A Review of the Efficacy of Nanomaterial-Based Natural Photosensitizers to Overcome Multidrug Resistance in Cancer
Abstract
:1. Introduction
1.1. MDR in Cancers
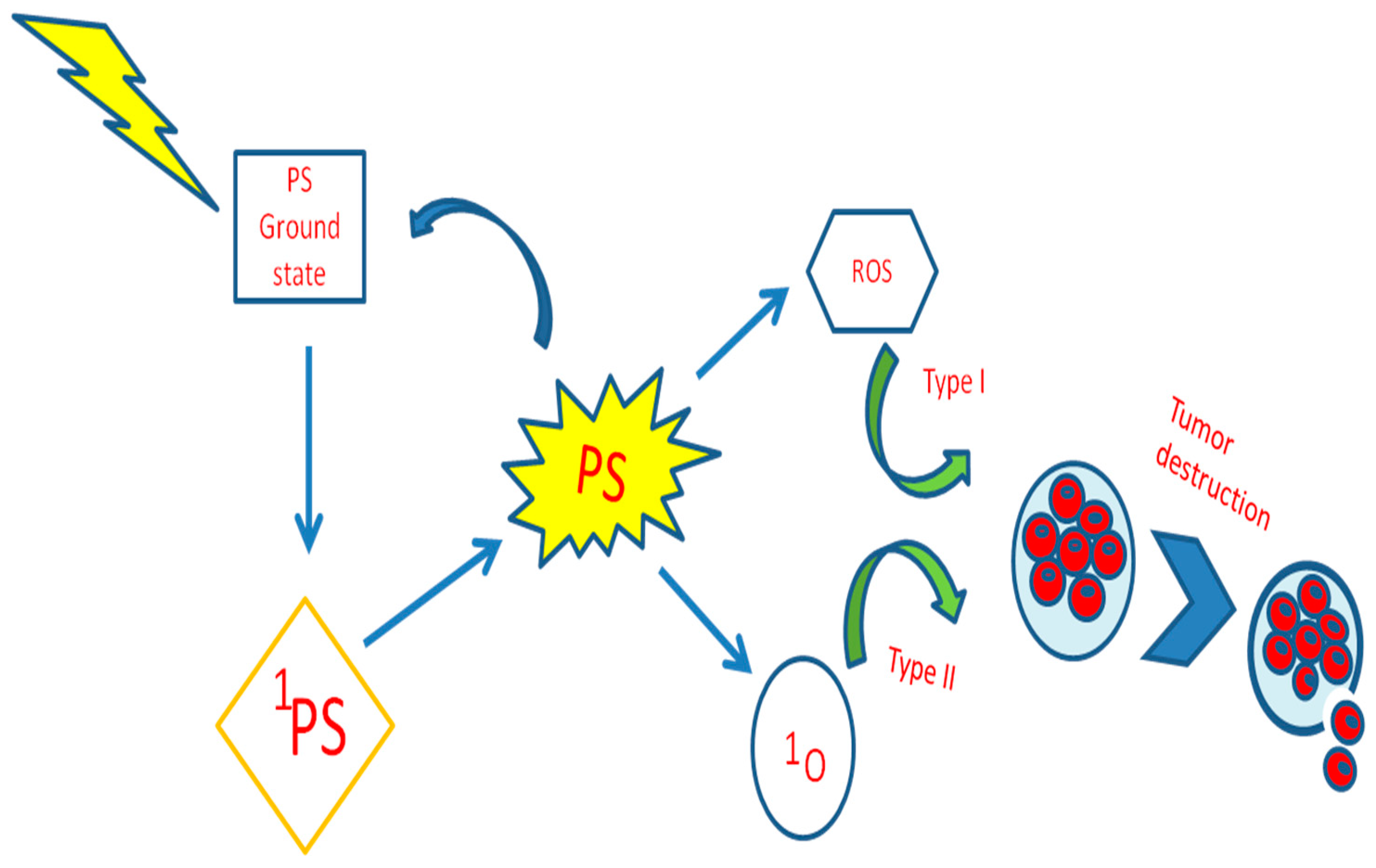
1.2. Photodynamic Therapy
1.3. Role of PS in Resistance Cell Death
1.4. Limitations of Photodynamic Therapy
2. Naturally Occurring PSs
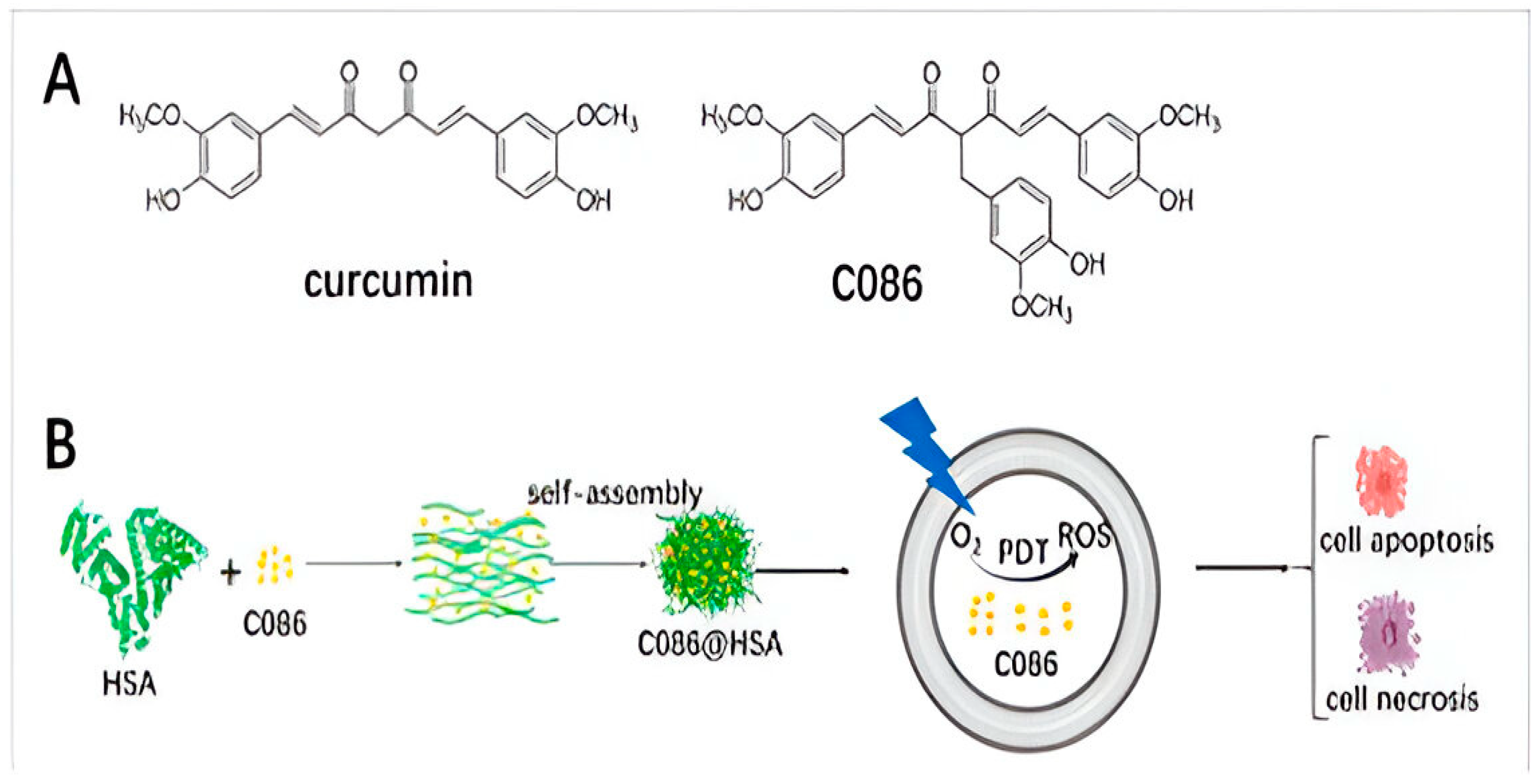
2.1. Curcumin
2.2. Porphyrin

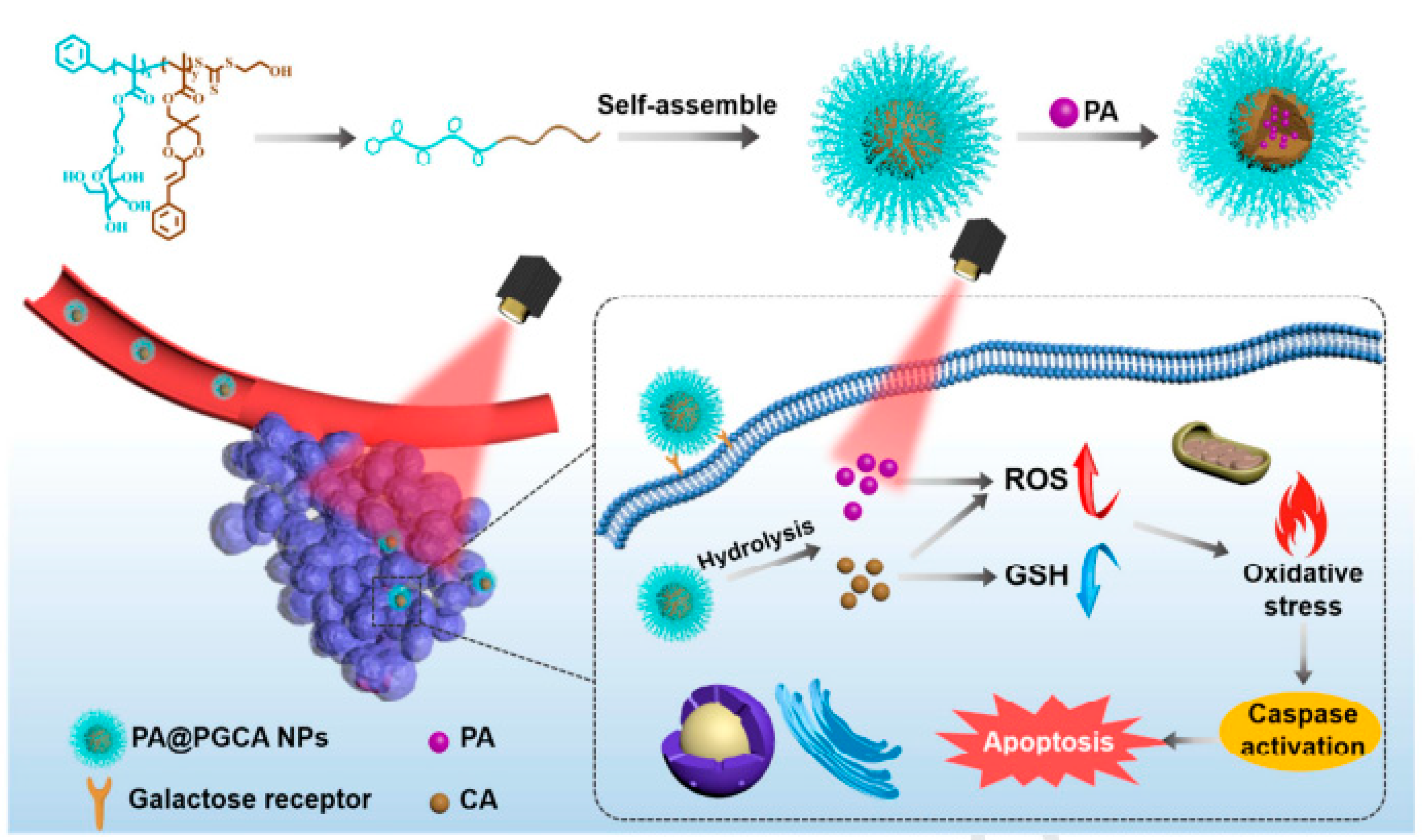
2.3. Pheophorbide a (PA)
2.4. Hypocrellin
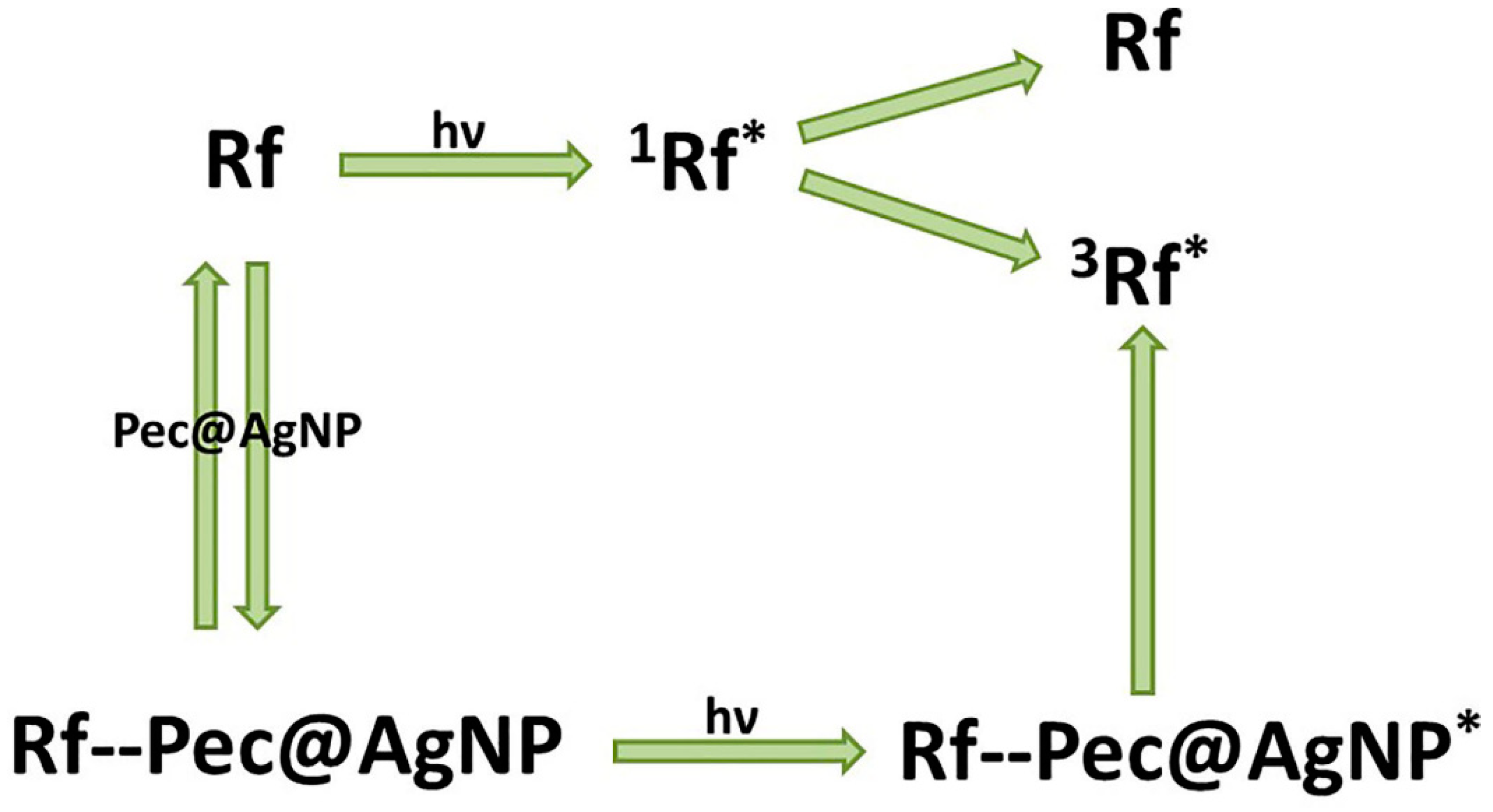
2.5. Riboflavin
2.6. Hypericin
2.7. 5-Aminolevulinic Acid
2.8. Pheophytin
2.9. Bacteriochlorin
2.10. Berberine
3. Strategies to Address Challenges in PDT with Natural PSs against Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug resistance |
| PSs | Photosensitizers |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| MoS2 | Molybdenum disulfide |
| ICG | Indocyanine green |
| ROS | Reactive oxygen species |
| CUR | Curcumin |
| ABC | ATP-binding cassette |
| NIR | Near-infrared light |
| MRI | Magnetic resonance imaging |
| HY | Hypericin |
| UCL | Upconversion luminescence imaging |
| FL | Fluorescence imaging |
| PAT | Photoacoustic imaging |
| PET | Positron emission tomography |
| PEG | Polyethylene glycol |
| Pa | Pheophorbide a |
| PpIX | Porphyrin |
| BER | Berberine |
| Rf | Riboflavin |
| SLNs | Solid lipid nanoparticles |
| 5-ALA | 5-Aminolevulinic acid |
| MNPs | Magnetic nanoparticles |
| HAS | Human serum albumin |
| PLGA | Poly(lactic-co-glycolic acid) |
| MSNs | Mesoporous silica nanoparticles |
| CMC | Carboxymethylchitosan |
| FA | Folic acid |
| HA | Hyaluronic acid |
| MOF | Metal–organic framework |
| MB | Methylene blue |
| DNA | Deoxyribonucleic acid |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-xl | B-cell lymphoma-extra-large |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ER | Endoplasmic reticulum |
| EPR | Enhanced permeability and retention effect |
References
- Weiss, C. One in Four Dies of Cancer. Questions About the Epidemiology of Malignant Tumours. Recent. Results Cancer Res. 2021, 218, 15–29. [Google Scholar] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef]
- López-Estévez, A.M.; Lapuhs, P.; Pineiro-Alonso, L.; Alonso, M.J. Personalized Cancer Nanomedicine: Overcoming Biological Barriers for Intracellular Delivery of Biopharmaceuticals. Adv. Mater. 2024, 36, 2309355. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; El-Shakankery, K.H.; Lee, J.Y. PARP inhibitors in ovarian cancer: Overcoming resistance with combination strategies. J. Gynecol. Oncol. 2022, 33, e44. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Wang, X.; Ramamurthy, G.; Shirke, A.A.; Walker, E.; Mangadlao, J.; Wang, Z.; Wang, Y.; Shan, L.; Schluchter, M.D.; Dong, Z.; et al. Photodynamic Therapy Is an Effective Adjuvant Therapy for Image-Guided Surgery in Prostate Cancer. Cancer Res. 2020, 80, 156–162. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef]
- Petrakou, K.; Iatrou, G.; Lamari, F.N. Ethnopharmacological survey of medicinal plants traded in herbal markets in the Peloponnisos, Greece. J. Herb. Med. 2020, 19, 100305. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Hu, J.; Wang, C.; Hagedoorn, P.-L.; Li, N.; Zhou, X. Hypericin: Source, Determination, Separation, and Properties. Sep. Purif. Rev. 2020, 51, 1–10. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Cossari, S.; Panzanelli, P.; Dosio, F.; Serpe, L. Ultrasound Triggers Hypericin Activation Leading to Multifaceted Anticancer Activity. Pharmaceutics 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Kaur, G.; Jain, S.; Gupta, S.; Tripathi, S.K. Characteristics and mechanism associated with drug conjugated inorganic nanoparticles. J. Drug Target. 2019, 27, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Ye, D. Activatable Multimodal Probes for In Vivo Imaging and Theranostics. Angew. Chem. Int. Ed. 2022, 61, e202209512. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: A critical review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.; Fan, Q.; Ma, Q.; Dong, Z.; Tao, W.; Tao, H.; Liu, Z.; Wang, C. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020, 13, 564–569. [Google Scholar] [CrossRef]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Whitlock, B.D.; Leslie, E.M. Efflux transporters in anti-cancer drug resistance: Molecular and functional identification and characterization of multidrug resistance proteins (MRPs/ABCCs). In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 31–65. [Google Scholar]
- Yang, X.; Yu, Y.; Huang, X.; Chen, Q.; Wu, H.; Wang, R.; Qi, R.; Miao, Y.; Qiu, Y. Delivery of platinum (II) drugs with bulky ligands in trans-geometry for overcoming cisplatin drug resistance. Mater. Sci. Eng. C 2019, 96, 96–104. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular Mechanisms of Cancer Drug Resistance: Emerging Biomarkers and Promising Targets to Overcome Tumor Progression. Cancers 2022, 14, 1614. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Guo, Q. Drug Resistance in Cancers: A Free Pass for Bullying. Cells 2022, 11, 3383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar]
- Fan, J.; To, K.K.W.; Chen, Z.-S.; Fu, L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updates 2023, 66, 100905. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Haderk, F.; Kumaraswamy, A.; Baughn, L.B.; Van Ness, B.; Liu, S.; Marathe, H.; Alumkal, J.J.; Bivona, T.G.; Chan, K.S.; et al. Understanding Drug Sensitivity and Tackling Resistance in Cancer. Cancer Res. 2022, 82, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Khaled, J.; Kopsida, M.; Lennernäs, H.; Heindryckx, F. Drug Resistance and Endoplasmic Reticulum Stress in Hepatocellular Carcinoma. Cells 2022, 11, 632. [Google Scholar] [CrossRef]
- Icard, P.; Damotte, D.; Alifano, M. New Therapeutic Strategies for Lung Cancer. Cancers 2021, 13, 1937. [Google Scholar] [CrossRef]
- Famta, P.; Shah, S.; Chatterjee, E.; Singh, H.; Dey, B.; Guru, S.K.; Singh, S.B.; Srivastava, S. Exploring new Horizons in overcoming P-glycoprotein-mediated multidrug-resistant breast cancer via nanoscale drug delivery platforms. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100054. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Tang, S.; Yu, H.; Zhang, H.; Zhang, Y.; Wan, Y.; Cai, F. The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer. Front. Oncol. 2022, 12, 964934. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.H.; Chang, Y.J.; Hsu, W.H.; Tu, Y.T.; Chen, Y.R.; Lee, M.C.; Tsai, K.W. Breast Cancer with Increased Drug Resistance, Invasion Ability, and Cancer Stem Cell Properties through Metabolism Reprogramming. Int. J. Mol. Sci. 2022, 23, 12875. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. Mitochondrial Dynamics in Ovarian Cancer: Pathophysiology and Therapeutic Implications. J. Mol. Pathol. 2023, 4, 275–293. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Song, C.; Duan, C. Upregulation of FAM3B Promotes Cisplatin Resistance in Gastric Cancer by Inducing Epithelial-Mesenchymal Transition. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921002. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Yfantis, A.; Mylonis, I.; Chachami, G.; Nikolaidis, M.; Amoutzias, G.D.; Paraskeva, E.; Simos, G. Transcriptional Response to Hypoxia: The Role of HIF-1-Associated Co-Regulators. Cells 2023, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eichhorn, P.J.A.; Thiery, J.P. TGF-β, EMT, and resistance to anti-cancer treatment. In Seminars in Cancer Biology Semin; Academic Press: Cambridge, MA, USA, 2023; Volume 97, pp. 1–11. [Google Scholar]
- Singh, S.; Gomez, H.J.; Thakkar, S.; Singh, S.P.; Parihar, A.S. Overcoming Acquired Drug Resistance to Cancer Therapies through Targeted STAT3 Inhibition. Int. J. Mol. Sci. 2023, 24, 4722. [Google Scholar] [CrossRef] [PubMed]
- Afjei, R.; Sadeghipour, N.; Kumar, S.U.; Pandrala, M.; Kumar, V.; Malhotra, S.V.; Massoud, T.F.; Paulmurugan, R. A New Nrf2 Inhibitor Enhances Chemotherapeutic Effects in Glioblastoma Cells Carrying p53 Mutations. Cancers 2022, 14, 6120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Chen, Q.; He, J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int. 2020, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Hou, J.; An, Q.; Assaraf, Y.G.; Wang, X. Towards the overcoming of anticancer drug resistance mediated by p53 mutations. Drug Resist. Updates 2020, 49, 100671. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656. [Google Scholar] [CrossRef]
- Engler, C.; Herzog, R.O. Zur chemischen Erkenntnis biologischer Oxydationsreaktionen. Hoppe-Seyler’s Z. Für Physiol. Chem. 1909, 59, 327–375. [Google Scholar] [CrossRef]
- Davila, M.L. Photodynamic Therapy. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, H.; Li, Q.; Bai, C.; Zeng, Y.; Lai, G.; Guo, S.; Gu, X.; Li, W.; Zhang, H. Clinical application of photodynamic therapy for malignant airway tumors in China. Thorac. Cancer 2020, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, D.R.; George, B.P.; Abrahamse, H. Photodynamic Therapy Induced Cell Death Mechanisms in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 10506. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, L.; Xing, D.; Chen, Q. Bid Is Required in NPe6-PDT-induced Apoptosis. Photochem. Photobiol. 2007, 84, 250–257. [Google Scholar] [CrossRef]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting mitochondria for cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef] [PubMed]
- Gitego, N.; Agianian, B.; Mak, O.W.; Kumar Mv, V.; Cheng, E.H.; Gavathiotis, E. Chemical modulation of cytosolic BAX homodimer potentiates BAX activation and apoptosis. Nat. Commun. 2023, 14, 8381. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Shi, L.; Hu, F.; Duan, Y.; Wu, W.; Dong, J.; Meng, X.; Zhu, X.; Liu, B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano 2020, 14, 2183–2190. [Google Scholar] [CrossRef]
- Kralova, J.; Kolar, M.; Kahle, M.; Truksa, J.; Lettlova, S.; Balusikova, K.; Bartunek, P. Glycol porphyrin derivatives and temoporfin elicit resistance to photodynamic therapy by different mechanisms. Sci. Rep. 2017, 7, 44497. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Yoo, H.; Park, S.; Kim, J.; Yum, K.; Kim, K.; Kim, H. Overcoming anticancer resistance by photodynamic therapy-related efflux pump deactivation and ultrasound-mediated improved drug delivery efficiency. Nano Converg. 2020, 7, 30. [Google Scholar] [CrossRef]
- Martins, W.K.; Belotto, R.; Silva, M.N.; Grasso, D.; Suriani, M.D.; Lavor, T.S.; Itri, R.; Baptista, M.S.; Tsubone, T.M. Autophagy Regulation and Photodynamic Therapy: Insights to Improve Outcomes of Cancer Treatment. Front. Oncol. 2020, 10, 610472. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Arévalo, D.E.; Sanabria, L.M.; Carrión, F.D.C.; Fanelli, M.A.; Rivarola, V.A. Heat shock protein 27 modulates autophagy and promotes cell survival after photodynamic therapy. Photochem. Photobiol. Sci. 2019, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Hu, J.; Yu, S.; Zhou, Y.; Shi, T.; Zhao, Y.; Wang, X.; Liu, X. A Mitochondrial Oxidative Stress Amplifier to Overcome Hypoxia Resistance for Enhanced Photodynamic Therapy. Small Methods 2021, 5, 2100581. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef]
- Mossakowska, B.J.; Shahmoradi Ghahe, S.; Cysewski, D.; Fabisiewicz, A.; Tudek, B.; Siedlecki, J.A. Mechanisms of Resistance to Photodynamic Therapy (PDT) in Vulvar Cancer. Int. J. Mol. Sci. 2022, 23, 4117. [Google Scholar] [CrossRef] [PubMed]
- Hönigsmann, H. History of phototherapy in dermatology. Photochem. Photobiol. Sci. 2013, 12, 16–21. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Zoulikha, M.; Boafo, G.F.; Magar, K.T.; Ju, Y.; He, W. Multifunctional nanoparticle-mediated combining therapy for human diseases. Signal Transduct. Target. Ther. 2024, 9, 1. [Google Scholar] [CrossRef]
- Majerník, M.; Jendželovský, R.; Vargová, J.; Jendželovská, Z.; Fedoročko, P. Multifunctional Nanoplatforms as a Novel Effective Approach in Photodynamic Therapy and Chemotherapy, to Overcome Multidrug Resistance in Cancer. Pharmaceutics 2022, 14, 1075. [Google Scholar] [CrossRef]
- Kamuhabwa, A.A.R.; Cosserat-Gerardin, I.; Didelon, J.; Notter, D.; Guillemin, F.; Roskams, T.; D’Hallewin, M.-A.; Baert, L.; de Witte, P.A.M. Biodistribution of hypericin in orthotopic transitional cell carcinoma bladder tumors: Implication for whole bladder wall photodynamic therapy. Int. J. Cancer 2001, 97, 253–260. [Google Scholar] [CrossRef]
- Liao, J.; Li, P.; Wu, C. Screening new photosensitizers from Chinese medicinal herbs and searching for herbal photodynamic killing effects on human stomach cancer cells. PubMed 1997, 17, 726–729. [Google Scholar]
- Wu, J.Y. Chinese Herbal Composition for the Treatment of Macular Degeneration and the Process for Manufacturing the Same. U.S. Patent No. 8,372,450, 12 February 2013. [Google Scholar]
- Qiu, H.; Kim, M.M.; Rozhin, P.; Finlay, J.C.; Busch, T.M.; Wang, T.; Guo, W.; Cengel, K.A.; Simone, C.B.; Glatstein, E.; et al. A Comparison of Dose Metrics to Predict Local Tumor Control for Photofrin-mediated Photodynamic Therapy. Photochem. Photobiol. 2017, 93, 1115–1122. [Google Scholar] [CrossRef]
- Udrea, A.M.; Smarandache, A.; Dinache, A.; Mares, C.; Nistorescu, S.; Avram, S.; Staicu, A. Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment. Pharmaceutics 2023, 15, 2124. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Fateh, M. Curcumin intercalated layered double hydroxide nanohybrid as a potential drug delivery system for effective photodynamic therapy in human breast cancer cells. RSC Adv. 2015, 5, 93987–93994. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K. Methylene blue, curcumin and ion pairing nanoparticles effects on photodynamic therapy of MDA-MB-231 breast cancer cell. Photodiagnosis Photodyn. Ther. 2017, 18, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Dulal, M.; Vanitha, R.; Md Kausar, R.; Upadhyay, A.; Pal, M.; Amit, K.; Roy, M. La (iii)—Curcumin-functionalized gold nanocomposite as a red light-activatable mitochondria-targeting PDT agent. Inorg. Chem. Front. 2022, 9, 686–701. [Google Scholar]
- Zhong, Y.; Zhang, X.; Yang, L.; Liang, F.; Zhang, J.; Jiang, Y.; Chen, X.; Ren, F. Hierarchical dual-responsive cleavable nanosystem for synergetic photodynamic/photothermal therapy against melanoma. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2021, 131, 112524. [Google Scholar] [CrossRef] [PubMed]
- Vadarevu, H.; Juneja, R.; Lyles, Z.; Vivero-Escoto, J.L. Light-Activated Protoporphyrin IX-Based Polysilsesquioxane Nanoparticles Induce Ferroptosis in Melanoma Cells. Nanomaterials 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ghazaeian, M.; Khorsandi, K.; Hosseinzadeh, R.; Naderi, A.; Abrahamse, H. Curcumin–silica nanocomplex preparation, hemoglobin and DNA interaction and photocytotoxicity against melanoma cancer cells. J. Biomol. Struct. Dyn. 2020, 39, 6606–6616. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Liu, C.; He, J.; Tian Tian, L.; Fu, C.; Meng, X.; Shao, H. Enhanced Photothermal-Photodynamic Therapy by Indocyanine Green and Curcumin-Loaded Layered MoS2 Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomed. 2021, 16, 433–442. [Google Scholar] [CrossRef]
- Halevas, E.; Arvanitidou, M.; Mavroidi, B.; Hatzidimitriou, A.G.; Politopoulos, K.; Alexandratou, E.; Pelecanou, M.; Sagnou, M. A novel curcumin gallium complex as photosensitizer in photodynamic therapy: Synthesis, structural and physicochemical characterization, photophysical properties and in vitro studies against breast cancer cells. J. Mol. Struct. 2021, 1240, 130485. [Google Scholar] [CrossRef]
- Magaela, N.B.; Matshitse, R.; Babu, B.; Managa, M.; Prinsloo, E.; Nyokong, T. Sn(IV) porphyrin-biotin decorated nitrogen doped graphene quantum dots nanohybrids for photodynamic therapy. Polyhedron 2022, 213, 115624. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, L.; Gu, Z.; Tian, G.; Yan, L.; Ren, W.; Yin, W.; Liu, X.; Zhang, X.; Hu, Z.; et al. A new near infrared photosensitizing nanoplatform containing blue-emitting up-conversion nanoparticles and hypocrellin A for photodynamic therapy of cancer cells. Nanoscale 2013, 5, 11910. [Google Scholar] [CrossRef] [PubMed]
- Seung Hong, C.; Seung Wook, B.; Chang, S.-J.; Song, Y.; Rafique, R.; Lee, K.; Tae Gwan, P. Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosens. Bioelectron. 2017, 93, 267–273. [Google Scholar]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin photoactivation by upconversion nanoparticles for cancer treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, Q.; Liu, W.; Nan, F.; Zheng, X.; Ding, Y.; Ren, H.; Wu, J.; Ge, J. Hypocrellin Derivative-Loaded Calcium Phosphate Nanorods as NIR Light-Triggered Phototheranostic Agents with Enhanced Tumor Accumulation for Cancer Therapy. ChemMedChem 2019, 15, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.-S.; Wang, P. Hypocrellin-Based Multifunctional Phototheranostic Agent for NIR-Triggered Targeted Chemo/Photodynamic/Photothermal Synergistic Therapy against Glioblastoma. ACS Appl. Bio Mater. 2020, 3, 3817–3826. [Google Scholar] [CrossRef]
- Xu, F.; Li, J.; Zhu, T.-T.; Yu, S.-S.; Zuo, C.; Yao, R.-S.; Qian, H.-S. A new trick (hydroxyl radical generation) of an old vitamin (B2) for near-infrared-triggered photodynamic therapy. RSC Adv. 2016, 6, 102647–102656. [Google Scholar] [CrossRef]
- Rivas Aiello, M.B.; Castrogiovanni, D.; Parisi, J.; Azcárate, J.C.; García Einschlag, F.S.; Gensch, T.; Bosio, G.N.; Mártire, D.O. Photodynamic Therapy in HeLa Cells Incubated with Riboflavin and Pectin-coated Silver Nanoparticles. Photochem. Photobiol. 2018, 94, 1159–1166. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Khoshgard, K.; Arkan, E.; Hosseinzadeh, L.; Hemati Azandaryani, A. Evaluating the photodynamic therapy efficacy using 5-aminolevulinic acid and folic acid-conjugated bismuth oxide nanoparticles on human nasopharyngeal carcinoma cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S514–S523. [Google Scholar] [CrossRef]
- Leite, P.; Miguel, N.; Pierre, M.B. Microemulsions Improve Topical Protoporphyrin IX (PpIX) Delivery for Photodynamic Therapy of Skin Cancer. Braz. J. Pharm. Sci. 2023, 59, e21920. [Google Scholar] [CrossRef]
- Shivashankarappa, A.; Sanjay, K.R. Photodynamic therapy on skin melanoma and epidermoid carcinoma cells using conjugated 5-aminolevulinic acid with microbial synthesised silver nanoparticles. J. Drug Target. 2019, 27, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, S.; Sanjay, K.R. Conjugation study of 5-aminolevulinic acid with microbial synthesized gold nanoparticles to evaluate its effect on skin melanoma and epidermoid carcinoma cell lines using photodynamic cancer therapy. Gold. Bull. 2017, 51, 11–19. [Google Scholar] [CrossRef]
- Wu, J.-N.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and Proof of Programmed 5-Aminolevulinic Acid Prodrug Nanocarriers for Targeted Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Xu, H.; Wang, B.; Yao, C. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 2015, 20, 051043. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jia, Q.; Nan, F.; Zheng, X.; Liu, W.; Wu, J.; Ren, H.; Ge, J.; Wang, P. Pheophytin Derived Near-Infrared-Light Responsive Carbon Dot Assembly as a New Phototheranotic Agent for Bioimaging and Photodynamic Therapy. Chem. Asian J. 2019, 14, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Cong, Y.; Lei, Y.; Sun, F.; Xu, M.; Zhang, J.; Fang, L.; Hong, H.; Cai, T. Transforming Passive into Active: Multimodal Pheophytin—Based Carbon Dots Customize Protein Corona to Target Metastatic Breast Cancer. Adv. Healthc. Mater. 2022, 11, 2102270. [Google Scholar] [CrossRef] [PubMed]
- Petr, O.; Semkina, A.S.; Naumenko, V.; Plotnikova, E.A.; Melnikov, P.; Abakumova, T.O.; Yakubovskaya, R.I.; Mирoнoв, A.Φ.; Vodopyanov, S.S.; Abakumov, A.M.; et al. Synthesis and characterization of bacteriochlorin loaded magnetic nanoparticles (MNP) for personalized MRI guided photosensitizers delivery to tumor. J. Colloid. Interface Sci. 2019, 537, 132–141. [Google Scholar]
- Jiang, S.; Zhu, R.; He, X.; Wang, J.; Wang, M.; Qian, Y.; Wang, S. Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles. Int. J. Nanomed. 2016, 12, 167–178. [Google Scholar] [CrossRef]
- Barras, A.; Boussekey, L.; Courtade, E.; Boukherroub, R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale 2013, 5, 10562. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Li, H.; Dong, L.; Zhou, Y.; Zhu, J.; Yang, L.; Tao, J. Modified hollow mesoporous silica nanoparticles as immune adjuvant-nanocarriers for photodynamically enhanced cancer immunotherapy. Front. Bioeng. Biotechnol. 2022, 10, 1039154. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pang, X.; Liu, R.; Xiao, Q.; Wang, P.; Leung, A.W.; Luan, Y.; Xu, C. Design of an Amphiphilic iRGD Peptide and Self-Assembling Nanovesicles for Improving Tumor Accumulation and Penetration and the Photodynamic Efficacy of the Photosensitizer. ACS Appl. Mater. Interfaces 2018, 10, 31674–31685. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kumar, S.; Kumar, R.; Gaharwar, U.S.; Rajamani, P. PLGA-CTAB curcumin nanoparticles: Fabrication, characterization and molecular basis of anticancer activity in triple negative breast cancer cell lines (MDA-MB-231 cells). Biomed. Pharmacother. 2017, 94, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Raschpichler, M.; Preis, E.; Shashank Reddy, P.; Baghdan, E.; Pourasghar, M.; Schneider, M. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, Y.; Li, L.; Shi, X.; Chen, Z.; Wu, F.; Liu, Y.; Zhang, Z.; Wang, S. Pheophorbide co-encapsulated with Cisplatin in folate-decorated PLGA nanoparticles to treat nasopharyngeal carcinoma: Combination of chemotherapy and photodynamic therapy. Colloids Surf. B Biointerfaces 2021, 208, 112100. [Google Scholar] [CrossRef]
- Venkateshwaran, K.; Chandrasekar, P.; Senthilkumar, S.; Muthuselvam, P.; Ragupathy, M.; Rajaguru, P.; Kandasamy, R.; Subramanian, N. Development of copolymeric nanoparticles of hypocrellin B: Enhanced phototoxic effect and ocular distribution. Eur. J. Pharm. Sci. 2018, 116, 26–36. [Google Scholar]
- Rajkumar, S.; Prabaharan, M. Theranostics Based on Iron Oxide and Gold Nanoparticles for Imaging-Guided Photothermal and Photodynamic Therapy of Cancer. Curr. Top. Med. Chem. 2017, 17, 1858–1871. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Cao, Y.; Wang, Y.; Wang, F.; Zhang, F.; Zheng, S. Biodegradable Hypocrellin B nanoparticles coated with neutrophil membranes for hepatocellular carcinoma photodynamics therapy effectively via JUNB/ROS signaling. Int. Immunopharmacol. 2021, 99, 107624. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, J.; Zhang, M.; Ge, Y.; Wei, J.; Li, Y.; Wang, W.; Li, M.; Jin, Y. Chemo-photodynamic therapy by pulmonary delivery of gefitinib nanoparticles and 5-aminolevulinic acid for treatment of primary lung cancer of rats. Photodiagnosis Photodyn. Ther. 2020, 31, 101807. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Yu, K.-H.; Huang, Y.-C.; Lee, C.-I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Luo, L.; Li, W.; Yang, J.; Zhu, C.; Jiang, M.; Qin, B.; Yuan, X.; Yin, H.; Lu, Y.; et al. A cabazitaxel liposome for increased solubility, enhanced antitumor effect and reduced systemic toxicity. Asian J. Pharm. Sci. 2019, 14, 658–667. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, G.; Zhang, J.; Niu, J.; Huang, P.; Wang, Z.; Wang, Y.; Wang, W.; Li, C.; Kong, D. Redox- and light-responsive alginate nanoparticles as effective drug carriers for combinational anticancer therapy. Nanoscale 2017, 9, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Qin, Y.; Song, H.; Huang, P.; Wang, W.; Wang, C.; Li, C.; Wang, Y.; Kong, D. Co-delivery of doxorubicin and pheophorbide A by pluronic F127 micelles for chemo-photodynamic combination therapy of melanoma. J. Mater. Chem. B 2018, 6, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Kye, Y.-C.; Yun, C.-H. The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses. Pharmaceutics 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Moukheiber, D.; Upendra, C.; Carter, K.A.; Luo, D.; Sun, B.; Goel, S.; Ferreira, C.A.; Engle, J.W.; Wang, D.; Geng, J.; et al. Surfactant-Stripped Pheophytin Micelles for Multimodal Tumor Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 2, 544–554. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Liu, X.; Song, H.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials 2020, 255, 120210. [Google Scholar] [CrossRef]
- Bouramtane, S.; Bretin, L.; Pinon, A.; Leger, D.; Liagre, B.; Perez, D.D.S.; Launay, Y.; Brégier, F.; Sol, V.; Chaleix, V. Acetylxylan-pheophorbide-a nanoparticles designed for tumor-targeted photodynamic therapy. J. Appl. Polym. Sci. 2021, 138, 50799. [Google Scholar] [CrossRef]
- Siwawannapong, K.; Zhang, R.; Lei, H.; Jin, Q.; Tang, W.; Dong, Z.; Lai, R.-Y.; Liu, Z.; Kamkaew, A.; Cheng, L. Ultra-small Pyropheophorbide-a Nanodots for Near-infrared Fluorescence/Photoacoustic Imaging-guided Photodynamic Therapy. Theranostics 2020, 10, 62–73. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.; Wang, K.; Lu, Z.; Su, X.; Li, L.; Yuan, C.; Feng, J.; Yan, S.; Kong, B.; et al. Hypocrellin B-loaded, folate-conjugated polymeric micelle for intraperitoneal targeting of ovarian cancer in vitro and in vivo. Cancer Sci. 2018, 109, 1958–1969. [Google Scholar] [CrossRef]
- Ji Eun, C.; Hyun Jong, C.; Yi, E.; Dae Duk, K.; Sanghoon, J. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid–ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B Biol. 2016, 158, 113–121. [Google Scholar]
- Damke, G.; Souza, R.P.; Montanha, M.C.; Damke, E.; Gonçalves, R.S.; César, G.B.; Kimura, E.; Caetano, W.; Hioka, N.; Consolaro, M.E.L. Selective Photodynamic Effects on Breast Cancer Cells Provided by p123 Pluronic®-Based Nanoparticles Modulating Hypericin Delivery. Anti-Cancer Agents Med. Chem. 2020, 20, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
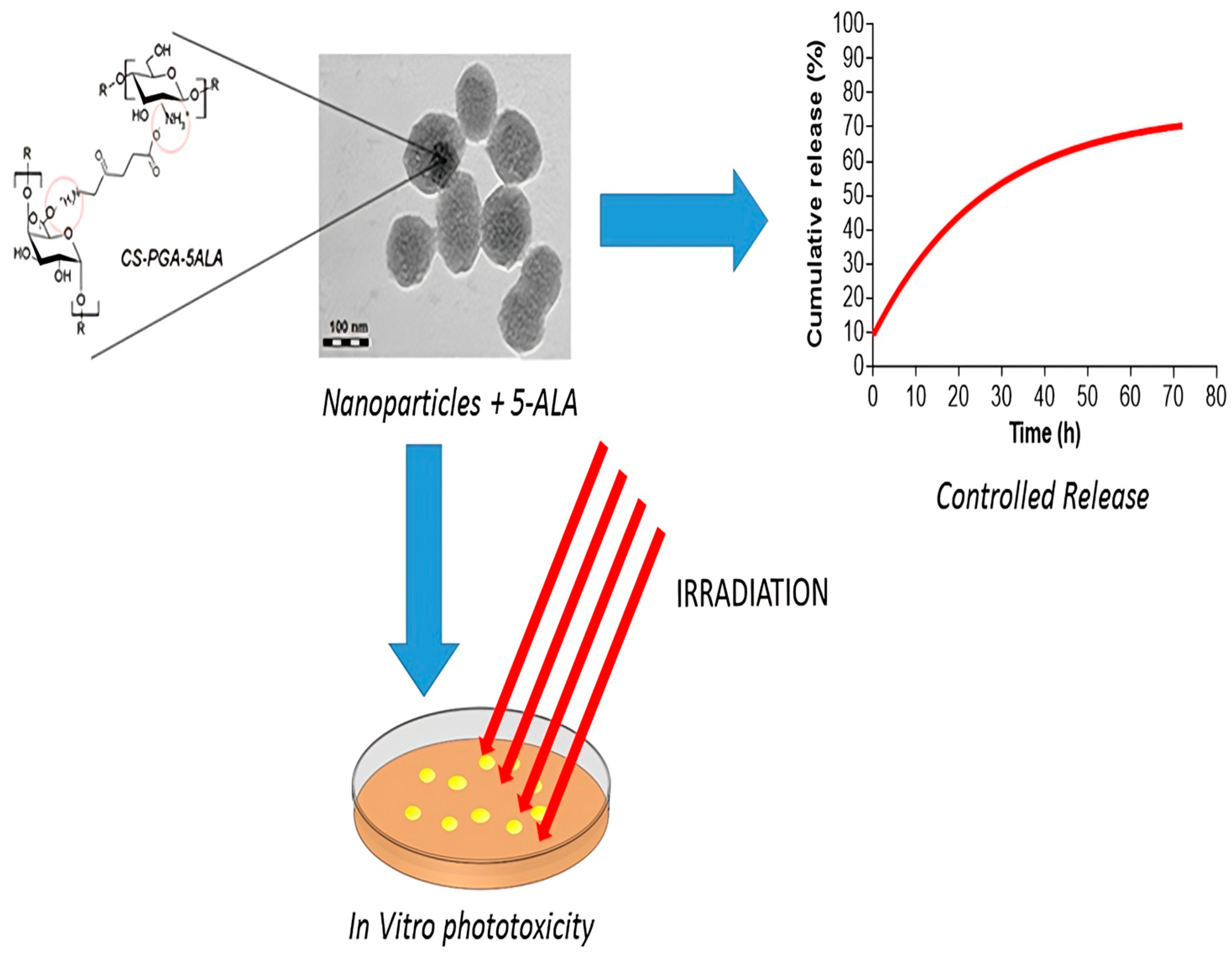
- Antonio Di, M.; Pavelkova, A.; Postnikov, P.S.; Vladimir, S. Enhancement of 5-aminolevulinic acid phototoxicity by encapsulation in polysaccharides based nanocomplexes for photodynamic therapy application. J. Photochem. Photobiol. B Biol. 2017, 175, 226–234. [Google Scholar]
- Wu, M.; Liu, Z.; Zhang, W. An ultra-stable bio-inspired bacteriochlorin analogue for hypoxia-tolerant photodynamic therapy. Chem. Sci. 2021, 12, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ghosh, S.; He, X.; Huang, W.-C.; Quinn, B.; Tian, M.; Dushyant, J.; Mabrouk, M.T.; Ortega, J.; Zhang, Y.; et al. Anti-cancer liposomal chemophototherapy using bilayer-localized photosensitizer and cabazitaxel. Nano Res. 2022, 15, 4302–4309. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, K.; Wang, Y.; Jiang, W.; Cheng, H.; Wang, Q.; Xiang, T.; Zhang, Z.; Liu, J.; Shi, J. Intracellular Self-Assembly Driven Nucleus-Targeted Photo-Immune Stimulator with Chromatin Decompaction Function for Robust Innate and Adaptive Antitumor Immunity. Adv. Funct. Mater. 2022, 32, 2108883. [Google Scholar] [CrossRef]
- Shen, Y.; Fang, Z.; Yang, E.; Du, Y.; Gao, D.; Wu, G.; Zhang, Y. Biomimetic smart nanoplatform for dual imaging-guided synergistic cancer therapy. J. Mater. Chem. B 2022, 10, 966–976. [Google Scholar]
- Li, J.; Wang, S.; Lin, X.; Cao, Y.; Cai, Z.; Wang, J.; Zhang, Z.; Liu, X.; Wu, M.; Yao, C. Red Blood Cell-Mimic Nanocatalyst Triggering Radical Storm to Augment Cancer Immunotherapy. Nano-Micro Lett. 2022, 14, 57. [Google Scholar] [CrossRef]
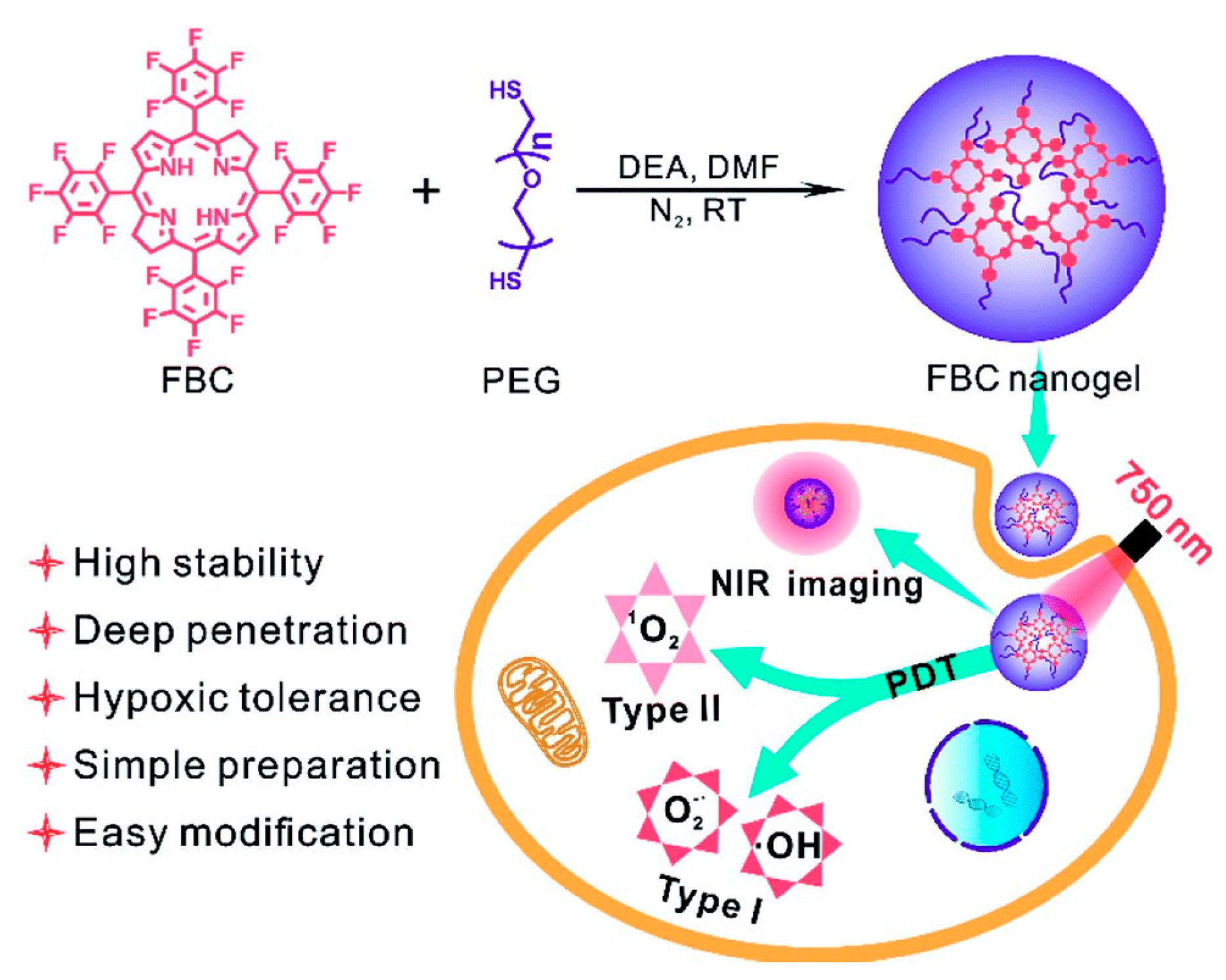
- Luo, T.K.; Ni, K.Y.; Culbert, A.; Lan, G.X.; Li, Z.; Jiang, X.M.; Kaufmann, M.; Lin, W.B. Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 7334–7339. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine-A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Carolina Alves, R.; Perosa Fernandes, R.; Fonseca-Santos, B.; Damiani Victorelli, F.; Chorilli, M. A Critical Review of the Properties and Analytical Methods for the Determination of Curcumin in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2018, 49, 138–149. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef] [PubMed]
- Alkahtany, M.F. Efficacy of curcumin-mediated photodynamic therapy for root canal therapy procedures: A systematic review. Photodiagnosis Photodyn. Ther. 2023, 41, 103252. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Gupta, A.P.; Pandotra, P.; Sharma, R.; Kushwaha, M.; Gupta, S. Marine Resource. Stud. Nat. Prod. Chem. 2013, 40, 229–325. [Google Scholar]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, L.; Liu, W.; Huang, Y.; Hu, P.; Dai, T.; Xu, J.; Chen, Z. Albumin-based nanoparticles combined with photodynamic therapy enhance the antitumor activity of curcumin derivative C086. Dye. Pigment. 2021, 189, 109258. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jalde, S.S.; Anil Kumar, C.; Ji Hoon, L.; Pankaj Kumar, C.; Park, J.-S.; Kim, Y.-W. Synthesis of novel Chlorin e6-curcumin conjugates as photosensitizers for photodynamic therapy against pancreatic carcinoma. Eur. J. Med. Chem. 2018, 147, 66–76. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.J.; Zhang, J.F.; Dai, W.J.; Li, R.L.; Lu, Z.Y.; Duan, J.L.; Li, J.P.; Chen, X.P.; Fan, J.P.; et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget 2017, 8, 50747–50760. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Park, J.M.; Hong, K.-I.; Lee, H.; Jang, W.-D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B Biol. 2023, 248, 112796. [Google Scholar] [CrossRef]
- Terver, J.; Sase, T.; Gungsat, N.; Wufem, B.; Bioltif, Y.; Kendeson, C.; Naanogot, D.; Moses, I. Porphyrin and its use as Photosensitizers in Photodynamic Therapy 1*. Int. J. Trend Res. Dev. 2022, 8, 52–67. [Google Scholar]
- Pathak, P.; Zarandi, M.A.; Zhou, X.; Jayawickramarajah, J. Synthesis and Applications of Porphyrin-Biomacromolecule Conjugates. Front. Chem. 2021, 9, 764137. [Google Scholar] [CrossRef] [PubMed]
- Koifman, O.; Ageeva, T.; Kuzmina, N.; Otvagin, V.; Nyuchev, A.; Dorov, A.; Belykh, D.; Lebedeva, N.; Yurina, E.; Syrbu, S.; et al. Synthesis Strategy of Tetrapyrrolic Photosensitizers for Their Practical Application in Photodynamic Therapy. Macroheterocycles 2022, 15, 207–304. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Meng, Q.; Chen, C.; Pang, M. Facile Synthesis of a Cubic Porphyrin-Based Covalent Organic Framework for Combined Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 56873–56880. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef]
- Silva, L.B.; Castro, K.; Botteon, C.E.A.; Oliveira, C.L.P.; da Silva, R.S.; Marcato, P.D. Hybrid Nanoparticles as an Efficient Porphyrin Delivery System for Cancer Cells to Enhance Photodynamic Therapy. Front. Bioeng. Biotechnol. 2021, 9, 679128. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Tian, Z.; Liu, X.; Guo, Y.; He, J.; Wang, Z.; Zhou, T.; Liu, Y. Porphyrin-Based Nanoparticles: A Promising Phototherapy Platform. ChemPlusChem 2022, 87, e202200156. [Google Scholar] [CrossRef]
- Yue, J.; Mei, Q.; Wang, P.; Miao, P.; Dong, W.-F.; Li, L. Light-triggered multifunctional nanoplatform for efficient cancer photo-immunotherapy. J. Nanobiotechnol. 2022, 20, 181. [Google Scholar] [CrossRef]
- Hou, Y.; Kuang, Y.; Jiang, Q.; Zhou, S.; Yu, J.; He, Z.; Sun, J. Arginine-peptide complex-based assemblies to combat tumor hypoxia for enhanced photodynamic therapeutic effect. Nano Res. 2022, 15, 5183–5192. [Google Scholar] [CrossRef]
- Pan, W.-L.; Tan, Y.; Meng, W.; Huang, N.-H.; Zhao, Y.-B.; Yu, Z.-Q.; Huang, Z.; Zhang, W.-H.; Sun, B.; Chen, J.-X. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials 2022, 283, 121449. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, J.; Santos, H.A. Recent advances in Fenton and Fenton-like reaction mediated nanoparticle in cancer therapy. Biomed. Technol. 2023, 3, 40–51. [Google Scholar] [CrossRef]
- Konan, Y.N.; Berton, M.; Gurny, R.; Allémann, E. Enhanced photodynamic activity of meso-tetra(4-hydroxyphenyl)porphyrin by incorporation into sub-200 nm nanoparticles. Eur. J. Pharm. Sci. 2003, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liang, X.; Li, X.; Lin, L.; Yang, Y.; Yue, X.; Dai, Z. Mn-porphyrin conjugated Au nanoshells encapsulating doxorubicin for potential magnetic resonance imaging and light triggered synergistic therapy of cancer. Theranostics 2014, 4, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Liu, Y.; Wang, H.; Li, Z.T.; Zhang, D.W. Water-Soluble Porphyrin-Based Nanoparticles Derived from Electrostatic Interaction for Enhanced Photodynamic Therapy. ACS Appl. Bio Mater. 2022, 5, 881–888. [Google Scholar] [CrossRef]
- Tang, P.M.-K.; Bui-Xuan, N.-H.; Wong, C.-K.; Fong, W.-P.; Fung, K.-P. Pheophorbide a-Mediated Photodynamic Therapy Triggers HLA Class I-Restricted Antigen Presentation in Human Hepatocellular Carcinoma. Transl. Oncol. 2010, 3, 114–122. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Yeo, S.; Yoon, I.; Woo Kyoung, L. Design and Characterisation of pH-Responsive Photosensitiser-Loaded Nano-Transfersomes for Enhanced Photodynamic Therapy. Pharmaceutics 2022, 14, 210. [Google Scholar] [CrossRef]
- Moret, F.; Luca, M.; Battan, M.; Tedesco, D.; Columbaro, M.; Guerrini, A.; Avancini, G.; Ferroni, C.; Varchi, G. Pheophorbide A and Paclitaxel Bioresponsive Nanoparticles as Double-Punch Platform for Cancer Therapy. Pharmaceutics 2021, 13, 1130. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-Immunotherapy: A New Trend in Cancer Treatment. Cancers 2023, 15, 2912. [Google Scholar] [CrossRef]
- Law, S.; Lo, C.; Han, J.; Leung, A.W.; Xu, C. Antimicrobial photodynamic therapy with hypocrellin B against SARS-CoV-2 infection? Photodiagnosis Photodyn. Ther. 2021, 34, 102297. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiao, Q.; Niu, C.; Jin, N.; Ouyang, J.; Xiao, X.; He, D. Multifunctional core–shell upconversion nanoparticles for targeted tumor cells induced by near-infrared light. J. Mater. Chem. B 2013, 1, 2757. [Google Scholar] [CrossRef] [PubMed]
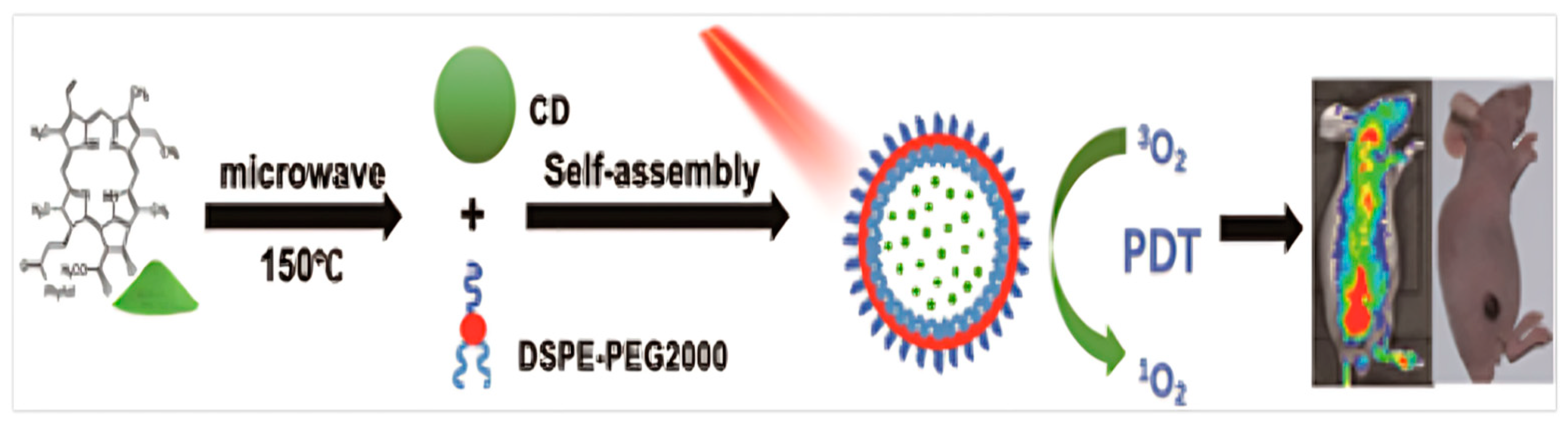
- Lin, X.; Yan, S.-Z.; Qi, S.-S.; Xu, Q.; Han, S.-S.; Guo, L.-Y.; Zhao, N.; Chen, S.-L.; Yu, S.-Q. Transferrin-Modified Nanoparticles for Photodynamic Therapy Enhance the Antitumor Efficacy of Hypocrellin, A. Front. Pharmacol. 2017, 8, 815. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Venkateshwaran, K.; Chandrasekar, P.; Madi, M.; Woo, T.; Rajaguru, P. Hypocrellin B and nano silver loaded polymeric nanoparticles: Enhanced generation of singlet oxygen for improved photodynamic therapy. Mater. Sci. Eng. C 2017, 77, 935–946. [Google Scholar]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef]
- Wid, M.; Anunyaporn, P.; Komkrich, S.; Pattarapa, W.; Chutima, K.; Pimchai, C.; Kanlaya Prapainop, K. Dual Functions of Riboflavin-functionalized Poly(lactic-co-glycolic acid) Nanoparticles for Enhanced Drug Delivery Efficiency and Photodynamic Therapy in Triple-negative Breast Cancer Cells. Photochem. Photobiol. 2021, 97, 1548–1557. [Google Scholar]
- Bareford, L.M.; Phelps, M.A.; Foraker, A.B.; Swaan, P.W. Intracellular processing of riboflavin in human breast cancer cells. Mol. Pharm. 2008, 5, 839–848. [Google Scholar] [CrossRef]
- Rivas, B.; Fiorela, G.; Joaquín Martínez, P.; Giovanetti, L.J.; Patricia Laura, S.; Daniel Osvaldo, M. Riboflavin-Mediated Photooxidation of Gold Nanoparticles and Its Effect on the Inactivation of Bacteria. Langmuir 2020, 36, 8272–8281. [Google Scholar] [CrossRef]
- Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. [Google Scholar] [CrossRef]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, C.; Li, Y.; Liu, W.; Yao, N.; Gao, M.; Ji, Y.; Huang, D.; Yin, Z.; Sun, Z.; et al. Evaluation of hypericin: Effect of aggregation on targeting biodistribution. J. Pharm. Sci. 2015, 104, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jendželovská, Z.; Jendželovský, R.; Kuchárová, B.; Fedoročko, P. Hypericin in the Light and in the Dark: Two Sides of the Same Coin. Front. Plant Sci. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Miskovsky, P. Hypericin--a new antiviral and antitumor photosensitizer: Mechanism of action and interaction with biological macromolecules. Curr. Drug Targets 2002, 3, 55–84. [Google Scholar] [CrossRef]
- de Morais, F.A.P.; De Oliveira, A.C.V.; Balbinot, R.B.; Lazarin-Bidóia, D.; Ueda-Nakamura, T.; de Oliveira Silva, S.; da Silva Souza Campanholi, K.; da Silva Junior, R.C.; Gonçalves, R.S.; Caetano, W.; et al. Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma. Polymers 2022, 15, 179. [Google Scholar] [CrossRef]
- Youssef, T.; Fadel, M.; Fahmy, R.; Kassab, K. Evaluation of hypericin-loaded solid lipid nanoparticles: Physicochemical properties, photostability and phototoxicity. Pharm. Dev. Technol. 2012, 17, 177–186. [Google Scholar] [CrossRef]
- Peng, Z.; Lu, J.; Liu, K.; Xie, L.; Wang, Y.; Cai, C.; Yang, D.; Xi, J.; Yan, C.; Li, X.; et al. Hypericin as a promising natural bioactive naphthodianthrone: A review of its pharmacology, pharmacokinetics, toxicity, and safety. Phytother. Res. PTR 2023, 37, 5639–5656. [Google Scholar] [CrossRef]
- Chen, E.; Chen, X.; Yuan, X.; Wei, S.; Zhou, L.; Zhou, J.; Shen, J. One-pot method to prepare a theranostic nanosystem with magnetic resonance imaging function and anticancer activity through multiple mechanisms. Dalton Trans. 2017, 46, 5151–5158. [Google Scholar] [CrossRef]
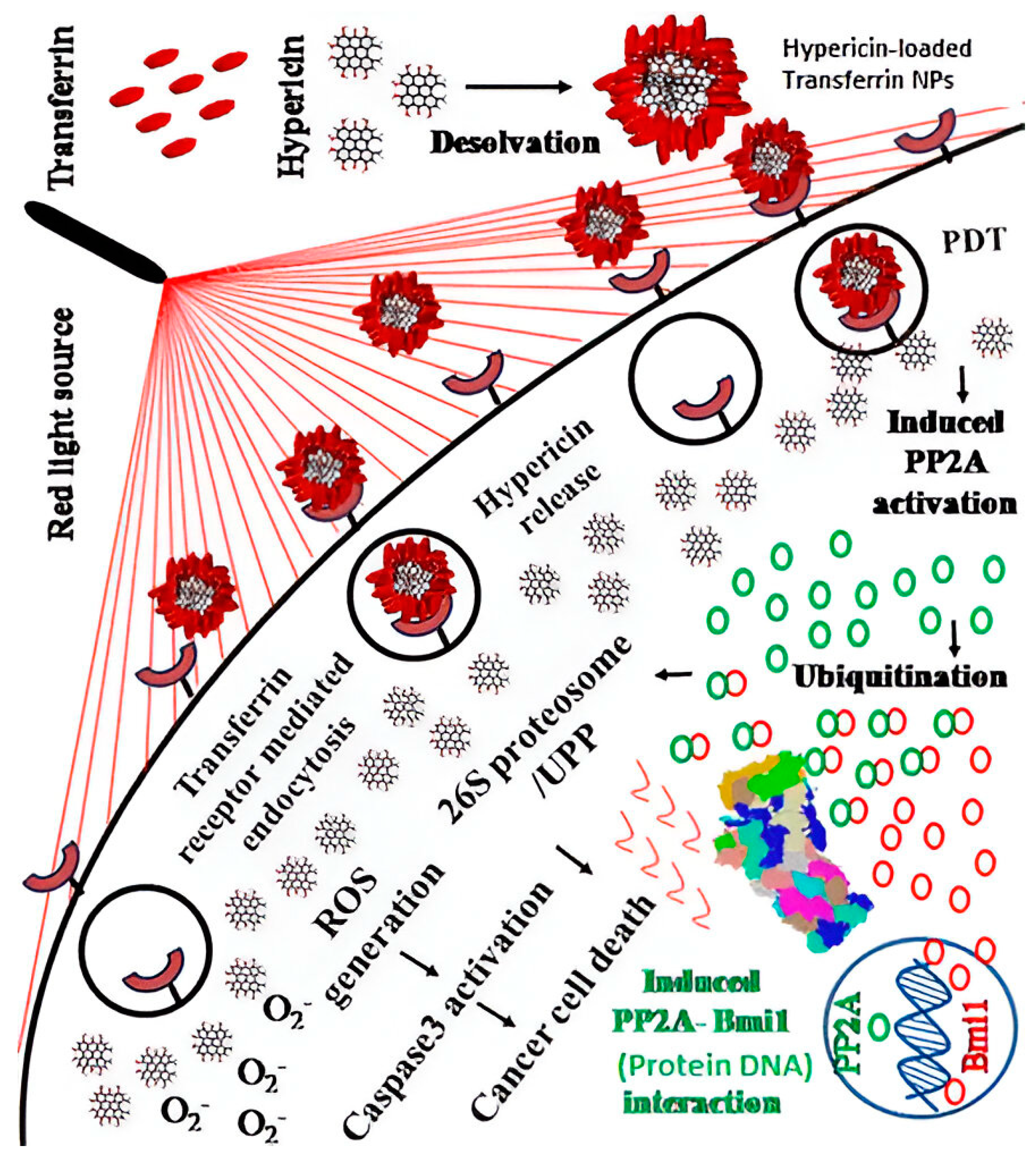
- Sardoiwala, M.N.; Kushwaha, A.C.; Dev, A.; Shrimali, N.; Guchhait, P.; Karmakar, S.; Roy Choudhury, S. Hypericin-Loaded Transferrin Nanoparticles Induce PP2A-Regulated BMI1 Degradation in Colorectal Cancer-Specific Chemo-Photodynamic Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3139–3153. [Google Scholar] [CrossRef]
- Taşkonak, B.; Aylaz, G.; Andac, M.; Güven, E.; Ozkahraman, B.; Perçin, I.; Kılıç Süloğlu, A. Hypericin-Loaded Chitosan Nanoparticles for Enhanced Photodynamic Therapy in A549 Lung Cancer Cells. BioNanoScience 2023, 13, 352–364. [Google Scholar] [CrossRef]
- Torres-Martínez, A.; Begoña, B.; Falomir, E.; Marín, M.J.; Angulo-Pachón, C.A.; Galindo, F. Non-Polymeric Nanogels as Versatile Nanocarriers: Intracellular Transport of the Photosensitizers Rose Bengal and Hypericin for Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 3658–3669. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, P.; Liu, J.; Yang, Y.; Chen, Q.; Zhang, Y.; Zhang, H.; Wang, X. Application of 5-aminolevulinic acid-photodynamic therapy in common skin diseases. Transl. Biophoton. 2020, 2, e201900028. [Google Scholar] [CrossRef]
- Ciampo, D.; Fábia Cristina, R.; Vitória, M.; Bernadete, M. Improved In vitro and In vivo Cutaneous Delivery of Protoporphyrin IX from PLGA-based Nanoparticles. Photochem. Photobiol. 2013, 89, 1176–1184. [Google Scholar]
- Akbarzadeh, F.; Khoshgard, K. Enhancement of the effect of novel targeted 5-aminolevulinic acid conjugated bismuth oxide nanoparticles-based photodynamic therapy by simultaneous radiotherapy on KB cells. Photodiagnosis Photodyn. Ther. 2024, 46, 104025. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, H.; Xu, X.; Wang, X.; Chen, Y.; Yin, L. Far-red light-mediated programmable anti-cancer gene delivery in cooperation with photodynamic therapy. Biomaterials 2018, 171, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Huang, K.-S.; Wang, Y.-T.; Shaw, J.-F. A Review of Bacteriochlorophyllides: Chemical Structures and Applications. Molecules 2021, 26, 1293. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar]
- Jin, Y.; Zhang, J.; Pan, Y.; Shen, W. Berberine Suppressed the Progression of Human Glioma Cells by Inhibiting the TGF-β1/SMAD2/3 Signaling Pathway. Integr. Cancer Ther. 2022, 21, 15347354221130303. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Sadeghi, S.; Alavi, S.M.A.; Alavi, S.S.; Jafari, A.; Khan, H.; Aschner, M.; Mirzaei, H.; Sharifi, M.; Asemi, Z. The therapeutic effects of berberine for gastrointestinal cancers. Asia-Pac. J. Clin. Oncol. 2024, 20, 152–167. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, K.-M.; Ko, E.; Han, W.; Lee, J.E.; Shin, I.; Bae, J.-Y.; Kim, S.; Noh, D.-Y. Berberine inhibits growth of the breast cancer cell lines MCF-7 and MDA-MB-231. Planta Medica 2008, 74, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, S.-H.; Cheong, H.-T.; Ra, C.-S.; Rhee, K.-J.; Jung, B. Berberine Induces p53-Dependent Apoptosis through Inhibition of DNA Methyltransferase3b in Hep3B Cells. Korean J. Clin. Lab. Sci. 2020, 52, 69–77. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid With Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef] [PubMed]
- Floriano, B.F.; Carvalho, T.; Lopes, T.Z.; Takahashi, L.A.U.; Rahal, P.; Tedesco, A.C.; Calmon, M.F. Effect of berberine nanoemulsion Photodynamic therapy on cervical carcinoma cell line. Photodiagnosis Photodyn. Ther. 2021, 33, 102174. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Yu, J.; Zhu, X.; Wu, Y.; Liu, J.; Zhang, Y. Photodynamic-based combinatorial cancer therapy strategies: Tuning the properties of nanoplatform according to oncotherapy needs. Coord. Chem. Rev. 2022, 461, 214495. [Google Scholar] [CrossRef]
- Hao, Y.; Chung, C.K.; Gu, Z.; Schomann, T.; Dong, X.; Veld, R.; Camps, M.G.M.; Ten Dijke, P.; Ossendorp, F.A.; Cruz, L.J. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol. Biomed. 2022, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A. Emerging Nanotherapeutic Approaches to Overcome Drug Resistance in Cancers with Update on Clinical Trials. Pharmaceutics 2022, 14, 866. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Zhang, T.; Jiang, X.; Hu, Y. Hybrid nanoparticle composites applied to photodynamic therapy: Strategies and applications. J. Mater. Chem. B 2020, 8, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Rees, T.W.; Liao, X.; Ji, L.; Chao, H. Oxygen self-sufficient photodynamic therapy. Coord. Chem. Rev. 2021, 432, 213714. [Google Scholar] [CrossRef]
- Liu, X.; Ye, N.; Xiao, C.; Wang, X.; Li, S.; Deng, Y.; Yang, X.; Li, Z.; Yang, X. Hyperbaric oxygen regulates tumor microenvironment and boosts commercialized nanomedicine delivery for potent eradication of cancer stem-like cells. Nano Today 2021, 40, 101248. [Google Scholar] [CrossRef]
- Mao, J.; Xu, Z.; Lin, W. Nanoscale metal–organic frameworks for photodynamic therapy and radiotherapy. Curr. Opin. Chem. Eng. 2022, 38, 100871. [Google Scholar] [CrossRef]
- Zhang, P.; Ouyang, Y.; Sohn, Y.S.; Fadeev, M.; Karmi, O.; Nechushtai, R.; Stein, I.; Pikarsky, E.; Willner, I. miRNA-Guided Imaging and Photodynamic Therapy Treatment of Cancer Cells Using Zn(II)-Protoporphyrin IX-Loaded Metal-Organic Framework Nanoparticles. ACS Nano 2022, 16, 1791–1801. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhong, H.; Pan, W.; Li, Y.; Chen, Y.; Li, N.; Tang, B. Programmed Release of Dihydroartemisinin for Synergistic Cancer Therapy Using a CaCO3 Mineralized Metal–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 14134–14139. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Chen, S.; Ahmadi, S.; Veedu, R.N. Aptamer-engineered (nano)materials for theranostic applications. Theranostics 2023, 13, 5183–5206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, D.; Zhang, Q.; Zhang, Y.; Peng, R.; Tan, W. Leveraging Aptamer-Based DNA Nanotechnology for Bioanalysis and Cancer Therapeutics. Acc. Mater. Res. 2024, 5, 438–452. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Lo, Y.; Shiu, S.C.; Kinghorn, A.B.; Tanner, J.A. Aptamer-Enabled Nanomaterials for Therapeutics, Drug Targeting and Imaging. Cells 2022, 11, 159. [Google Scholar] [CrossRef]
- Wong, K.-Y.; Wong, M.-S.; Liu, J. Aptamer-functionalized liposomes for drug delivery. Biomed. J. 2023, 47, 100685. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Liu, M.-C.; Chen, X.-L.; Lin, H.-C.; Liu, B.; Peng, Y.-B.; Zhang, L.-Y.; Shen, J.; Zhao, P. A dual-targeted nucleic acid moiety decorated SPION nanoparticles for chemo-photodynamic synergistic therapy. J. Lumin. 2019, 209, 387–397. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Sun, M.; Wang, J.; Wang, C.; Sun, Y. Cell Membrane-Camouflaged Nanocarriers for Cancer Diagnostic and Therapeutic. Front. Pharmacol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; He, W.; Zheng, M.; Zou, Y. Cell Membrane Camouflaged Biomimetic Nanoparticles as a Versatile Platform for Brain Diseases Treatment. Small Methods 2024, 2400096, advance online publication. [Google Scholar] [CrossRef]
- Li, A.; Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef] [PubMed]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, D.; Wen, X.; Chen, C.; Liu, G.; Lu, Z. Internal Light Sources-Mediated Photodynamic Therapy Nanoplatforms: Hope for the Resolution of the Traditional Penetration Problem. Adv. Healthc. Mater. 2024, 13, 2301326. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mu, M.; Chen, B.; Fan, R.; Chen, H.; Zou, B.; Han, B.; Guo, G. Metal-organic framework-based photodynamic combined immunotherapy against the distant development of triple-negative breast cancer. Biomater. Res. 2023, 27, 120. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Ahn, H.; Kim, J.H.; Lee, Y.J.; Kim, J.Y.; Lee, K.C.; Kang, C.S.; Kim, S. Cancer therapy by antibody-targeted Cerenkov light and metabolism-selective photosensitization. J. Control. Release 2022, 352, 25–34. [Google Scholar] [CrossRef] [PubMed]



| Nanomaterial Classification | Nanomaterial | Natural PS | Therapeutic Ability | Reference |
|---|---|---|---|---|
| Inorganic NPs | LDH | Curcumin | PDT | [76,77] |
| Inorganic NPs | Gold NPs | Curcumin, 5-ALA | PDT | [78,79,80] |
| Inorganic NPs | Silica | Curcumin | PDT | [81,82] |
| Inorganic NPs | MoS2 | Curcumin, ICG | PDT, PTT | [83] |
| Inorganic NPs | Ga | Curcumin | PDT | [84] |
| Inorganic NPs | Sn | Porphyrin | PDT | [85] |
| Inorganic NPs | Up conversion NPs | Hypocrellin b Hypocrellin a Riboflavin | PDT, MRI, FL, UCL Imaging | [86,87,88] |
| Inorganic NPs | Calcium phosphate | Hypocrellin b | PDT, FL IMAGING | [89] |
| Inorganic NPs | MSN | Riboflavin, Hypocrellin b, Curcumin | PDT | [90,91] |
| Inorganic NPs | Bismuth NPS | 5-ALA, Riboflavin | PDT | [92,93] |
| Inorganic NPs | Silver NPs | 5-ALA, Riboflavin | PDT | [94,95] |
| Inorganic NPs | Gold nanoclusters | 5-ALA | PDT | [96,97,98] |
| Inorganic NPs | Carbon- Dots | Pheophytin | PDT | [99,100] |
| Inorganic NPs | Magnetic NPs | Bacteriochlorine | PDT, MRI | [101] |
| Organic NPs | Lipid NPs | Curcumin, Hypericin | PDT | [102,103] |
| Organic NPs | Liposomes | porphyrin | PDT | [104] |
| Organic NPs | Micelles | Pheophorbide a, Hypericin | PDT | [85,105] |
| Organic NPs | Irgd peptide | Hypocrellin b | PDT | [106] |
| Polymers | PLGA | 5-ALA, Riboflavin, Hypocrellin b, Curcumin, Pheophorbide a | PDT, Chemotherapy FL | [107,108,109,110,111,112,113] |
| Polymers | Chitosan | curcumin | PDT, FL | [114] |
| Polymers | Arginine complex | porphyrin | PDT | [115] |
| Polymers | Alginate | Pheophorbide a | PDT, Chemotherapy, Immunotherapy | [116] |
| Polymers | Pluronic F127 | Surfactant-stripped Pheophytin, Pa | PDT, chemotherapy | [117] |
| Polymers | PEI | Pheophorbide a, Pheophytin | PDT | [118,119] |
| Polymers | polygalactose-co-polycinnamaldehyde polyprodrug | Pheophorbide a | PDT, Immunotherapy | [120] |
| Polymers | Xylan | Pheophorbide a | PDT | [121] |
| Polymers | PEG | Pheophorbide a | PDT | [122] |
| Polymers | PEG-PLA | Hypocrellin b | PDT | [123] |
| Polymers | hyaluronic acid–ceramide nanoparticles | Hypocrellin b | PDT, CD | [124] |
| Polymers | P123 nanomicelles | HYP | PDT | [125] |
| Polymers | Polysaccharides based nanocomplexes | 5-ALA | PDT | [126] |
| Polymers | SH–PEG–SH | tetrafluorophenyl porphyrin | PDT | [127] |
| Hybrid NPs | Metal organic framework | Porphyrin, Bacteriochlorin | PDT, PTT, Chemotherapy, Immunotherapy | [128,129,130,131,132] |
| Hybrid NPs | Polysilsesquioxane nanoparticles | 5-ALA | PDT | [80] |
| Transferrin Nanoparticles | Hypericin | PDT, Chemodynamic Therapy | [103] | |
| Nanoemulsion | berberine | PDT | [133] |
| Photosensitizers | Structure | Wavelength |
|---|---|---|
| Curcumin |  | 420–480 nm |
| Bacteriochlorin |  | 700–900 nm |
| Berberine | 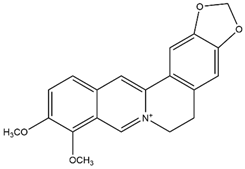 | 250–350 nm |
| 5-aminolevulinic acid | 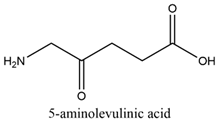 | 405 and 635 nm |
| Hypericin | 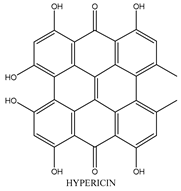 | 590 nm |
| Riboflavin |  | 365–445 nm |
| Pheophorbide a | 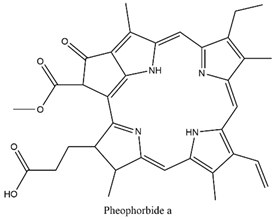 | 670 nm |
| Porphyrin |  | 600–900 nm |
| Pheophytin |  | 400–700 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaram, J.; Mende, L.K.; Kuthati, Y. A Review of the Efficacy of Nanomaterial-Based Natural Photosensitizers to Overcome Multidrug Resistance in Cancer. Pharmaceutics 2024, 16, 1120. https://doi.org/10.3390/pharmaceutics16091120
Rajaram J, Mende LK, Kuthati Y. A Review of the Efficacy of Nanomaterial-Based Natural Photosensitizers to Overcome Multidrug Resistance in Cancer. Pharmaceutics. 2024; 16(9):1120. https://doi.org/10.3390/pharmaceutics16091120
Chicago/Turabian StyleRajaram, Jagadeesh, Lokesh Kumar Mende, and Yaswanth Kuthati. 2024. "A Review of the Efficacy of Nanomaterial-Based Natural Photosensitizers to Overcome Multidrug Resistance in Cancer" Pharmaceutics 16, no. 9: 1120. https://doi.org/10.3390/pharmaceutics16091120







