Synergistic Strategies in Prostate Cancer Therapy: Electrochemotherapy and Electromagnetic Hyperthermia
Abstract
1. Introduction
2. Materials and Methods (Figure S1)
2.1. Cell Culture
2.2. Cytotoxicity Assays with WST-1
2.2.1. Chemotherapeutic Drugs
2.2.2. Determination of IC50 of Cisplatin, Bleomycin, and Combination of Both Drugs
2.2.3. Electrochemotherapy In Vitro Standardization
2.2.4. Determination of IC50 of Cisplatin and Bleomycin with Electrochemotherapy
2.2.5. SPION’s Heating Capability and Cytotoxicity
2.2.6. Electromagnetic Hyperthermia Standardization In Vitro
2.2.7. Electrochemotherapy Combined with Electromagnetic Hyperthermia In Vitro
2.3. Statistical Analysis
3. Results
3.1. Cytotoxic Effect of Cisplatin and Bleomycin on Prostate-Derived Cell Lines
3.2. Electroporation Enhances the Sensitivity of Prostate-Derived Cell Lines to Chemotherapeutics
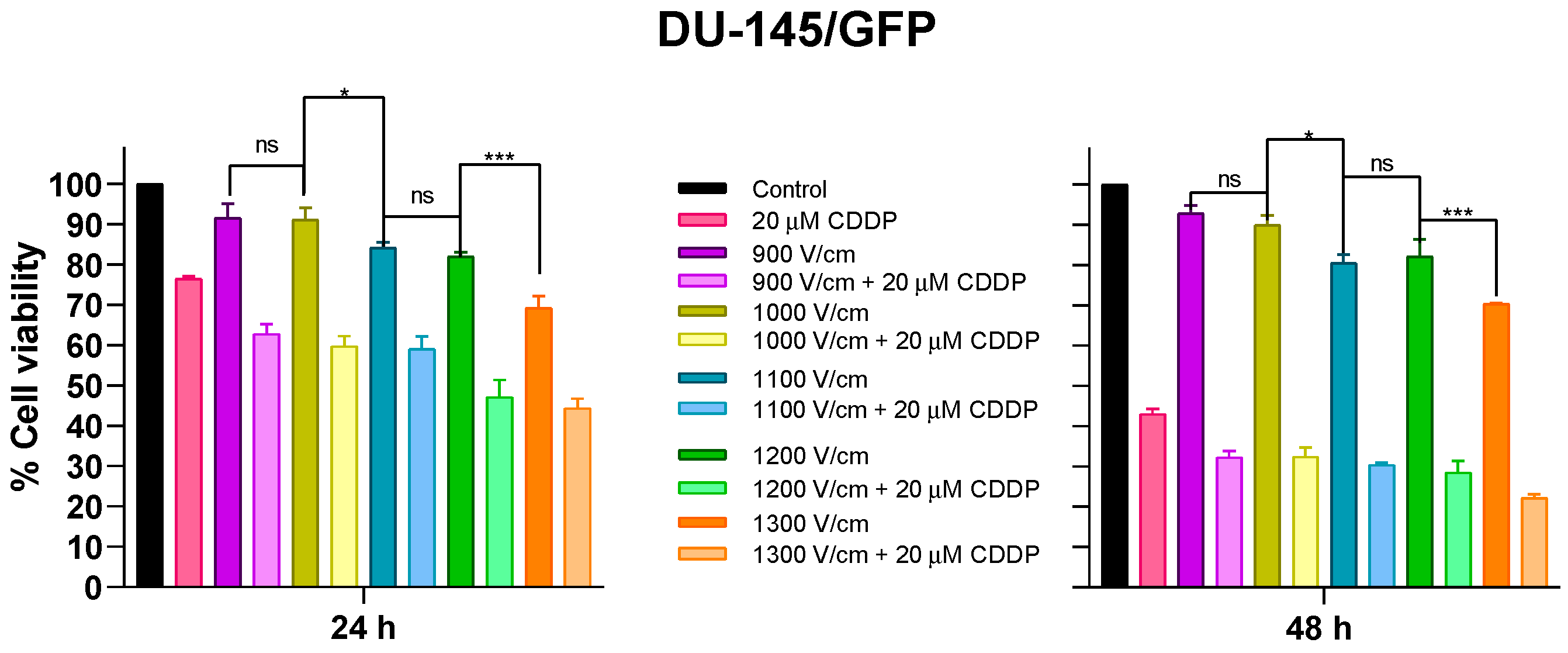
3.2.1. Effects of Different Electric Fields on Prostate Cancer-Derived Cell Line DU-145/GFP
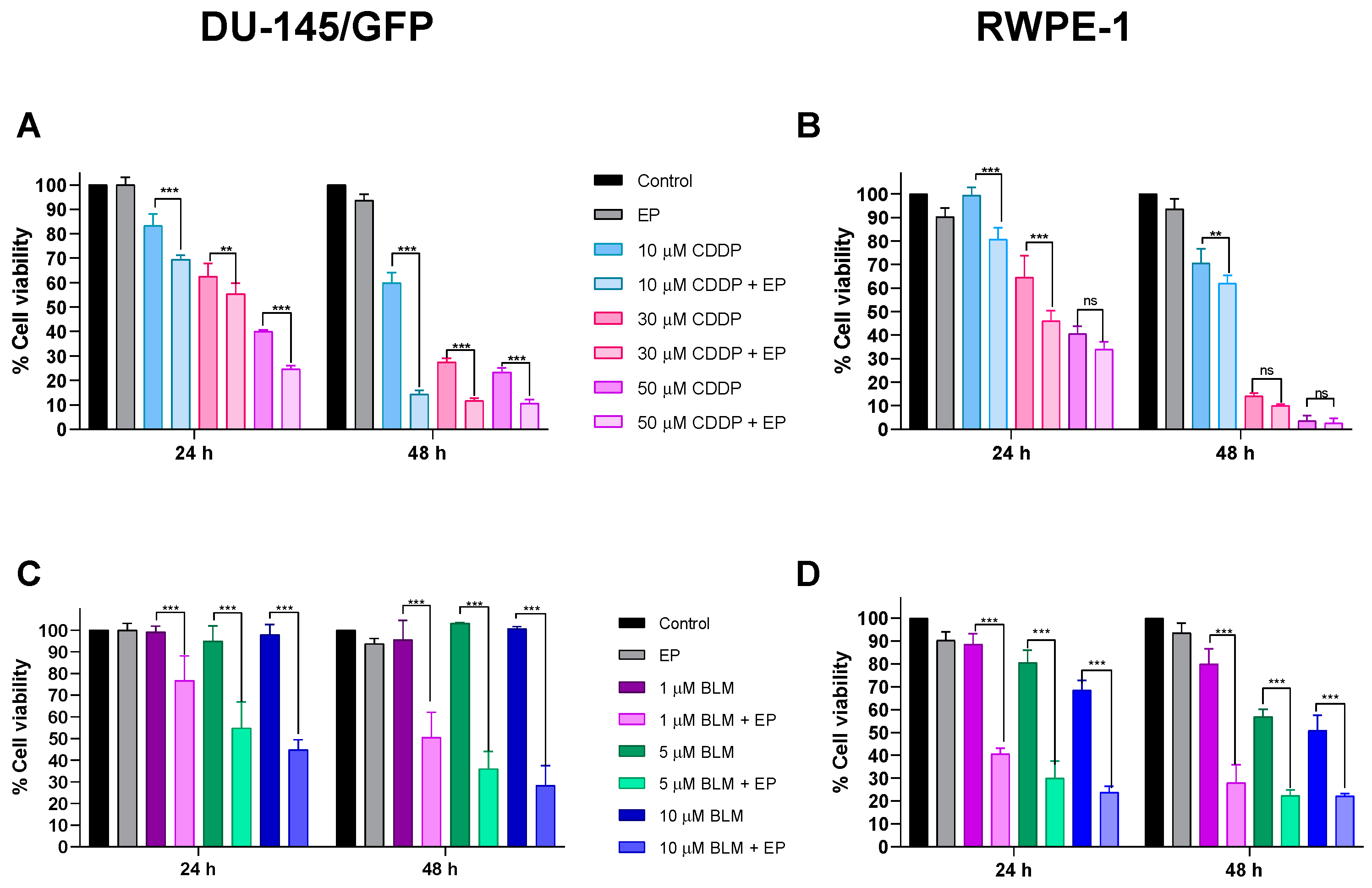
3.2.2. Cytotoxic Effect of Electrochemotherapy with Cisplatin and Bleomycin on Prostate-Derived Cell Lines
3.3. Irradiation Time of SPIONs Influences Inhibition of Cell Proliferation of Prostate Cancer Cells
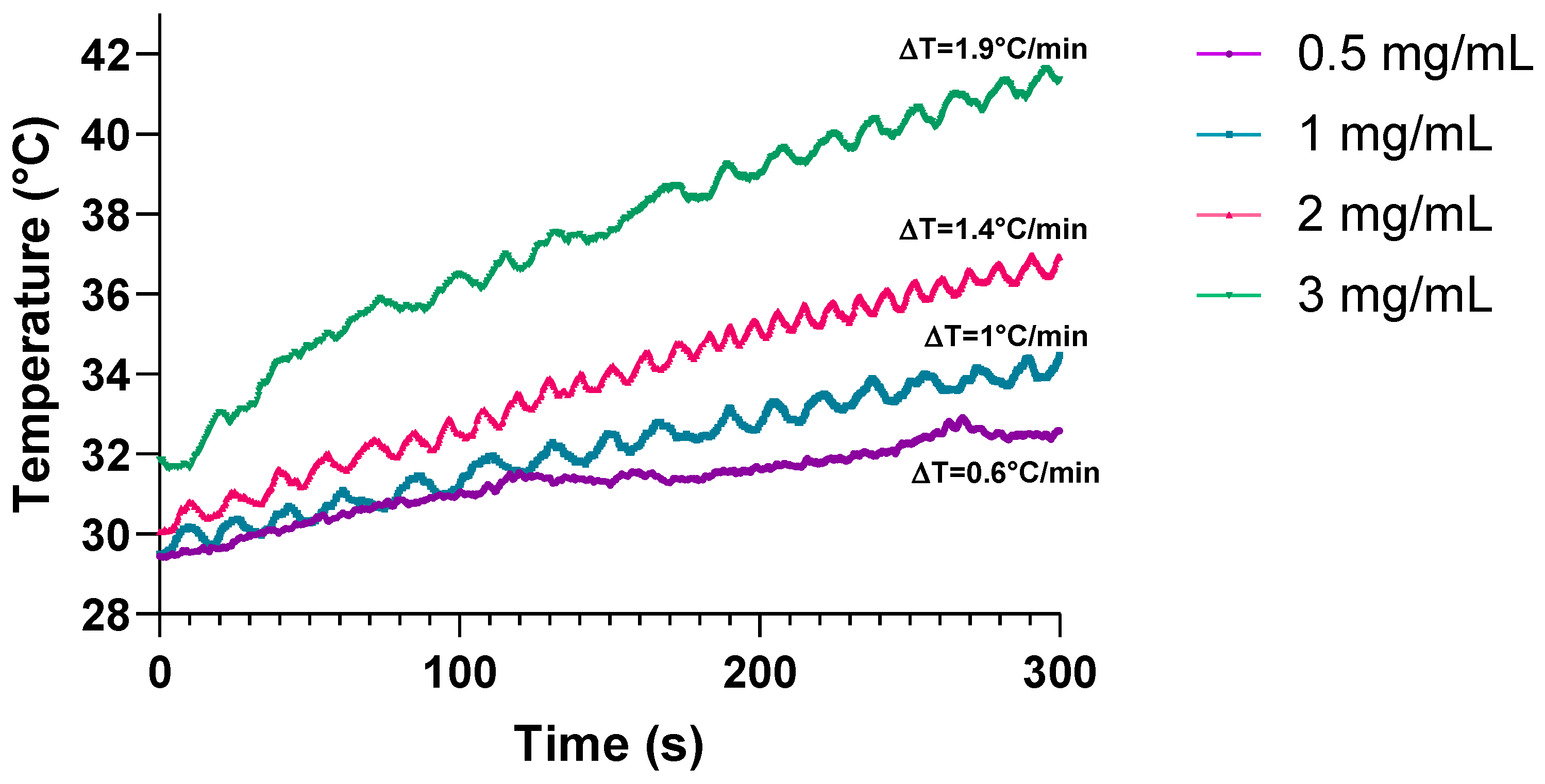
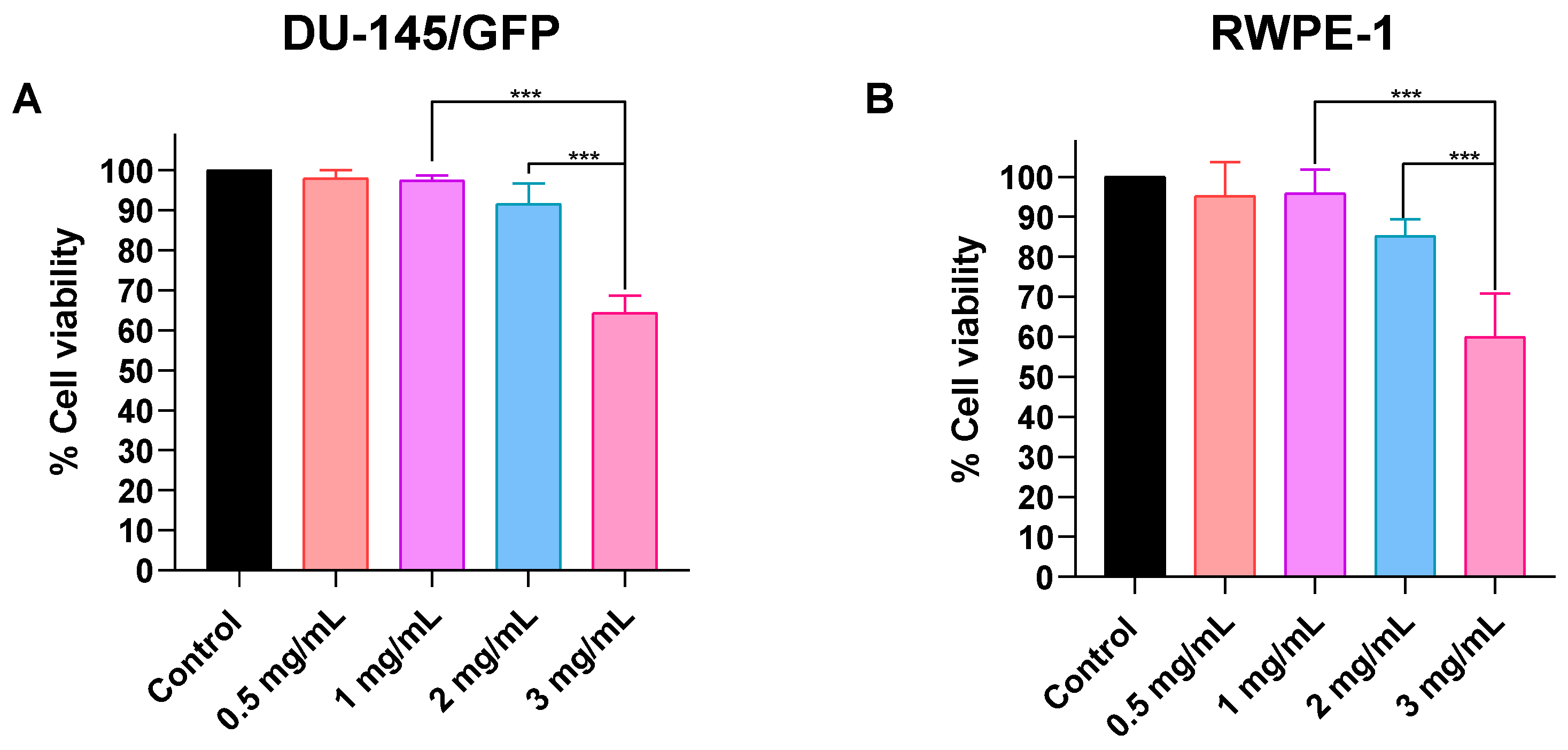
3.3.1. Determination of Optimal Concentration of SPIONs for Electromagnetic Hyperthermia on Prostate-Derived Cell Lines
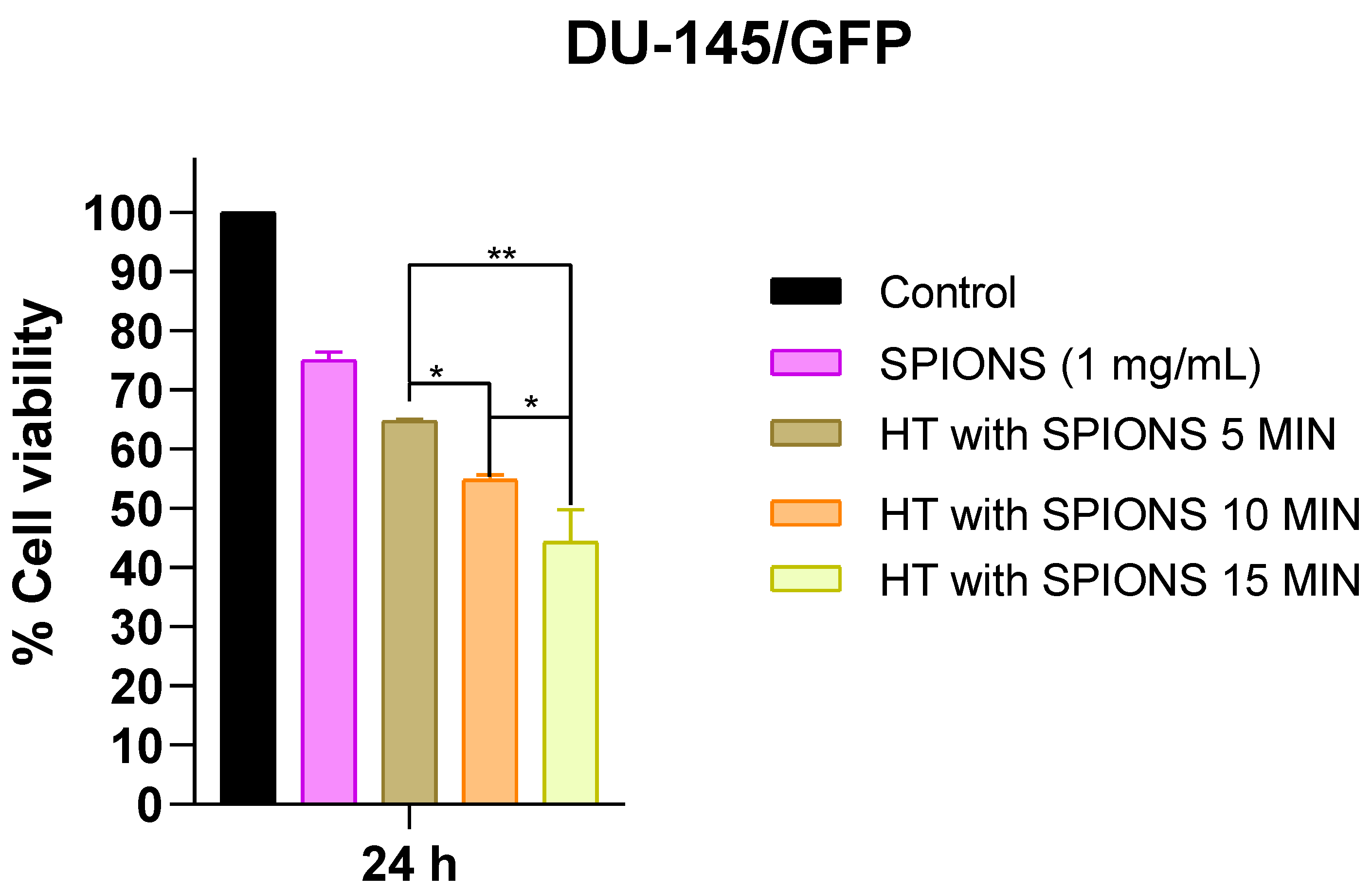
3.3.2. Effects of Different Irradiation Times on Cell Proliferation in Prostate Cancer-Derived Cell Line DU-145/GFP
3.4. Electrochemotherapy and Electromagnetic Hyperthermia Significantly Decrease Cell Proliferation of Prostate-Cancer Cells
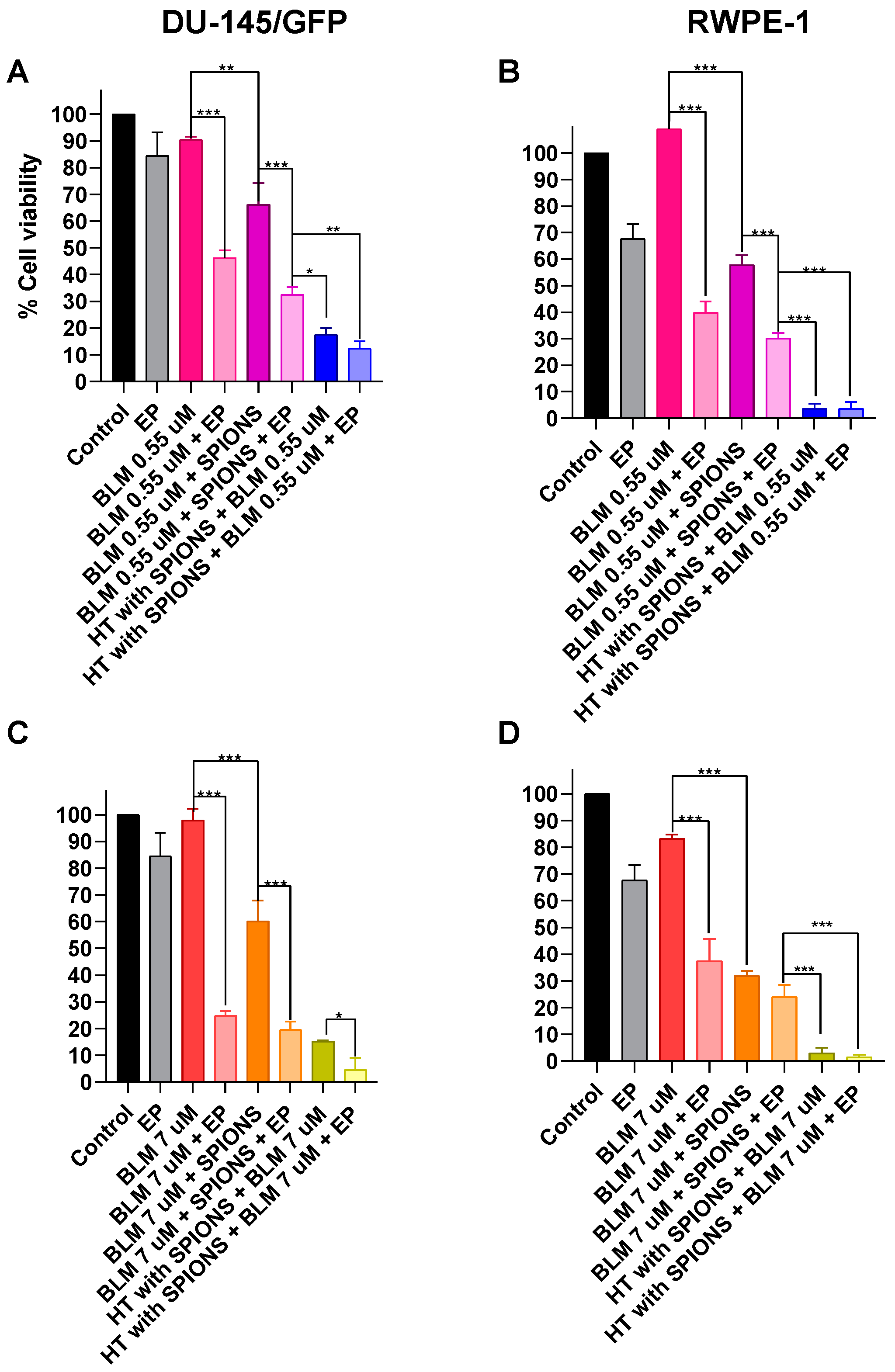
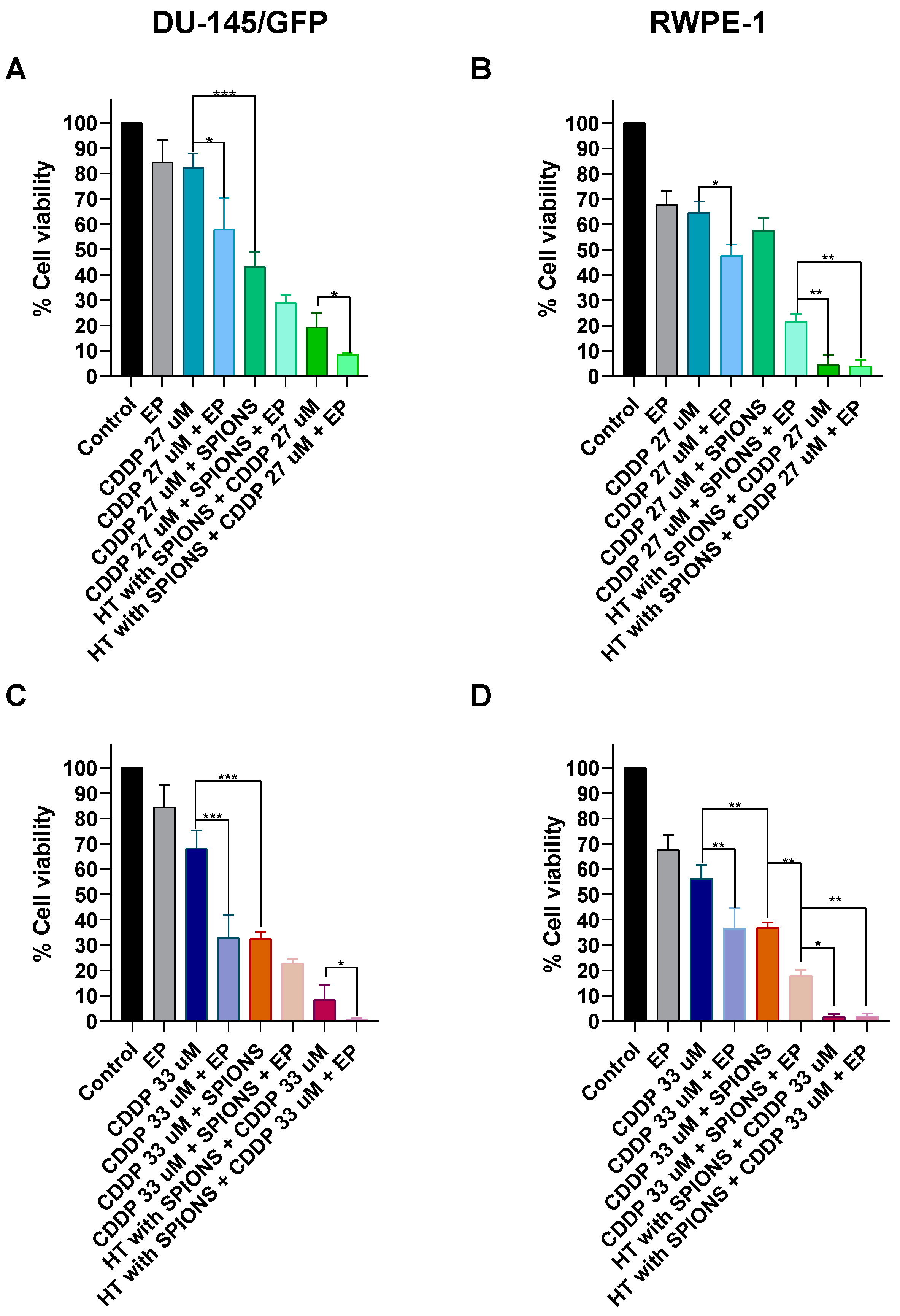
3.4.1. Electroporation Increases the Effectiveness of Electromagnetic Hyperthermia in Prostate-Derived Cell Lines
3.4.2. Effect of Electrochemotherapy with Bleomycin and Cisplatin in Combination with Electromagnetic Hyperthermia on Prostate-Derived Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 2024. Available online: https://gco.iarc.who.int/today (accessed on 3 April 2024).
- Lopez, W.; Nguyen, N.; Cao, J.; Eddow, C.; Shung, K.K.; Lee, N.S.; Chow, M.S.S. Ultrasound Therapy, Chemotherapy and Their Combination for Prostate Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211011965. [Google Scholar] [CrossRef]
- Sebesta, E.M.; Anderson, C.B. The Surgical Management of Prostate Cancer. Semin. Oncol. 2017, 44, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J. Treatment effects in prostate cancer. Mod. Pathol. 2018, 31 (Suppl. S1), S110–S121. [Google Scholar] [CrossRef] [PubMed]
- Dal Pra, A.; Souhami, L. Prostate cancer radiation therapy: A physician’s perspective. Phys. Med. 2016, 32, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Boulos, S.; Mazhar, D. The evolving role of chemotherapy in prostate cancer. Future Oncol. 2017, 13, 1091–1095. [Google Scholar] [CrossRef]
- Farha, N.G.; Kasi, A. TEAM Study: Upfront Docetaxel Treatment in Patients with Metastatic Hormone-Sensitive Prostate Cancer: A Real-World, Multicenter, Retrospective Analysis. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/30725927/ (accessed on 3 April 2024).
- Nightingale, G.; Ryu, J. Cabazitaxel (Jevtana): A Novel Agent for Metastatic Castration-Resistant Prostate Cancer. 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/23091336/ (accessed on 3 April 2024).
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A review of current status, alternative IGP approaches, and future perspectives. J. Healthc. Eng. 2019, 2018, 2784516. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–2013. [Google Scholar] [CrossRef]
- Ueki, T.; Uemura, H.; Nagashima, Y.; Ohta, S.; Ishiguro, H.; Kubota, Y. Antitumour effect of electrochemotherapy with bleomycin on human prostate cancer xenograft. BJU Int. 2008, 102, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Gunther, E.; Zapf, S.; El-Idrissi, R.; Atta, J.; Stehling, M. Prostate cancer infiltrating the bladder sphincter successfully treated with Electrochemotherapy: A case report. Clin. Case Rep. 2017, 5, 2127–2132. [Google Scholar] [CrossRef]
- Kima, K.; Lee, W.G. Electroporation for nanomedicine: A review. J. Mater. Chem. B Mater. Biol. Med. 2017, 5, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, O.; Casillas, N.; Knauth, P.; Lopez, Z.; Virgen-Ortiz, A.; Lozano, O.; Delgado-Enciso, I.; Sámano, A.; Rosales, S.; Martinez-Ceseña, L.; et al. An easily prepared ferrofluid with high power absorption density and low cytotoxicity for biomedical applications. Mater. Chem. Phys. 2020, 245, 122752. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, e2004788. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, P.; Tay, Z.W.; Hensley, D.; Zhou, X.Y.; Fung, B.K.; Colson, C.; Lu, Y.; Fellows, B.D.; Huynh, Q.; Saayujya, C.; et al. Using magnetic particle imaging systems to localize and guide magnetic hyperthermia treatment: Tracers, hardware, and future medical applications. Theranostics 2020, 10, 2965–2981. [Google Scholar] [CrossRef]
- Beola, L.L.; Asín, L.; Roma-Rodrigues, C.; Fernández-Afonso, Y.; Fratila, R.M.M.; Serantes, D.; Ruta, S.; Chantrell, R.W.; Fernandes, A.R.; Baptista, P.V.; et al. The Intracellular Number of Magnetic Nanoparticles Modulates the Apoptotic Death Pathway after Magnetic Hyperthermia Treatment. ACS Appl. Mater. Interfaces 2020, 12, 43474–43487. [Google Scholar] [CrossRef]
- Pucci, C.; Degl’Innocenti, A.; Gümüş, M.B.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- Mesárošová, M.; Kozics, K.; Bábelová, A.; Regendová, E.; Pastorek, M.; Vnuková, D.; Buliaková, B.; Rázga, F.; Gábelová, A. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol. Lett. 2014, 226, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Li, J.; Du, C.; Huang, Z.; Chen, G.; Yan, W. Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget 2017, 8, 9410–9424. [Google Scholar] [CrossRef]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Farzanegan, Z.; Tahmasbi, M. Evaluating the applications and effectiveness of magnetic nanoparticle-based hyperthermia for cancer treatment: A systematic review. Appl. Radiat. Isot. 2023, 198, 110873. [Google Scholar] [CrossRef]
- Rahban, D.; Doostan, M.; Salimi, A. Cancer Therapy; Prospects for Application of Nanoparticles for Magnetic-Based Hyperthermia. Cancer Ther. 2019, 38, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. Int. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Ellem, S.J.; De-Juan-Pardo, E.M.; Risbridger, G.P. In vitro modeling of the prostate cancer microenvironment. Adv. Drug Deliv. Rev. 2014, 79–80, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef]
- Johannsen, M.; Febu; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. [Google Scholar] [CrossRef]
- Singh, A.; Jain, S.; Sahoo, S.K. Magnetic nanoparticles for amalgamation of magnetic hyperthermia and chemotherapy: An approach towards enhanced attenuation of tumor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110695. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, O.; Lopez, Z.d.R.; Casillas, N.; Knauth, P.; Checa, N.; Cholico, F.A.; Hernandez-Gutiérrez, R.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules 2022, 27, 544. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Zalba, S.; Navarro, I.; de Ilarduya, C.T.; Garrido, M.J. Pharmacodynamics of cisplatin-loaded PLGA nanoparticles administered to tumor-bearing mice. Eur. J. Pharm. Biopharm. 2010, 74, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, M.; Orr, R.; Peacock, J. The role of apoptosis in cell killing by cisplatin: A flow cytometric study. Br. J. Cancer 1994, 69, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.N.; Horwitz, S.B. Characterization of the association of radiolabeled bleomycin A2 with HeLa cells. Cancer Res. 1984, 44, 1541–1546. [Google Scholar]
- Sleijfer, S. Bleomycin-Induced Pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef]
- Yamamoto, T. Bleomycin and the skin. Br. J. Dermatol. 2006, 155, 869–875. [Google Scholar] [CrossRef]
- Kiełbik, A.; Szlasa, W.; Michel, O.; Szewczyk, A.; Tarek, M.; Saczko, J.; Kulbacka, J. In Vitro Study of Calcium Microsecond Electroporation of Prostate Adenocarcinoma Cells. Molecules 2020, 25, 5406. [Google Scholar] [CrossRef]
- Todorovic, V.; Sersa, G.; Flisar, K.; Cemazar, M. Enhanced cytotoxicity of bleomycin and cisplatin after electroporation in murine colorectal carcinoma cells. Radiol. Oncol. 2009, 43, 264–273. [Google Scholar] [CrossRef]
- Saczko, J.; Pilat, J.; Choromanska, A.; Rembialkowska, N.; BAR, J.; Kaminska, I.; Zalewski, J.; Kulbacka, J. The effectiveness of chemotherapy and electrochemotherapy on ovarian cell lines in vitro. Neoplasma 2016, 63, 450–455. [Google Scholar] [CrossRef]
- Rosales, S.; Hernández-Gutiérrez, R.; Oaxaca, A.; López, Z.; Casillas, N.; Knauth, P.; Quintero, L.H.; Paz, J.A.; Cholico, F.; Velásquez, C.; et al. The Fluorescent Cell Line SW620-GFP Is a Valuable Model to Monitor Magnetic Hyperthermia. Bioengineering 2024, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Voinov, M.A.; Pagán, J.O.S.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-Mediated Production of Hydroxyl Radicals as a Mechanism of Iron Oxide Nanoparticle Biotoxicity. J. Am. Chem. Soc. 2011, 133, 35–41. [Google Scholar] [CrossRef]
- Asín, L.; Ibarra, M.R.; Tres, A.; Goya, G.F. Controlled Cell Death by Magnetic Hyperthermia: Effects of Exposure Time, Field Amplitude, and Nanoparticle Concentration. Pharm. Res. 2012, 29, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Jabalera, Y.; Chico-Lozano, M.A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Reactive oxygen species (ROS) production in HepG2 cancer cell line through the application of localized alternating magnetic field. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 7667–7676. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Salingova, B.; Simara, P.; Matula, P.; Zajickova, L.; Synek, P.; Jasek, O.; Veverkova, L.; Sedlackova, M.; Nichtova, Z.; Koutna, I. The Effect of Uncoated SPIONs on hiPSC-Differentiated Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 3536. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2016, 46, 4218–4244. [Google Scholar] [CrossRef]
- Hervaultab, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 2103–2121. [Google Scholar]
- Torres-Lugo, M.; Castillo, A.; Mendez, J.; Rinaldi, C.; Soto, O.; Alvarez-Berrios, M.P. Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity. Int. J. Nanomed. 2012, 8, 1003–1013. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Goya, G.F.; Asín, L.; Ibarra, M.R. Cell death induced by AC magnetic fields and magnetic nanoparticles: Current state and perspectives. Int. J. Hyperth. 2013, 29, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Seegenschmiedt, M.H.; Fessenden, P.; Vernon, C.C. Thermoradiotherapy and Thermochemotherapy: Biology, Physiology, Physics; Springer: Berlin/Heidelberg, Germany, 1995; Volume 1. [Google Scholar]
- Inaoka, Y.; Keii, T.; Mimura, A.; Murase, K. A Novel Therapeutic Strategy Combining Use of Intracellular Magnetic Nanoparticles under an Alternating Magnetic Field and Bleomycin. Open J. Appl. Sci. 2019, 9, 87–103. [Google Scholar] [CrossRef]
- Overgaard, J. Effect of hyperthermia on malignant cells in vivo: A review and a hypothesis. Cancer 1977, 39, 2637–2646. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizcarra-Ramos, S.; Molina-Pineda, A.; Gutiérrez-Ortega, A.; Herrera-Rodríguez, S.E.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; López, Z.; Cano, M.E.; Hernández-Gutiérrez, R. Synergistic Strategies in Prostate Cancer Therapy: Electrochemotherapy and Electromagnetic Hyperthermia. Pharmaceutics 2024, 16, 1109. https://doi.org/10.3390/pharmaceutics16091109
Vizcarra-Ramos S, Molina-Pineda A, Gutiérrez-Ortega A, Herrera-Rodríguez SE, Aguilar-Lemarroy A, Jave-Suárez LF, López Z, Cano ME, Hernández-Gutiérrez R. Synergistic Strategies in Prostate Cancer Therapy: Electrochemotherapy and Electromagnetic Hyperthermia. Pharmaceutics. 2024; 16(9):1109. https://doi.org/10.3390/pharmaceutics16091109
Chicago/Turabian StyleVizcarra-Ramos, Sayma, Andrea Molina-Pineda, Abel Gutiérrez-Ortega, Sara E. Herrera-Rodríguez, Adriana Aguilar-Lemarroy, Luis F. Jave-Suárez, Zaira López, Mario E. Cano, and Rodolfo Hernández-Gutiérrez. 2024. "Synergistic Strategies in Prostate Cancer Therapy: Electrochemotherapy and Electromagnetic Hyperthermia" Pharmaceutics 16, no. 9: 1109. https://doi.org/10.3390/pharmaceutics16091109
APA StyleVizcarra-Ramos, S., Molina-Pineda, A., Gutiérrez-Ortega, A., Herrera-Rodríguez, S. E., Aguilar-Lemarroy, A., Jave-Suárez, L. F., López, Z., Cano, M. E., & Hernández-Gutiérrez, R. (2024). Synergistic Strategies in Prostate Cancer Therapy: Electrochemotherapy and Electromagnetic Hyperthermia. Pharmaceutics, 16(9), 1109. https://doi.org/10.3390/pharmaceutics16091109









