Enhanced Antibacterial Efficacy of Bioceramic Implants Functionalized with Ciprofloxacin: An In Silico and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking Protocol
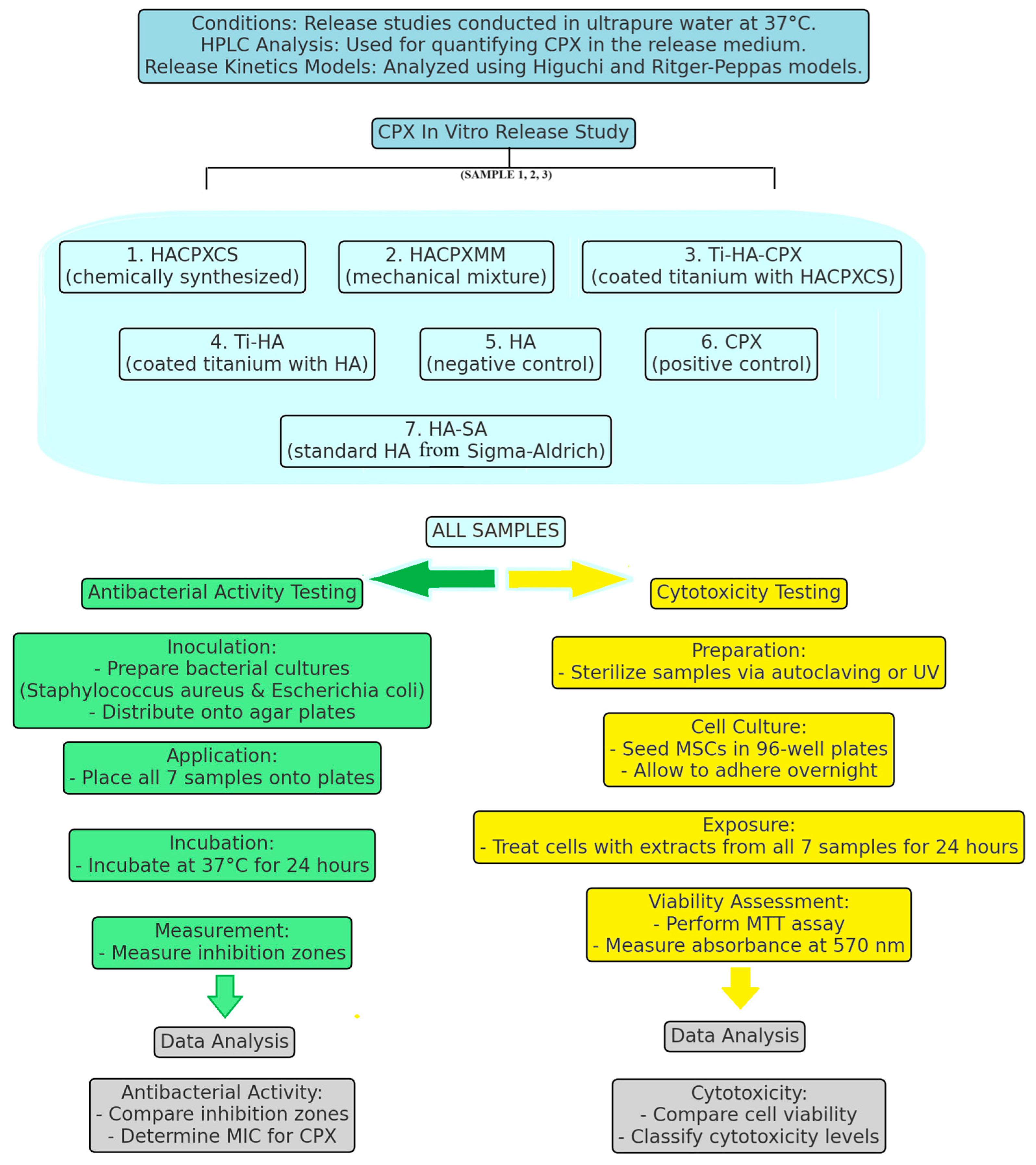
2.2. Design and Fabrication of Tested Tablets
Samples Preparation
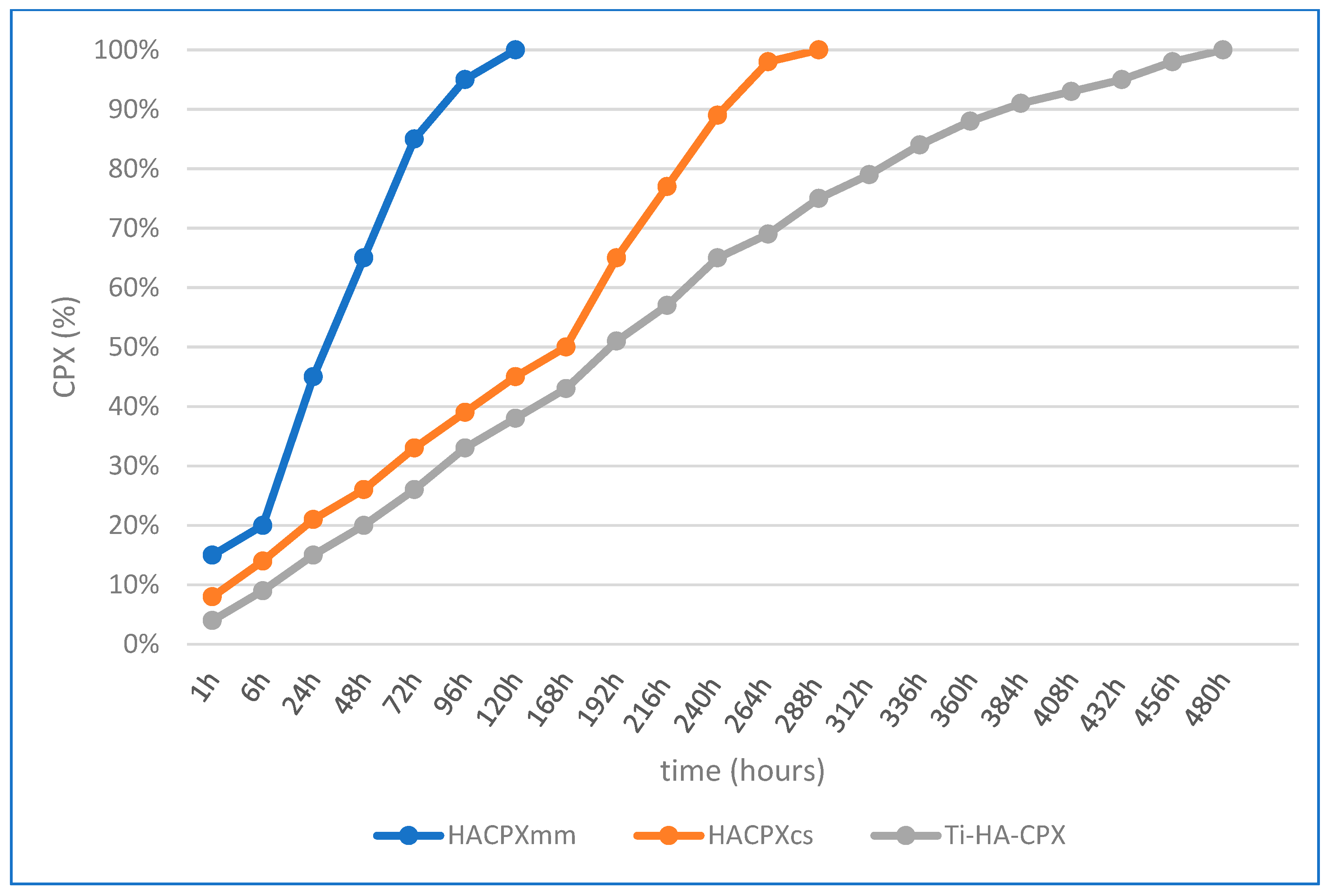
2.3. CPX in Vitro Release Study
2.3.1. Preparation and Conditions
2.3.2. Quantification of CPX in the Release Medium by HPLC
2.3.3. Calculation of CPX Release Kinetics
2.4. Cytotoxicity Test
2.5. Testing the Antibacterial Activity
2.5.1. Experimental Design
- –
- HA-coated Ti substrates in tablet form (Ti-HA) by MAPLE: 6 micrograms of HA.
- –
- HACPXCS-coated Ti substrates in tablet form (Ti-HA-CPX) by MAPLE: 1 microgram of HA and 5 micrograms of CPX.
- –
- tablets of HACPXCS composite obtained by chemical synthesis: 20 micrograms of HA and 5 micrograms of CPX.
- –
- tablets made from a mechanical mixture of CPX and HA (HACPXMM): 20 micrograms of HA and 5 micrograms of CPX.
- –
- tablets from synthetic HA (HA-synthesis): 25 micrograms.
- –
- tablets of standard CPX (Sigma-Aldrich)-CPX-SA: 5 micrograms.
- –
- tablets of standard HA from Sigma-Aldrich, HA-SA: 25 micrograms (Figure 3).
2.5.2. Preparation of the Culture Medium and Inoculation
2.5.3. Determination of the Minimum Inhibitory Concentration of CPX
2.5.4. Performing Antibiogram Testing
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, J.; Diefenbeck, M.; McNally, M. The use of local antibiotic carriers in bone and joint infection. J. Bone Jt. Infect. 2017, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Colston, J.; Atkins, B. Bone and joint infection. Clin. Med. 2018, 18, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zapata, D.; Higgs, J.; Wittholt, H.; Chittimalli, K.; Brooks, A.E.; Mulinti, P. Nanotechnology in the Diagnosis and Treatment of Osteomyelitis. Pharmaceutics 2022, 14, 1563. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, T.; Turk, H.; Hrenovic, J.; Schauperl, Z.; Ivankovic, M.; Ressler, A. Antibacterial activity of silver doped hydroxyapatite toward multidrug-resistant clinical isolates of Acinetobacter baumannii. J. Hazard. Mater. 2023, 458, 131867. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, H.; Chen, H.; Xiu, P.; Zeng, J.; Song, Y.; Li, T. Recent Advances in Nanotechnology-Based Strategies for Bone Tuberculosis Management. Pharmaceuticals 2024, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021, 8, 193–202. [Google Scholar] [CrossRef]
- Kushner, B.; Allen, P.D.; Crane, B.T. Frequency and Demographics of Gentamicin Use. Otol. Neurotol. 2016, 37, 190–195. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. BIOVIA Workbook, Release 2017; BIOVIA Pipeline Pilot, Release 2017; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.-Q. Insights into protein-ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Medvecky, L.; Giretova, M.; Stulajterova, R.; Luptakova, L.; Sopcak, T. Tetracalcium Phosphate/Monetite/Calcium Sulfate Hemihydrate Biocement Powder Mixtures Prepared by the One-Step Synthesis for Preparation of Nanocrystalline Hydroxyapatite Biocement-Properties and In vitro Evaluation. Materials 2021, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Iosif, A.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Bioactive Hydroxyapatite-Magnesium Phosphate Coatings Deposited by MAPLE for Preventing Infection and Promoting Orthopedic Implants Osteointegration. Materials 2022, 15, 7337. [Google Scholar] [CrossRef] [PubMed]
- Bolashikov, Z.D.; Melikov, A.K. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009, 44, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/ cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, L.T. Correlation of the Theoretical Study with the Experimental Determination of the Antibacterial Effect of Vaccinium myrtillus Folium (VM-f) Plant Extract. Rev. Chim. 2019, 70, 990–992. [Google Scholar] [CrossRef]
- Văruț, R.M.; Manda, V.; Gingu, O.; Sima, G.; Teișanu, C.; Neamtu, J. The chemisorption-release and antibacterial potential studies of gentamicin from hydroxyapatite-based implants. JOSA 2020, 2, 459–466. [Google Scholar]
- Honório, K.M.; Da Silva, A.B.F. An AM1 study on the electron-donating and electron-accepting character of biomolecules. Int. J. Quantum Chem. 2003, 95, 126–132. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Lgaz, H.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Masroor, S.; Cho, Y. New 8-Hydroxyquinoline-Bearing Quinoxaline Derivatives as Effective Corrosion Inhibitors for Mild Steel in HCl: Electrochemical and Computational Investigations. Coatings 2020, 10, 811. [Google Scholar] [CrossRef]
- Fei, J.; Mao, Q.; Peng, L.; Ye, T.; Yang, Y.; Luo, S. The Internal Relation between Quantum Chemical Descriptors and Empirical Constants of Polychlorinated Compounds. Molecules 2018, 23, 2935. [Google Scholar] [CrossRef]
- Rathi, P.C.; Ludlow, F.; Verdonk, M.J. Practical High-Quality Electrostatic Potential Surfaces for Drug Discovery Using a Graph-Convolutional Deep Neural Network. Med. Chem. 2020, 63, 8778–8790. [Google Scholar] [CrossRef]
- Obot, I.B.; Ebenso, E.E.; Kabanda, M.M. Metronidazole as Environmentally Safe Corrosion Inhibitor for Mild Steel in 0.5 M HCl: Experimental and Theoretical Investigation. J. Environ. Chem. Eng. 2013, 10, 431–439. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Wang, Y.; Li, J. Relationship between HOMO-LUMO gap and antibacterial activity: A combined experimental and theoretical study. J. Chem. Inf. Model. 2018, 58, 1121–1129. [Google Scholar]
- Ishihara, M.; Wakabayashi, H.; Motohashi, N.; Sakagami, H. Estimation of relationship between the structure of tri-haloacetylazulene derivatives determined by a semiempirical molecular-orbital method (PM5) and their cytotoxicity. Anticancer Res. 2010, 30, 837–842. [Google Scholar]
- Shah, M.; Kumar, A.; Singh, A.K.; Singh, H.; Narasimhan, B.; Kumar, P. In Silico studies of indole derivatives as anti-bacterial agents. J. Pharmacopunct. 2023, 26, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, K.; Schläppi, M.; Meier, C.; Wahl, P. Local antibiotic treatment with calcium sulfate as carrier material improves the outcome of debridement, antibiotics, and implant retention procedures for periprosthetic joint infections after hip arthroplasty—A retrospective study. J. Bone Jt. Infect. 2022, 7, 11–21. [Google Scholar] [CrossRef]
- Hashimoto, A.; Miyamoto, H.; Kobatake, T.; Nakashima, T.; Shobuike, T.; Ueno, M.; Murakami, T.; Noda, I.; Sonohata, M.; Mawatari, M. The combination of silver-containing hydroxyapatite coating and vancomycin has a synergistic antibacterial effect on methicillin-resistant Staphylococcus aureus biofilm formation. Bone Jt. Res. 2020, 9, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of hydroxyapatite with antibacterial properties using a microwave-assisted combustion method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Aslani, Z.; Nazemi, N.; Rajabi, N.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Najafinezhad, A.; Ismail, A.F.; Sharif, S.; Berto, F. Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications. Materials 2022, 15, 4786. [Google Scholar] [CrossRef]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Sedláček, R.; Kopecký, F.; Veverka, P.; Kalbáčová, M.H.; Hradil, J. Vancomycin-loaded collagen/hydroxyapatite layers electrospun on 3D printed titanium implants prevent bone destruction associated with S. epidermidis infection and enhance osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.S.; Devesa, S.; Macêdo, A.A. Influence of polymeric blends on bioceramics of hydroxyapatite. Crystals 2023, 13, 1429. [Google Scholar] [CrossRef]
- Prasanna, A.P.S.; Venkatasubbu, G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N. Development of novel tetracycline and ciprofloxacin loaded silver doped hydroxyapatite suspensions for biomedical applications. Antibiotics 2022, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Praphakar, R.A.; Sumathra, M.; Ebenezer, R.S.; Vignesh, S.; Shakila, H.; Rajan, M. Fabrication of bioactive rifampicin loaded κ-Car-MA-INH/nano hydroxyapatite composite for tuberculosis osteomyelitis infected tissue regeneration. Int. J. Pharm. 2019, 565, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica micro-spheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci. Rep. 2017, 7, 41808. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Espinar, F.O.; Méndez, J.B. The application of microencapsulation techniques in the treatment of endodontic and periodontal diseases. Pharmaceutics 2011, 3, 538–571. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.A.; Adole, V.A.; Shinde, R.S.; Desale, B.S.; Jagdale, B.S. Synthesis, antibacterial, antifungal and computational study of (E)-4-(3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-3-oxoprop-1-en-1-yl)benzonitrile. Results Chem. 2022, 4, 100553. [Google Scholar] [CrossRef]
- Ghosh, M.; Pradhan, S.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced antibacterial activity of a novel protein-arginine deiminase type-4 (PADI4) inhibitor after conjugation with a biocompatible nanocarrier. J. Drug Deliv. Sci. Technol. 2022, 74, 103549. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical applications of TiO2 nanostructures: Recent advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Wu, X. Applications of titanium dioxide materials. In Titanium Dioxide; Ali, H.M., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Pupo, Y.M.; Leite, L.M.B.; Senegaglia, A.C.; Antunes, L.; Nadal, J.M.; de Lara, E.L.; Saito, R.E.; Antunes, S.R.M.; Lacerda, W.F.; Farago, P.V. Effect of hydroxyapatite microspheres, amoxicillin–hydroxyapatite and collagen–hydroxyapatite composites on human dental pulp-derived mesenchymal stem cells. Materials 2021, 14, 7515. [Google Scholar] [CrossRef] [PubMed]
- Song, Y. Design and fabrication of drug-loaded alginate/hydroxyapatite/collagen scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1121–1129. [Google Scholar]
- Han, J.; Zhang, F.; Van Meerbeek, B.; Vleugels, J.; Braem, A.; Castagne, S. Laser surface texturing of zirconia-based ceramics for dental applications: A review. Mater. Sci. Eng. C 2021, 123, 112034. [Google Scholar] [CrossRef]




| Microorganism Test | R (mm) | IS (mm) | S (mm) |
|---|---|---|---|
| Staphylococcus aureus (ATCC 25923) | ≤15 | 16–20 | ≥21 |
| Escherichia coli (ATCC 25922) | ≤15 | 16–20 | ≥21 |
| Sample | Heat of Formation (kcal/mol) | Dipole Moment (Debye) | Total Energy (kcal/mol) |
|---|---|---|---|
| CPX | −98.52 | 7.85 | −945.24 |
| HACPXCS | −395.03 | 15.22 | −1968.04 |
| Ti-HA-CPX | −514.53 | 10.96 | −2025.25 |
| Sample | Electrostatic Potential | HOMO Orbitals | LUMO Orbitals |
|---|---|---|---|
| CPX | 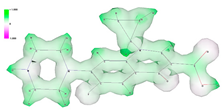 |  |  |
| HACPXCS |  |  |  |
| Ti-HA-CPX |  |  |  |
| Sample | HOMO (eV) | LUMO (eV) | ΔE |
|---|---|---|---|
| CPX | −9.3214 | 1.8151 | 11.13 |
| HACPXCS | −8.3535 | −0.7128 | 7.64 |
| Ti-HA-CPX | −7.3649 | −1.6377 | 5.72 |
| Ligands | Escherichia coli | Staphylococcus aureus |
|---|---|---|
| CPX/HACPXCS/Ti-HA-CPX | −4.68 ± 0.01 | −5.46 ± 0.03 |
| DZI mm | Product Tested | Staphylococcus aureus | Escherichia coli |
| Ti-HA | 17.5 ± 0.5 ** | 18 ± 0.5 ** | |
| Ti-HA-CPX | 33.5 ± 0.3 *** | 27.5 ± 0.4 *** | |
| HACPXCS | 26 ± 0.2 *** | 26± 0.3 *** | |
| HACPXMM | 26.5 ± 0.2 *** | 26± 0.2 *** | |
| HA-synthesis | 15 ± 0.3 * | 17.5± 0.4 ** | |
| CPX- SA | 29 ± 0.4 *** | 27.5± 0.3 *** | |
| HA-SA | 14.5 0.3 * | 17± 0.2 ** |
| Product Tested | Cell Viability (%) | Cytotoxicity Level |
|---|---|---|
| Ti-HA | 95% | Low cytotoxicity |
| Ti-HA-CPX | 80% | Moderate cytotoxicity |
| HACPXCS (chemical synthesis) | 75% | Moderate cytotoxicity |
| HACPXMM (mechanical mixture) | 75 | Moderate cytotoxicity |
| HA-synthesis | 95% | Low cytotoxicity |
| CPX-SA | 50 | High cytotoxicity |
| HA-SA | 95% | Low cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Văruț, R.-M.; Rotaru, L.T.; Cimpoesu, D.; Corlade, M.; Singer, C.E.; Popescu, A.I.S.; Popescu, C.; Iulian-Nicolae, I.; Mocanu, A.; Popescu, M.; et al. Enhanced Antibacterial Efficacy of Bioceramic Implants Functionalized with Ciprofloxacin: An In Silico and In Vitro Study. Pharmaceutics 2024, 16, 998. https://doi.org/10.3390/pharmaceutics16080998
Văruț R-M, Rotaru LT, Cimpoesu D, Corlade M, Singer CE, Popescu AIS, Popescu C, Iulian-Nicolae I, Mocanu A, Popescu M, et al. Enhanced Antibacterial Efficacy of Bioceramic Implants Functionalized with Ciprofloxacin: An In Silico and In Vitro Study. Pharmaceutics. 2024; 16(8):998. https://doi.org/10.3390/pharmaceutics16080998
Chicago/Turabian StyleVăruț, Renata-Maria, Luciana Teodora Rotaru, Diana Cimpoesu, Mihaela Corlade, Cristina Elena Singer, Alin Iulian Silviu Popescu, Cristina Popescu, Iliescu Iulian-Nicolae, Adriana Mocanu, Mihaela Popescu, and et al. 2024. "Enhanced Antibacterial Efficacy of Bioceramic Implants Functionalized with Ciprofloxacin: An In Silico and In Vitro Study" Pharmaceutics 16, no. 8: 998. https://doi.org/10.3390/pharmaceutics16080998
APA StyleVăruț, R.-M., Rotaru, L. T., Cimpoesu, D., Corlade, M., Singer, C. E., Popescu, A. I. S., Popescu, C., Iulian-Nicolae, I., Mocanu, A., Popescu, M., Butoi, M. A., & Nicolaescu, O. E. (2024). Enhanced Antibacterial Efficacy of Bioceramic Implants Functionalized with Ciprofloxacin: An In Silico and In Vitro Study. Pharmaceutics, 16(8), 998. https://doi.org/10.3390/pharmaceutics16080998









