast, in vivo) | >20 h | >20 h | >20 h | >20 h |
| Estimated Half-life (E. coli, in vivo) | >10 h | >10 h | >10 h | >10 h |
| Instability Index (II) | 15.64 | 50.49 | 66.81 | 31.74 |
| Stability | Stable | Unstable | Unstable | Stable |
| Aliphatic Index | 46.96 | 33.39 | 75.73 | 42.1 |
| GRAVY | −0.564 | −0.653 | −0.257 | −0.571 |
| Antigenicity Score (VaxiJen 2.0) | 0.52 | 1.0026 | 0.9305 | 1.0933 |
| Antigenicity Score (ANTIGENpro) | 0.83 | 0.88 | 0.86 | 0.87 |
| Allergen Status | Probable Non-Allergen | Probable Non-Allergen | Probable Non-Allergen | Probable Non-Allergen |
| Toxin Status | Non-Toxin | Non-Toxin | Non-Toxin | Non-Toxin |
| Solubility | 0.563 | 0.520 | 0.435 | 0.294 |
| Improbability of expression in inclusion bodies | 0.964 | 0.965 | 0.92 | 0.978 |
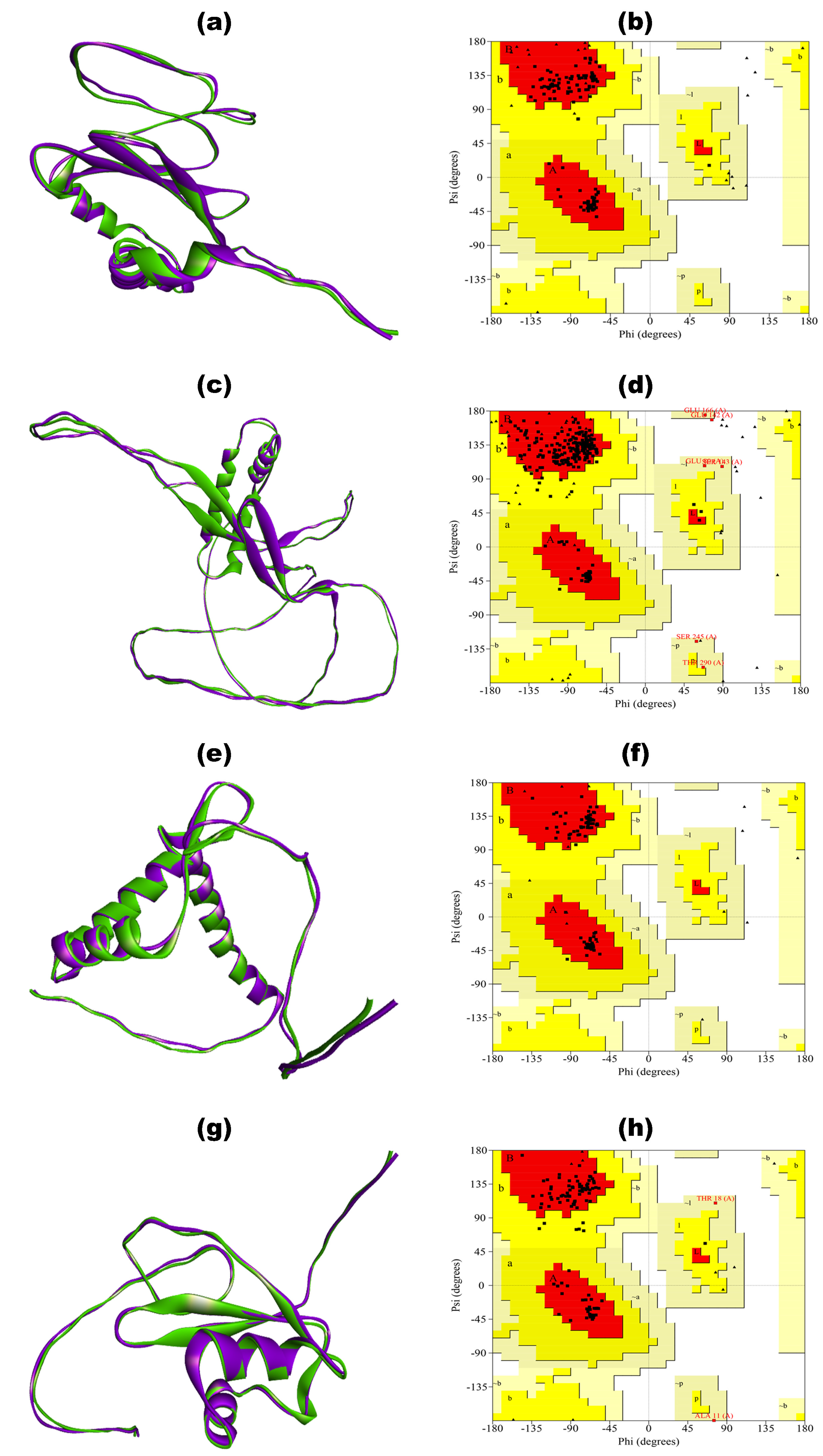
| Ramachandran Plot | BGTV | CDPV | GMPV | LDPV | ||||
|---|---|---|---|---|---|---|---|---|
| Unrefined | Refined | Unrefined | Refined | Unrefined | Refined | Unrefined | Refined | |
| Residues in most favored regions | 60.90% | 95.50% | 30.80% | 92.40% | 57.60% | 97.80% | 42.90% | 91.20% |
| Residues in additional allowed regions | 23.60% | 4.50% | 31.20% | 4.90% | 17.40% | 2.20% | 41.80% | 6.60% |
| Residues in generously allowed regions | 13.60% | 0.00% | 25.00% | 2.20% | 15.20% | 0.00% | 11.00% | 2.20% |
| Residues in disallowed regions | 1.80% | 0.00% | 12.90% | 0.40% | 9.80% | 0.00% | 4.40% | 0.00% |
| Docking Complex | Interface Residues | Interface Area (Å2) | dG (kcal mol−1) | Kd (M) at 25 °C | Binding Affinity in kcalmol−1 (Center, Lower Energy) | Electrostatic-Favored Binding Affinity in kcal mol−1 (Center, Lower Energy) | Hydrophobic-Favored Binding Affinity in kcal mol−1 (Center, Lower Energy) | Van-der Waal and Electrostatic Binding Affinity in kcal mol−1 (Center, Lower Energy) | Salt Bridges | Hydrogen Bonds | Non-Bonded Contacts |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BGTV-TLR2 | 28–17 | 1056–1187 | −13.5 | 1.2 × 10−10 | −1077.2, −1124.9 | −1084.7, −1134.4 | −1857.4, −2124.7 | −191.5, −264.3 | 2 | 12 | 150 |
| CDPV-TLR2 | 29–10 | 785–1154 | −14.5 | 2.2 × 10−11 | −1059.4, −1114.9 | −1003.9, −1100.0 | −1645.9, −1783.9 | −201.9, −247.2 | 4 | 146 | |
| GMPV-TLR2 | 31–13 | 864–1238 | −12.7 | 5.2 × 10−10 | −1062.7, −1205.8 | −1184.4, −1271.5 | −1883.9, −1962.8 | −214.3, −253.6 | 6 | 153 | |
| LDPV-TLR2 | 39–27 | 1376–1709 | −15.1 | 8.6 × 10−12 | −1233.6, −1453.9 | −1231.6, −1546.6 | −1926.6, −2129.6 | −242.2, −288.3 | 3 | 17 | 215 |
| BGTV-TLR4 | 30–22 | 1205–1418 | −13.5 | 1.4 × 10−10 | −902.6, −993.5 | −987.4, −1030.1 | −941.4, −1189.2 | −212.6, −212.6 | 6 | 14 | 150 |
| CDPV-TLR4 | 51–51 | 2264–2314 | −16.4 | 8.9 × 10−13 | −916.9, −959.9 | −883.9, −977.3 | −988.9, −1092.8 | −188.6, −219.4 | 3 | 30 | 317 |
| GMPV-TLR4 | 39–33 | 1670–1791 | −14.8 | 1.4 × 10−11 | −911.4, −911.4 | −817.3, −1016.6 | −1062.7, −1174 | −208.9, −213.5 | 4 | 17 | 226 |
| LDPV-TLR4 | 49–38 | 1932–2137 | −20.4 | 1.1 × 10−15 | −1109.5, −1234.4 | −1050.2, −1324.2 | −1205.8, −1390.1 | −210.1, −235.8 | 2 | 29 | 229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elalouf, A.; Maoz, H.; Rosenfeld, A.Y. Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum. Pharmaceutics 2024, 16, 983. https://doi.org/10.3390/pharmaceutics16080983
Elalouf A, Maoz H, Rosenfeld AY. Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum. Pharmaceutics. 2024; 16(8):983. https://doi.org/10.3390/pharmaceutics16080983
Chicago/Turabian StyleElalouf, Amir, Hanan Maoz, and Amit Yaniv Rosenfeld. 2024. "Bioinformatics-Driven mRNA-Based Vaccine Design for Controlling Tinea Cruris Induced by Trichophyton rubrum" Pharmaceutics 16, no. 8: 983. https://doi.org/10.3390/pharmaceutics16080983






