Nanosized Complexes of the Proteolytic Enzyme Serratiopeptidase with Cationic Block Copolymer Micelles Enhance the Proliferation and Migration of Human Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
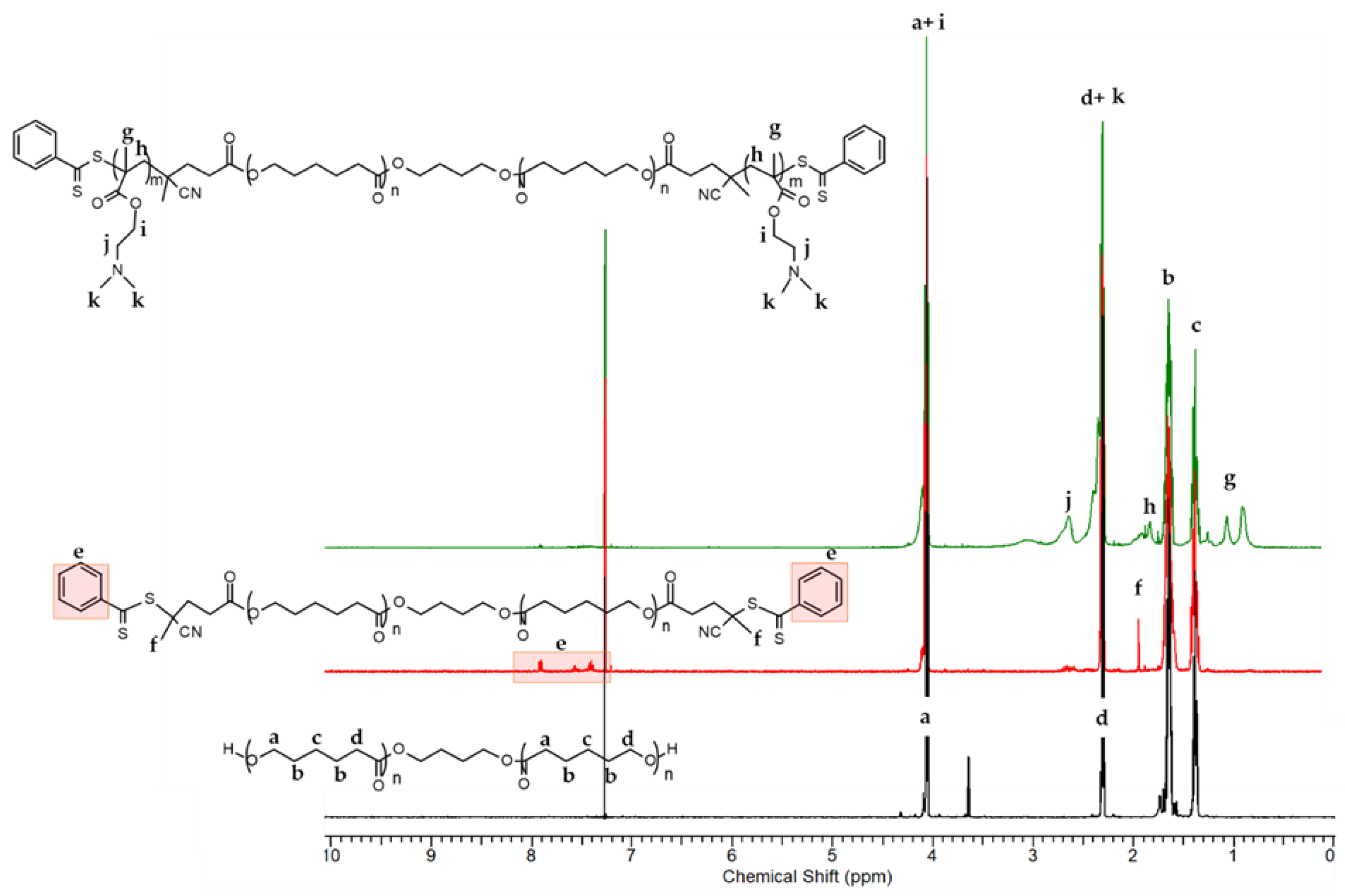
2.2.1. Synthesis of PCL-Based Macro-RAFT Agent
2.2.2. Synthesis of Amphiphilic PDMAEMA9-b-PCL35-b-PDMAEMA9 Triblock Copolymer by RAFT Polymerization
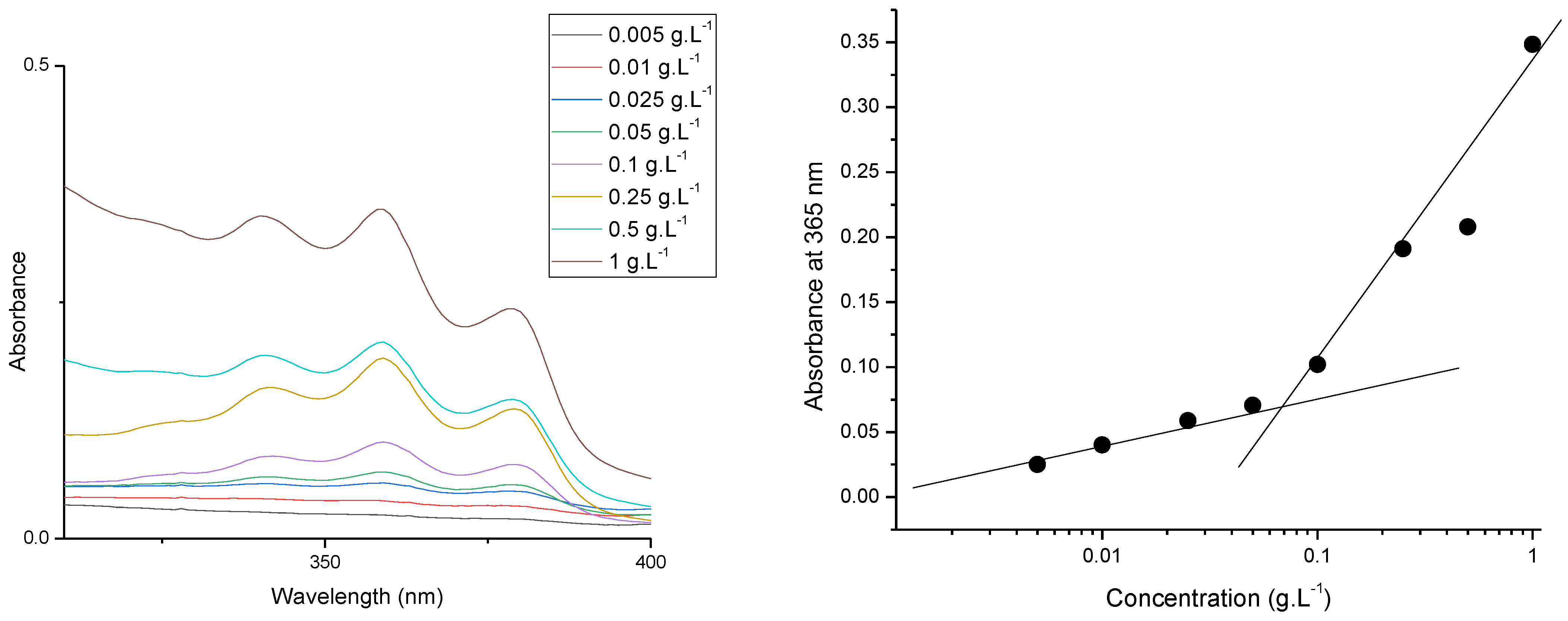
2.2.3. Preparation of PDMAEMA9-b-PCL35-b-PDMAEMA9 Block Copolymer Micelles and Determination of Critical Micelle Concentration
2.2.4. Preparation of Complexes of PDMAEMA9-b-PCL35-b-PDMAEMA9 Micelles and SER
2.2.5. Characterization of Polymers
2.2.6. Particle Size and Zeta Potential
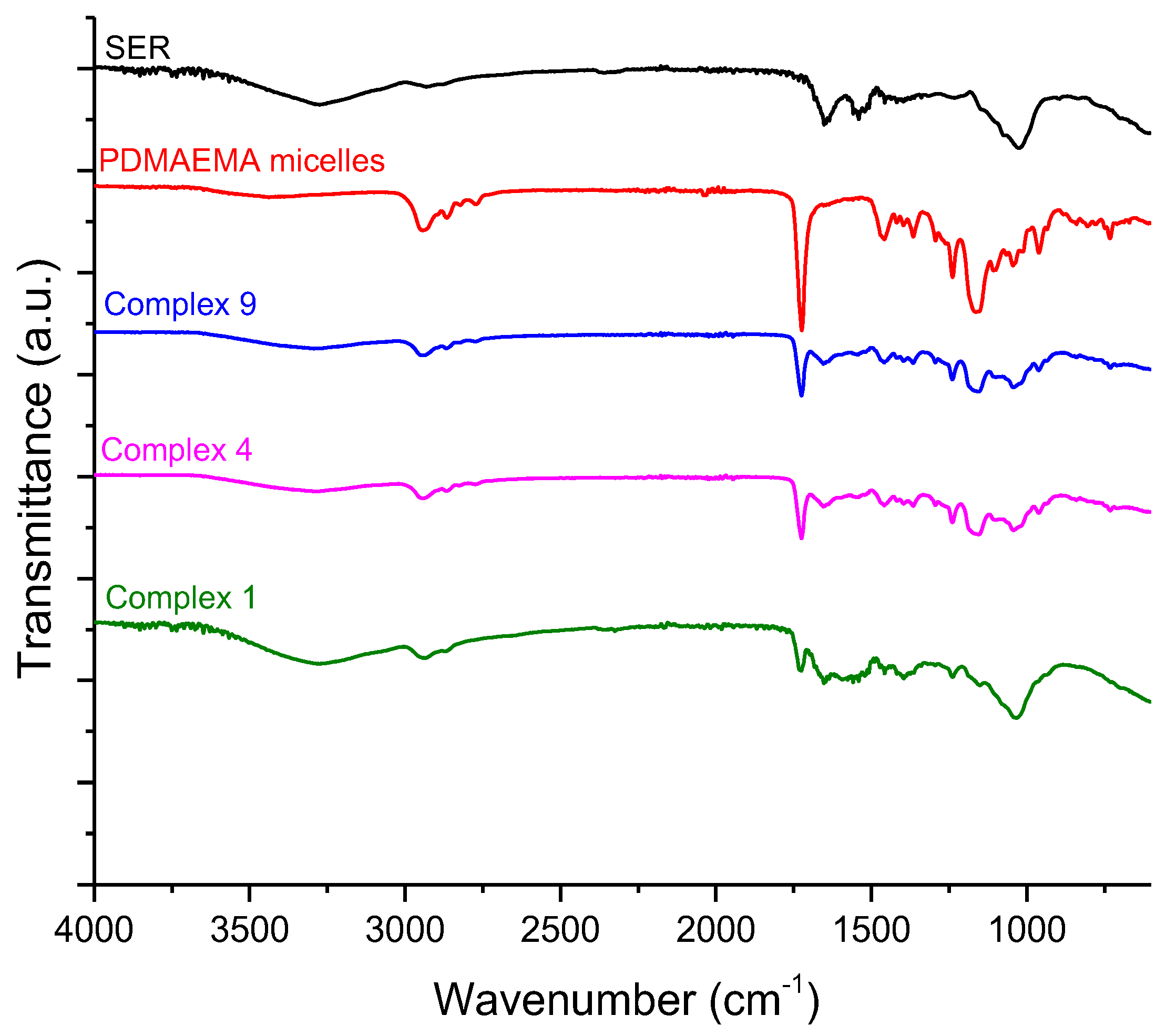
2.2.7. Fourier Transforms Infrared Spectroscopy (FTIR)
2.2.8. UV–Vis Spectrophotometry
2.2.9. Enzyme Activity
2.2.10. In Vitro Drug Release Study
2.2.11. Cell Cultures
2.2.12. Cell Viability Test
2.2.13. Wound Assay
2.3. Statistics
3. Results and Discussion
3.1. Synthesis and Characterization of the Triblock Copolymer
3.2. Preparation and Characterization of PDMAEMA9-b-PCL35-b-PDMAEMA9 Micelles
3.3. Preparation and Characterization of Complexes of PDMAEMA9-b-PCL35-b-PDMAEMA9 Micelles and SER
3.4. In Vitro Release Study
3.5. Cytotoxicity and Wound Healing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, Y.H.; Han, H.K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Vardaxi, A.; Kafetzi, M.; Pispas, S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers 2022, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Marras, A.E.; Ting, J.M.; Stevens, K.C.; Tirrell, M.V. Advances in the Structural Design of Polyelectrolyte Complex Micelles. J. Phys. Chem. 2021, 125, 7076–7089. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. 2019, 107, 978–990. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gómez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9181. [Google Scholar] [CrossRef]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M. The role of serratiopeptidase in the resolution of inflammation. Asian J. Pharm. Sci. 2017, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Pulicherla, K.K. Enzyme promiscuity in Earthworm serine protease-Substrate versatility and therapeutic potential. Amino Acids 2016, 48, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cirino, P.C. Recent advances in engineering proteins for biocatalysis. Biotechnol. Bioeng. 2014, 111, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.B.; Shah, N.; Rathi, A.; Rathi, V.; Rathi, A. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol. Rep. 2020, 28, e00544. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Agarwal, Y.K. Nanosuspension: An approach to enhance solubility of drug. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar] [PubMed]
- Maghraby, Y.; El-Shabasy, R.; Ibrahim, A.; Azzazy, H. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Pozdyshev, D.V.; Semenyuk, P.I. Polyelectrolytes for Enzyme Immobilization and the Regulation of Their Properties. Polymers 2022, 14, 4204. [Google Scholar] [CrossRef]
- Dong, Z.; Wei, H.; Mao, J.; Wang, D.; Yang, M.; Bo, S.; Ji, X. Synthesis and responsive behavior of poly(N,N-dimethylaminoethyl methacrylate) brushes grafted on silica nanoparticles and their quaternized derivatives. Polymer 2012, 53, 2074–2084. [Google Scholar] [CrossRef]
- Schepelina, O.; Zharov, I. Poly(2-(dimethylamino)ethyl methacrylate)-modified nanoporous Colloidal films with pH and ion response. Langmuir 2008, 24, 14188–14194. [Google Scholar] [CrossRef]
- Stawski, D. Poly(N,N-dimethylaminoethyl methacrylate) as a bioactive polyelectrolyte-production and properties. R. Soc. Open Sci. 2023, 10, 230188. [Google Scholar] [CrossRef]
- Lei, H.; Wang, M.; Tang, Z.; Luan, Y.; Liu, W.; Song, B.; Chen, H. Control of Lysozyme Adsorption by pH on Surfaces Modified with Polyampholyte Brushes. Langmuir 2013, 30, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, S.; Yoshimoto, K.; Tomita, S.; Sakuma, H.; Matsuoka, T.; Shiraki, K.; Nagasaki, Y. Regulation of lysozyme activity based on thermotolerant protein/smart polymer complex formation. J. Am. Chem. Soc. 2009, 131, 6549–6553. [Google Scholar] [CrossRef]
- Haladjova, E.; Kyulavska, M.; Doumanov, J.; Topouzova-Hristova, T.; Petrov, P. Polymeric vehicles for transport and delivery of DNA via cationic micelle template method. Colloid. Polym. Sci. 2017, 295, 2197–2205. [Google Scholar] [CrossRef]
- Kamenova, K.; Haladjova, E.; Grancharov, G.; Kyulavska, M.; Tzankova, V.; Aluani, D.; Yoncheva, K.; Pispas, S.; Petrov, P. Co-assembly of block copolymers as a tool for developing novel micellar carriers of insulin for controlled drug delivery. Eur. Polym. J. 2018, 104, 1–9. [Google Scholar] [CrossRef]
- Sentoukas, T.; Pispas, S. Poly(2-(dimethylamino)ethyl methacrylate)-b-poly (hydroxypropyl methacrylate) copolymers/bovine serum albumin complexes in aqueous solutions. J. Polym. Sci. 2020, 58, 1241–1252. [Google Scholar] [CrossRef]
- Sentoukas, T.; Pispas, S. Poly(2-[dimethylamino]ethyl methacrylate)-b-poly (hydroxypropyl methacrylate)/DNA polyplexes in aqueous solutions. J. Polym. Sci. 2020, 58, 2335–2346. [Google Scholar] [CrossRef]
- Gupte, V.; Luthra, U. Analytical techniques for serratiopeptidase: A review. J. Pharm. Anal. 2017, 7, 203–207. [Google Scholar] [CrossRef]
- Nair, S.R. Serratiopeptidase: An integrated View of Multifaceted Therapeutic Enzyme. Biomolecules 2022, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Morihara, K. Serralysin and related bacterial proteinases. Methods Enzymol. 1995, 248, 395–413. [Google Scholar] [PubMed]
- Bhagat, S.; Agarwal, M.; Roy, V. Serratiopeptidase: A systematic review of the existing evidence. Int. J. Surg. 2013, 11, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mali, N.; Wavikar, P.; Vavia, P. Serratiopeptidase loaded chitosan nanoparticles by polyelectrolyte complexation: In vitro and in vivo evaluation. AAPS PharmSciTech. 2015, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Miyata, K.; Tomoda, K. A Manganese Superoxide Dismutase from Serratia marcescens. Agric. Biol. Chem. 1983, 47, 1537–1543. [Google Scholar] [CrossRef]
- Santhosh, K. The emerging role of serratiopeptidase in oral surgery: Literature update. Asian J. Clin. Pharm. Res. 2018, 11, 19–23. [Google Scholar]
- Jadav, S.P.; Patel, N.H.; Shah, T.G.; Gajera, M.V.; Trivedi, H.R.; Shah, B.K. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J. Pharmacol. Pharmacother. 2010, 1, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Sharm, C.; Jha, N.K.; Meeran, M.F.N.; Patil, C.R.; Goyal, S.N.; Ojha, S. Serratiopeptidase, a Serine Protease Anti-Inflammatory, Fibrinolytic, and Mucolytic Drug, Can Be a Useful Adjuvant for Management in COVID-19. Front. Pharmacol. 2021, 12, 603997. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jana, A.K.; Dhamija, I.; Singla, Y.; Maiti, M. Preparation, characterization and targeted delivery of serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 413–426. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, A. Design, development and characterization of serratiopeptidase loaded albumin nanoparticles. J. App. Pharm. Sci. 2015, 5, 103–109. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M.R. Development of antibiotic and debriding enzyme-loaded PLGA microspheres entrapped in PVA-gelatin hydrogel for complete wound management. Artif. Cell. Blood Sub. Biotechnol. 2012, 40, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hire, N.N.; Deore, A.B.; Derle, D.V.; Nathe, R. Formulation and evaluation of serratiopeptidase microspheres using eudragit rs100 polymer. World J. Pharm. 2014, 3, 3207–3218. [Google Scholar]
- Sandhya, K.V.; Devi, G.S.; Mathew, S.T. Liposomal formulations of serratiopeptidase: In vitro studies using PAMPA and Caco-2 models. Mol. Pharm. 2008, 5, 92–97. [Google Scholar]
- Cupp-Enyard, C. Sigma’s Non-specific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 17, 899. [Google Scholar]
- ISO 10993-5:2009; In Vitro Cytotoxicity of Medical Devices. International Organization for Standardization: Geneve, Switzerland, 2009.
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sung, T.-J.; Wang, Y.-Y.; Liu, K.-L.; Chou, C.-H.; Lai, P.-S.; Hsieh, C.-W. Pholiota Nameko Polysaccharides Promotes Cell Proliferation and Migration and Reduces ROS Content in H2O2-Induced L929 Cells. Antioxidants 2020, 9, 65. [Google Scholar] [CrossRef]
- Walter, M.N.M.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E.B. Mesenchymal Stem Cell-Conditioned Medium Accelerates Skin Wound Healing: An in Vitro Study of Fibroblast and Keratinocyte Scratch Assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for Microscopy. BioTechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Grădinaru, V.R.; Bucatariu, F.; Mihai, M. Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications. Chemistry 2022, 4, 1312–1338. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Kolapalli, V.R. Polyelectrolyte Complexes: A Review of their Applicability in Drug Delivery Technology. Indian. J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef]
- Blažic, R.; Kučić Grgić, D.; Kraljić Roković, M.; Vidović, E. Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels 2022, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, D.; Karayianni, M.; Skandalis, A.; Haladjova, E.; Forys, A.; Trzebicka, B.; Rangelov, S.; Pispas, S. Complexation of poly(methacrylic acid) star polyelectrolytes with lysozyme. Eur. Polym. J. 2024, 206, 112773. [Google Scholar] [CrossRef]
- Segger, D.; Aßmus, U.; Brock, M.; Erasmy, J.; Finkel, P.; Fitzner, A.; Heuss, H.; Kortemeier, U.; Munke, S.; Rheinländer, T.; et al. Multicenter Study on Measurement of the Natural PH of the Skin Surface. Int. J. Cosmet. Sci. 2008, 30, 75. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant. Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Kwan, P.; Desmoulière, A.; Tredget, E.E. 45—Molecular and Cellular Basis of Hypertrophic Scarring. In Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 455–465. [Google Scholar]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, D.; Abbot, V. A Review on Pharmaceutical, Pharmacological and Chemical Aspects of Serratiopeptidase as Anti-Inflammatory Agent. Mater Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Chandrasekaran, S.D.; Selvakumar, J.N.; Vaithilingam, M. Serratiopeptidase: A Multifaceted Microbial Enzyme in Health Care. In Biotechnology of Microorganisms; Apple Academic Press: Point Pleasant, NJ, USA, 2019; p. 16. [Google Scholar]
- Rouhani, M.; Valizadeh, V.; Bakhshandeh, H.; Hosseinzadeh, S.A.; Molasalehi, S.; Atyabi, S.M.; Norouzian, D. Improved Anti-Biofilm Activity and Long-Lasting Effects of Novel Serratiopeptidase Immobilized on Cellulose Nanofibers. Appl. Microbiol. Biotechnol. 2023, 107, 6487–6496. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound Healing and the Role of Fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar] [CrossRef]
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and Wound Healing: An Update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef] [PubMed]










| Sample Code | MnNMR g mol−1 | MnGPC g mol−1 | Mw/Mn |
|---|---|---|---|
| HO-PCL35-OH | 4000 | 6290 | 1.54 |
| CTA-PCL35-CTA | 4550 | 8402 | 1.56 |
| PDMAEMA9-b-PCL35-b-PDMAEMA9 | 6820 | 15000 | 1.32 |
| SER g/L | Activity of Free SER U/mL | Activity of PDMAEMA/SER Complex U/mL |
|---|---|---|
| 0.05 | 1.31 | 1.46 |
| 0.1 | 2.15 | 1.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenova, K.; Prancheva, A.; Radeva, L.; Yoncheva, K.; Zaharieva, M.M.; Najdenski, H.M.; Petrov, P.D. Nanosized Complexes of the Proteolytic Enzyme Serratiopeptidase with Cationic Block Copolymer Micelles Enhance the Proliferation and Migration of Human Cells. Pharmaceutics 2024, 16, 988. https://doi.org/10.3390/pharmaceutics16080988
Kamenova K, Prancheva A, Radeva L, Yoncheva K, Zaharieva MM, Najdenski HM, Petrov PD. Nanosized Complexes of the Proteolytic Enzyme Serratiopeptidase with Cationic Block Copolymer Micelles Enhance the Proliferation and Migration of Human Cells. Pharmaceutics. 2024; 16(8):988. https://doi.org/10.3390/pharmaceutics16080988
Chicago/Turabian StyleKamenova, Katya, Anna Prancheva, Lyubomira Radeva, Krassimira Yoncheva, Maya M. Zaharieva, Hristo M. Najdenski, and Petar D. Petrov. 2024. "Nanosized Complexes of the Proteolytic Enzyme Serratiopeptidase with Cationic Block Copolymer Micelles Enhance the Proliferation and Migration of Human Cells" Pharmaceutics 16, no. 8: 988. https://doi.org/10.3390/pharmaceutics16080988








