ERCC1 and ERCC2 Polymorphisms Predict the Efficacy and Toxicity of Platinum-Based Chemotherapy in Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Clinical Assessment
2.3. SNPs Selection and Genotyping
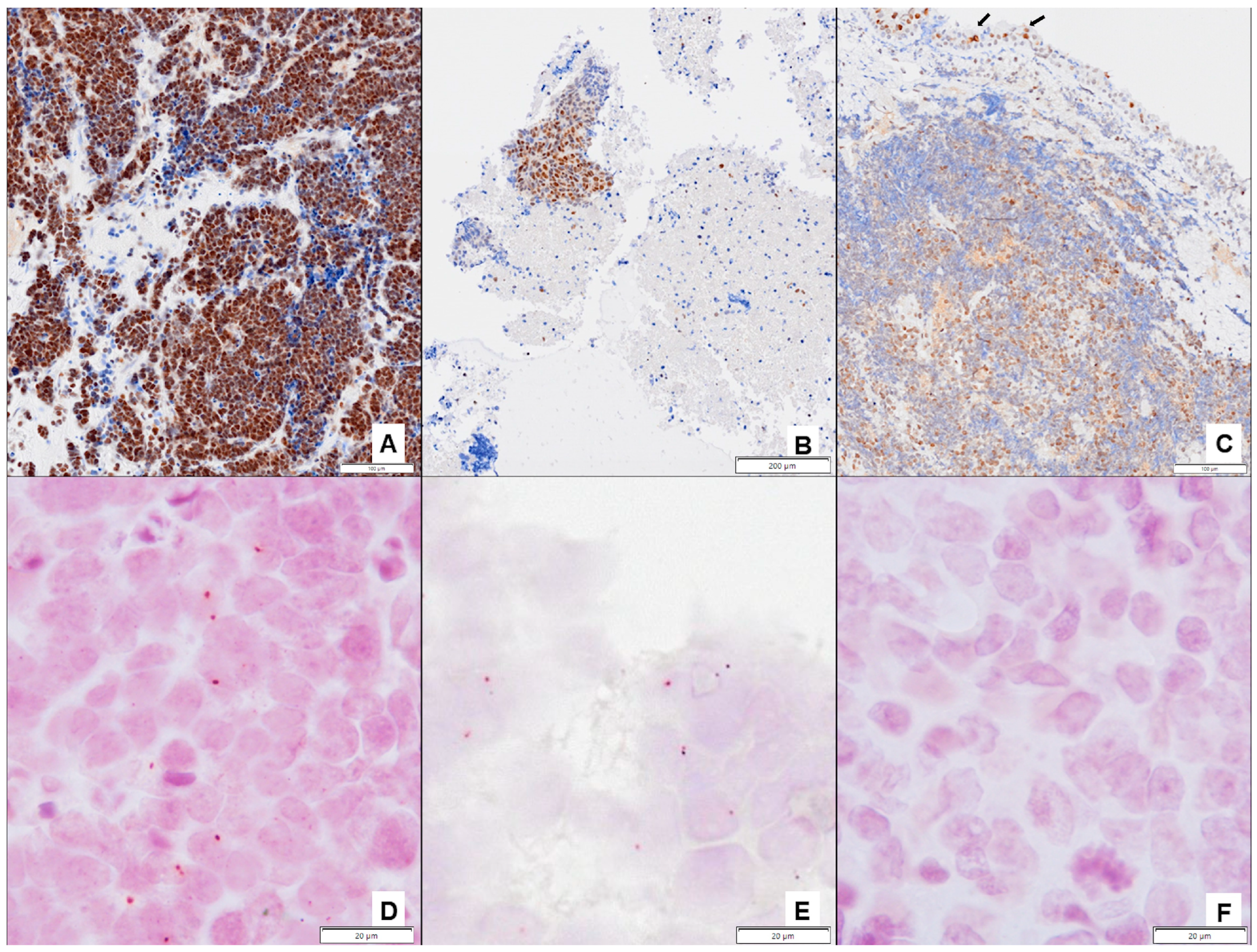
2.4. Immunohistochemical Studies
2.5. Proximity Ligation Assay (PLA)
2.6. Statistics
3. Results
3.1. Clinical Results
3.2. Survival and Objective Response Rate Analyses
3.2.1. Analyses of Genetic Variants
3.2.2. Analyses of ERCC1 and ERCC1-XPF Complex Expression
3.3. Safety Analyses
Analyses of Genetic Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindan, R.; Page, N.; Morgensztern, D.; Read, W.; Tierney, R.; Vlahiotis, A.; Spitznagel, E.L.; Piccirillo, J. Changing Epidemiology of Small-Cell Lung Cancer in the United States over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J. Clin. Oncol. 2006, 24, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Snee, M.; Ashcroft, L.; Appel, W.; Barlesi, F.; Bhatnagar, A.; Bezjak, A.; Cardenal, F.; Fournel, P.; Harden, S.; et al. Concurrent Once-Daily versus Twice-Daily Chemoradiotherapy in Patients with Limited-Stage Small-Cell Lung Cancer (CONVERT): An Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol. 2017, 18, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum–Etoposide versus Platinum–Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without Tremelimumab, plus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: 3-Year Overall Survival Update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Franco, F.; Carcereny, E.; Guirado, M.; Ortega, A.L.; López-Castro, R.; Rodríguez-Abreu, D.; García-Campelo, R.; Del Barco, E.; Juan, O.; Aparisi, F.; et al. Epidemiology, Treatment, and Survival in Small Cell Lung Cancer in Spain: Data from the Thoracic Tumor Registry. PLoS ONE 2021, 16, 0251761. [Google Scholar] [CrossRef]
- Janssen-Heijnen, M.L.G.; Maas, H.A.A.M.; van de Schans, S.A.M.; Coebergh, J.W.W.; Groen, H.J.M. Chemotherapy in Elderly Small-Cell Lung Cancer Patients: Yes We Can, but Should We Do It? Ann. Oncol. 2011, 22, 821–826. [Google Scholar] [CrossRef]
- Seigneurin, A.; Delafosse, P.; Trétarre, B.; Woronoff, A.S.; Velten, M.; Grosclaude, P.; Guizard, A.V.; Lapôtre-Ledoux, B.; Bara, S.; Molinié, F.; et al. Are Comorbidities Associated with Long-Term Survival of Lung Cancer? A Population-Based Cohort Study from French Cancer Registries. BMC Cancer 2018, 18, 1091. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, J.; Meng, Y.; Zhou, Y.; Li, W. Higher Pretreatment Lactate Dehydrogenase Concentration Predicts Worse Overall Survival in Patients with Lung Cancer. Medicine 2018, 97, e12524. [Google Scholar] [CrossRef]
- Aarts, M.J.; Aerts, J.G.; Van Den Borne, B.E.; Biesma, B.; Lemmens, V.E.P.P.; Kloover, J.S. Comorbidity in Patients with Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin. Lung Cancer 2015, 16, 282–291. [Google Scholar] [CrossRef]
- Sawant, A.; Floyd, A.M.; Dangeti, M.; Lei, W.; Sobol, R.W.; Patrick, S.M. Differential Role of Base Excision Repair Proteins in Mediating Cisplatin Cytotoxicity. DNA Repair 2017, 51, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Slyskova, J.; Sabatella, M.; Ribeiro-Silva, C.; Stok, C.; Theil, A.F.; Vermeulen, W.; Lans, H. Base and Nucleotide Excision Repair Facilitate Resolution of Platinum Drugs-Induced Transcription Blockage. Nucleic Acids Res. 2018, 46, 9537–9549. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-X.; Wang, J.; Huang, L.-H.; Li, J.-G.; Chen, X. Impact of Single-Nucleotide Polymorphisms on Radiation Pneumonitis in Cancer Patients. Mol. Clin. Oncol. 2016, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Karwowski, B.T. The Clustered DNA Lesions—Types, Pathways of Repair and Relevance to Human Health. Curr. Med. Chem. 2018, 25, 2722–2735. [Google Scholar] [CrossRef]
- Duan, M.; Ulibarri, J.; Liu, K.J.; Mao, P. Role of Nucleotide Excision Repair in Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 9248. [Google Scholar] [CrossRef]
- De Silva, I.U.; McHugh, P.J.; Clingen, P.H.; Hartley, J.A. Defects in Interstrand Cross-Link Uncoupling Do Not Account for the Extreme Sensitivity of ERCC1 and XPF Cells to Cisplatin. Nucleic Acids Res. 2002, 30, 3848–3856. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kothandapani, A.; Tillison, K.; Kalman-Maltese, V.; Patrick, S.M. Downregulation of XPF-ERCC1 Enhances Cisplatin Efficacy in Cancer Cells. DNA Repair 2010, 9, 745–753. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA Repair by ERCC1 in Non–Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Hubner, R.A.; Riley, R.D.; Billingham, L.J.; Popat, S. Excision Repair Cross-Complementation Group 1 (ERCC1) Status and Lung Cancer Outcomes: A Meta-Analysis of Published Studies and Recommendations. PLoS ONE 2011, 6, e25164. [Google Scholar] [CrossRef]
- Huang, Z.L.; Cao, X.; Luo, R.Z.; Chen, Y.F.; Zhu, L.C.; Wen, Z. Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as Predictors of Prognosis in Patients with Non-Small Cell Lung Cancer Who Received Cisplatin-Based Adjuvant Chemotherapy: A Prospective Study. Oncol. Lett. 2016, 11, 299–305. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, Y.W.; Han, J.H.; Kim, J.H.; Jung, J.H.; Jeong, S.H.; Kang, S.Y.; Choi, J.H.; Oh, Y.T.; Park, K.J.; et al. Expression of Excision Repair Cross-Complementation Group 1 Protein Predicts Poor Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Platinum-Based Doublet Chemotherapy. Lung Cancer 2009, 65, 377–382. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Yang, N.; Feng, R.; Xian, L. The Prognostic Value of Excision Repair Cross-Complementation Group 1 (ERCC1) in Patients with Small Cell Lung Cancer (SCLC) Receiving Platinum-Based Chemotherapy: Evidence from Meta-Analysis. PLoS ONE 2014, 9, e111651. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Rath, B. Pharmacogenetics of Platinum-Based Chemotherapy in Non-Small Cell Lung Cancer: Predictive Validity of Polymorphisms of ERCC1. Expert Opin. Drug Metab. Toxicol. 2018, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zhang, G.; Zhang, H.; Cui, S.; Zhang, L.; Yu, T.; Xiao, M.; Li, L.; Lu, X. A MiR-15a Related Polymorphism Affects NSCLC Prognosis via Altering ERCC1 Repair to Platinum-Based Chemotherapy. J. Cell. Mol. Med. 2022, 26, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, X.; Liu, J.; Yuan, P.; Tan, W.; Guo, Y.; Sun, T.; Zhao, D.; Yang, M.; Liu, J.; et al. Characterization of Functional Excision Repair Cross-Complementation Group 1 Variants and Their Association with Lung Cancer Risk and Prognosis. Clin. Cancer Res. 2008, 14, 2878–2886. [Google Scholar] [CrossRef]
- Nicoś, M.; Rolska-Kopińska, A.; Krawczyk, P.; Grenda, A.; Bozyk, A.; Szczyrek, M.; Milanowski, J. Effect of TOP2A and ERCC1 Gene Polymorphisms on the Efficacy and Toxicity of Cisplatin and Etoposidebased Chemotherapy in Small Cell Lung Cancer Patients. Arch. Med. Sci. 2021, 17, 474–480. [Google Scholar] [CrossRef]
- Anai, S.; Iwama, E.; Yoneshima, Y.; Otsubo, K.; Tanaka, K.; Nakanishi, Y.; Okamoto, I. Association of Nephrotoxicity during Platinum-Etoposide Doublet Therapy with UGT1A1 Polymorphisms in Small Cell Lung Cancer Patients. Lung Cancer 2018, 126, 156–161. [Google Scholar] [CrossRef]
- Negoro, Y.; Yano, R.; Yoshimura, M.; Suehiro, Y.; Yamashita, S.; Kodawara, T.; Watanabe, K.; Tsukamoto, H.; Nakamura, T.; Kadowaki, M.; et al. Influence of UGT1A1 Polymorphism on Etoposide plus Platinum-Induced Neutropenia in Japanese Patients with Small-Cell Lung Cancer. Int. J. Clin. Oncol. 2019, 24, 256–261. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ishii, G.; Goto, K.; Ota, S.; Kubota, K.; Murata, Y.; Mishima, M.; Saijo, N.; Nishiwaki, Y.; Ochiai, A. Expression of Breast Cancer Resistance Protein Is Associated with a Poor Clinical Outcome in Patients with Small-Cell Lung Cancer. Lung Cancer 2009, 65, 105–111. [Google Scholar] [CrossRef]
- Skov, B.G.; Holm, B.; Erreboe, A.; Skov, T.; Mellemgaard, A. ERCC1 and Ki67 in Small Cell Lung Carcinoma and Other Neuroendocrine Tumors of the Lung: Distribution and Impact on Survival. J. Thorac. Oncol. 2010, 5, 453–459. [Google Scholar] [CrossRef]
- Lee, G.W.; Go, S.I.; Cho, Y.J.; Jeong, Y.Y.; Kim, H.C.; Duk Lee, J.; Hwang, Y.S.; Ko, G.H.; Lee, J.H.; Kim, D.C.; et al. Hypoxia-Inducible Factor-1α and Excision Repair Cross-Complementing 1 in Patients with Small Cell Lung Cancer Who Received Front-Line Platinum-Based Chemotherapy: A Retrospective Study. J. Thorac. Oncol. 2012, 7, 528–534. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0. 2009. Available online: https://www.meddra.org/mapping (accessed on 15 June 2022).
- He, K.; Zhang, S.; Pang, J.; Yin, J.C.; Mu, D.; Wang, J.; Ge, H.; Ma, J.; Yang, Z.; Zheng, X.; et al. Genomic Profiling Reveals Novel Predictive Biomarkers for Chemo-Radiotherapy Efficacy and Thoracic Toxicity in Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 928605. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 Overcame Resistance to Topoisomerase Inhibitors in Small Cell Lung Cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef]
- Pérez-Ramírez, C.; Cañadas-Garre, M.; Alnatsha, A.; Villar, E.; Delgado, J.R.; Faus-Dáder, M.J.; Calleja-Hernández, M.Á. Pharmacogenetic Predictors of Toxicity to Platinum Based Chemotherapy in Non-Small Cell Lung Cancer Patients. Pharmacol. Res. 2016, 111, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.M.; Qiu, C.F.; Zhu, T.; Jin, Y.X.; Li, X.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Genetic Polymorphisms and Platinum-Based Chemotherapy Treatment Outcomes in Patients with Non-Small Cell Lung Cancer: A Genetic Epidemiology Study Based Meta-Analysis. Sci. Rep. 2017, 7, 5593. [Google Scholar] [CrossRef]
- Marcuello, E.; Páez, D.; Paré, L.; Salazar, J.; Sebio, A.; Del Rio, E.; Baiget, M. A Genotype-Directed Phase I-IV Dose-Finding Study of Irinotecan in Combination with Fluorouracil/Leucovorin as First-Line Treatment in Advanced Colorectal Cancer. Br. J. Cancer 2011, 105, 53–57. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [PubMed]
- Furuta, T.; Ueda, T.; Aune, G.; Sarasin, A.; Kraemer, K.H.; Pommier, Y. Transcription-Coupled Nucleotide Excision Repair as a Determinant of Cisplatin Sensitivity of Human Cells. Cancer Res. 2002, 62, 4899–4902. [Google Scholar]
- Yu, S.N.; Liu, G.F.; Li, X.F.; Fu, B.H.; Dong, L.X.; Zhang, S.H. Evaluation of Prediction of Polymorphisms of DNA Repair Genes on the Efficacy of Platinum-Based Chemotherapy in Patients With Non-Small Cell Lung Cancer: A Network Meta-Analysis. J. Cell. Biochem. 2017, 118, 4782–4791. [Google Scholar] [CrossRef]
- Yu, J.J.; Lee, K.B.; Mu, C.; Li, Q.; Abernathy, T.V.; Bostick-Bruton, F.; Reed, E. Comparison of Two Human Ovarian Carcinoma Cell Lines (A2780/CP70 and MCAS) That Are Equally Resistant to Platinum, but Differ at Codon 118 of the ERCC1 Gene. Int. J. Oncol. 2000, 16, 555–560. [Google Scholar] [CrossRef]
- Takenaka, T.; Yano, T.; Kiyohara, C.; Miura, N.; Kouso, H.; Ohba, T.; Kometani, T.; Shoji, F.; Yoshino, I.; Maehara, Y. Effects of Excision Repair Cross-Complementation Group 1 (ERCC1) Single Nucleotide Polymorphisms on the Prognosis of Non-Small Cell Lung Cancer Patients. Lung Cancer 2010, 67, 101–107. [Google Scholar] [CrossRef]
- Britten, R.A.; Liu, D.; Tessier, A.; Hutchison, M.J.; Murray, D. ERCC1 Expression as a Molecular Marker of Cisplatin Resistance in Human Cervical Tumor Cells. Int. J. Cancer 2000, 89, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, T.; Li, X.; Haura, E.; Sharma, A.; Bepler, G. DNA Synthesis and Repair Genes RRM1 and ERCC1 in Lung Cancer. N. Engl. J. Med. 2007, 356, 800–808. [Google Scholar] [CrossRef]
- Friboulet, L.; Olaussen, K.A.; Pignon, J.-P.; Shepherd, F.A.; Tsao, M.-S.; Graziano, S.; Kratzke, R.; Douillard, J.-Y.; Seymour, L.; Pirker, R.; et al. ERCC1 Isoform Expression and DNA Repair in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2013, 368, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Falzon, M.; Blackhall, F.; Spicer, J.; Nicolson, M.; Chaudhuri, A.; Middleton, G.; Ahmed, S.; Hicks, J.; Crosse, B.; et al. Randomized Prospective Biomarker Trial of ERCC1 for Comparing Platinum and Nonplatinum Therapy in Advanced Non-Small-Cell Lung Cancer: ERCC1 Trial (ET). J. Clin. Oncol. 2017, 35, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, N.R.; Roginskaya, V.Y.; Acquafondata, M.B.; Dhir, R.; Wood, R.D.; Niedernhofer, L.J. Immunodetection of DNA Repair Endonuclease ERCC1-XPF in Human Tissue. Cancer Res. 2009, 69, 6831–6838. [Google Scholar] [CrossRef]
- Ceppi, P.; Longo, M.; Volante, M.; Novello, S.; Cappia, S.; Bacillo, E.; Selvaggi, G.; Saviozzi, S.; Calogero, R.; Papotti, M.; et al. Excision Repair Cross Complementing-1 and Topoisomerase IIα Gene Expression in Small-Cell Lung Cancer Patients Treated with Platinum and Etoposide: A Retrospective Study. J. Thorac. Oncol. 2008, 3, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Grønberg, B.H.; Killingberg, K.T.; Fløtten, Ø.; Brustugun, O.T.; Hornslien, K.; Madebo, T.; Langer, S.W.; Schytte, T.; Nyman, J.; Risum, S.; et al. High-Dose versus Standard-Dose Twice-Daily Thoracic Radiotherapy for Patients with Limited Stage Small-Cell Lung Cancer: An Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2021, 22, 321–331. [Google Scholar] [CrossRef]
- Trigo, J.; Subbiah, V.; Besse, B.; Moreno, V.; López, R.; Sala, M.A.; Peters, S.; Ponce, S.; Fernández, C.; Alfaro, V.; et al. Lurbinectedin as Second-Line Treatment for Patients with Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol. 2020, 21, 645–654. [Google Scholar] [CrossRef]
- Blanco, A.C.; Mendivil, A.F.N.; Doger de Spéville, B.; Colomé, E.Á.; De Miguel Luken, M.J.; Alvarez, R.M.; Garcia, V.M.; ramon, J.; Valles, M.A.; Corral de la Fuente, E.; et al. 1989MO Lurbinectedin (LUR) in Combination with Pembrolizumab (PBL) in Relapsed Small Cell Lung Cancer (SCLC): The Phase I/II LUPER Study. Ann. Oncol. 2023, 34, S1060–S1061. [Google Scholar] [CrossRef]
- Dowlati, A.; Cervantes, A.; Babu, S.; Hamilton, E.P.; Wong, S.F.; Tazbirkova, A.; Sullivan, I.G.; Van Marcke de Lummen, C.; Italiano, A.; Patel, J.; et al. 1990MO Sacituzumab Govitecan (SG) as Second-Line (2L) Treatment for Extensive Stage Small Cell Lung Cancer (ES-SCLC): Preliminary Results from the Phase II TROPiCS-03 Basket Trial. Ann. Oncol. 2023, 34, S1061–S1062. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Pujol, J.L.; Dafni, U.; Dómine, M.; Popat, S.; Reck, M.; Andrade, J.; Becker, A.; Moro-Sibilot, D.; Curioni-Fontecedro, A.; et al. Consolidation Nivolumab and Ipilimumab versus Observation in Limited-Disease Small-Cell Lung Cancer after Chemo-Radiotherapy—Results from the Randomised Phase II ETOP/IFCT 4-12 STIMULI Trial. Ann. Oncol. 2022, 33, 67–79. [Google Scholar] [CrossRef]
- Tibaldi, C.; Giovannetti, E.; Vasile, E.; Mey, V.; Laan, A.C.; Nannizzi, S.; Di Marsico, R.; Antonuzzo, A.; Orlandini, C.; Ricciardi, S.; et al. Correlation of CDA, ERCC1, and XPD Polymorphisms with Response and Survival in Gemcitabine/Cisplatin-Treated Advanced Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2008, 14, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, W.; Qiao, R.; Chen, D.; Wang, H.; Wang, Y.; Zhang, S.; Gao, G.; Gu, A.; Shen, J.; et al. Association of XPD Polymorphisms with Severe Toxicity in Non-Small Cell Lung Cancer Patients in a Chinese Population. Clin. Cancer Res. 2009, 15, 3889–3895. [Google Scholar] [CrossRef]
- Abdulrahman, W.; Iltis, I.; Radu, L.; Braun, C.; Maglott-Roth, A.; Giraudon, C.; Egly, J.M.; Poterszman, A. ARCH Domain of XPD, an Anchoring Platform for CAK That Conditions TFIIH DNA Repair and Transcription Activities. Proc. Natl. Acad. Sci. USA 2013, 110, E633–E642. [Google Scholar] [CrossRef]
- Rump, A.; Benet-Pages, A.; Schubert, S.; Kuhlmann, J.D.; Janavičius, R.; Macháčková, E.; Foretová, L.; Kleibl, Z.; Lhota, F.; Zemankova, P.; et al. Identification and Functional Testing of ERCC2 Mutations in a Multi-National Cohort of Patients with Familial Breast- and Ovarian Cancer. PLoS Genet. 2016, 12, e1006248. [Google Scholar] [CrossRef]
- Seker, H.; Bowman, E.D.; Rusin, M.; Harris, C.C.; Butkiewicz, D.; Hedayati, M.; Grossman, L. Functional Significance of XPD Polymorphic Variants: Attenuated Apoptosis in Human Lymphoblastoid Cells with the XPD 312 Asp/Asp Genotype. Cancer Res. 2001, 61, 7430–7434. [Google Scholar] [PubMed]
| Characteristic | Total Cohort (n = 145) n (%) | Limited-Stage Disease (n = 45) n (%) | Extensive-Stage Disease (n = 100) n (%) |
|---|---|---|---|
| Baseline characteristics | |||
| Sex | |||
| Male | 104 (71.7) | 31 (68.9) | 73 (73) |
| Female | 41 (28.3) | 14 (31.1) | 27 (27) |
| Age, median (range)years | 67.4 (38–89) | 67 (54–89) | 66 (38–86) |
| ECOG | |||
| 0 | 9 (6.2) | 4 (8.9) | 5 (5) |
| 1 | 79 (54.5) | 31 (68.9) | 48 (48) |
| 2 | 37 (25.5) | 6 (13.3) | 31 (31) |
| 3–4 | 15 (10.4) | 1 (2.2) | 14 (14) |
| Missing | 5 (3.4) | 3 (6.7) | 2 (2) |
| Smoking | |||
| Current | 89 (61.4) | 22 (48.9) | 67 (67) |
| Former | 55 (37.9) | 23 (51.1) | 32 (32) |
| Missing | 1 (0.7) | 0 | 1 (1) |
| Metastases | |||
| Brain | 20 (14.2) | 20 (20) | |
| Liver | 43 (29.7) | 43 (43) | |
| Treatment details | |||
| Chemotherapy regimen | |||
| Cisplatin | 51 (35.2) | 22 (48.9) | 29 (29) |
| Carboplatin | 94 (64.8) | 23 (51.1) | 71 (71) |
| Median cycles (range) | 4 (1–6) | 4 (1–5) | 6 (1–6) |
| Immune checkpoint inhibitors | 9 (6.2%) | 0 | 9 (9.0%) |
| Radiotherapy | |||
| Thoracic radiotherapy | 40 (27.6) | 40 (88.9) | |
| Concomitant | 22 (15.2) | 22 (48.9) | |
| Sequential | 18 (12.4) | 18 (40) | |
| Gene Symbol | Reference SNP | Variant Description | HGVS (Nucleotide) | MAF Cohort | References for Rationale |
|---|---|---|---|---|---|
| XRCC1 | rs25487 | Missense | NM_006297.3:c.1196A>G | 0.37 | [34] |
| ERCC1 | rs3212986 | 3′-UTR | NM_001369414.1:c.*197= | 0.23 | [23,26,37] |
| rs11615 | Synonymous | NM_001369414.1:c.354T>C | 0.37 | [23,26,37] | |
| ERCC2 | rs13181 | Missense | NM_000400.4:c.2251A>C | 0.34 | [37] |
| rs50872 | Intron | NM_000400.4:c.1238–1492T>C | 0.26 | [36] | |
| rs1799793 | Missense | NM_000400.4:c.934G>A | 0.33 | [36] | |
| ABCC3 | rs4793665 | 2KB upstream | NC_000017.11:g.50634726C>T | 0.46 | [36] |
| ABCB1 | rs1045642 | Synonymous | NM_001348944.2:c.3435T>C | 0.47 | [35,36] |
| rs2032582 | Missense | NM_001348944.2:c.2677T>G; c.2677T>A | 0.41/0.05 | [35,36] | |
| rs1128503 | Synonymous | NM_001348944.2:c.1236T>C | 0.43 | [35,36] | |
| UGT1A1 | rs3064744 | Upstream | NG_002601.2:g.175491_175505 | 0.36 | [27,28] |
| GSTP1 | rs1695 | Missense | NM_000852.4:c.313A>G | 0.34 | [37] |
| Progression-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| SNP | n | mPFS (95%CI), Months | HR (95% CI) | p-Value | n | mOS (95%CI), Months | HR (95% CI) | p-Value |
| Total Cohort | ||||||||
| ERCC1 rs11615 | ||||||||
| AA | 49 | 6.6 (5.8–7.4) | Reference (1) | 0.06 | 50 | 11.3 (7.9–14.7) | Reference (1) | 0.26 |
| AG | 52 | 7.0 (4.0–10.0) | 0.66 (0.44–1.0) | 53 | 11.8 (6.8–16.8) | 0.72 (0.48–1.09) | ||
| GG | 16 | 7.7 (5.8–9.6) | 0.57 (0.31–1.03) | 18 | 12.7 (8.9–16.4) | 0.75 (0.43–1.31) | ||
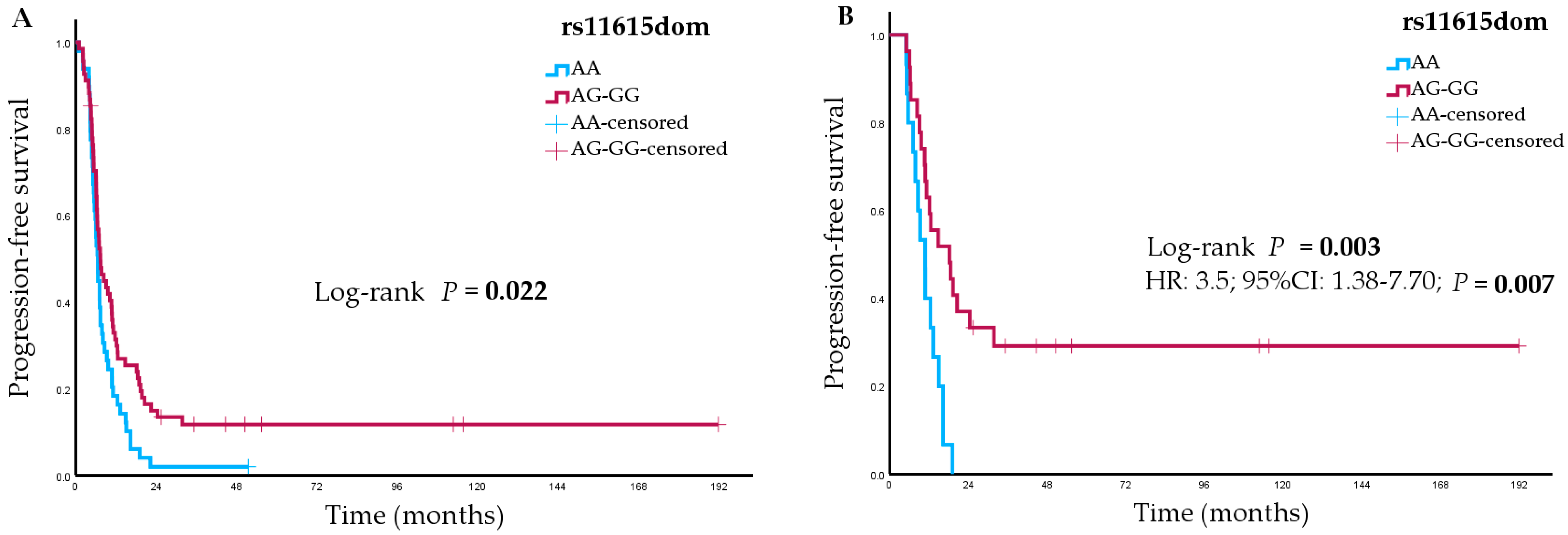
| AG-GG a | 68 | 7.4 (5.6–9.1) | 0.64 (0.43–0.94) | 0.02 | 71 | 12.7 (8.6–16.7) | 0.73 (0.50–1.07) | 0.1 |
| ABCC3 rs4793665 | ||||||||
| TT | 35 | 6.4 (5.4–7.4) | Reference (1) | 0.18 | 35 | 10.1 (7.5–12.7) | Reference (1) | 0.046 |
| TC | 59 | 7.7 (5.7–9.8) | 0.67 (0.43–1.03) | 62 | 14.6 (10.4–18.7) | 0.59 (0.38–0.90) | ||
| CC | 23 | 7.3 (6.1–8.6) | 0.80 (0.47–1.34) | 24 | 12.7 (7.3–18.0) | 0.77 (0.46–1.31) | ||
| Limited-stage disease | ||||||||
| ERCC1 rs11615 | ||||||||
| AA | 15 | 10.8 (8.0–13.6) | Reference (1) | 0.009 | 15 | 20.7 (14.3–27.1) | Reference (1) | 0.04 |
| AG | 17 | 18.2 (10.2–26.2) | 0.3 (0.13–0.68) | 18 | 33.8 (26.8–40.7) | 0.38 (0.17–0.83) | ||
| GG | 10 | 9.6 (0.0–28.5) | 0.43 (0.17–1.1) | 10 | 14.8 (0.0–45.4) | 0.73 (0.32–1.7) | ||
| AG-GG b | 27 | 18.2 (8.1–28.3) | 0.34 (0.16–0.71) | 0.003 | 28 | 33.8 (22.2–45.4) | 0.48 (0.24–0.96) | 0.03 |
| ERCC2 rs50872 | ||||||||
| GG | 23 | 13.3 (9.3–17.4) | Reference (1) | 0.03 | 24 | 31.8 (19.4–44.3) | Reference (1) | 0.16 |
| GA | 14 | 14.9 (5.5–24.3) | 0.66 (0.3–1.45) | 14 | 16.1 (0.0–44.6) | 1.00 (0.47–2.13) | ||
| AA | 5 | 7.8 (6.4–9.3) | 2.9 (1.03–8.17) | 5 | 15.3 (6.7–23.9) | 2.55 (0.92–7.09) | ||
| GG-GA | 37 | 14.8 (10.6–18.9) | 0.30 (0.11–0.82) | 0.01 | 38 | 28.7 (16.7–40.7) | 0.39 (0.15–1.06) | 0.06 |
| ABCC3 rs4793665 | ||||||||
| TT | 9 | 11.2 (9.6–12.8) | Reference (1) | 0.24 | 9 | 23.0 (0.0–55.8) | Reference (1) | 0.04 |
| TC | 25 | 14.8 (10.8–18.7) | 0.57 (0.26–1.29) | 25 | 35.2 (32.8–37.5) | 0.40 (0.17–0.92) | ||
| CC | 8 | 9.6 (4.5–14.7) | 1.05 (0.39–2.84) | 9 | 19.0 (7.0–30.9) | 0.96 (0.38–2.51) | ||
| UGT1A1*28 rs3064744 | ||||||||
| *1/*1 | 17 | 19.1 (15.1–23.1) | Reference (1) | 0.05 | 17 | 34.8 (29.1–40.6) | Reference (1) | 0.23 |
| *1/*28 | 15 | 10.8 (6.6–15.1) | 2.57 (1.17–5.64) | 16 | 15.3 (7.2–23.3) | 1.90 (0.89–4.07) | ||
| *28/*28 | 10 | 9.1 (5.7–12.5) | 1.84 (0.75–4.54) | 10 | 16.1 (0.0–45.4) | 1.17 (0.48–2.86) | ||
| *1/*28–*28/*28 a | 25 | 10.8 (8.0–13.6) | 2.24 (1.1–4.57) | 0.02 | 26 | 16.1 (8.8–23.4) | 1.559 (0.78–3.10) | 0.2 |
| Extensive-stage disease | ||||||||
| ERCC1 rs3212986 | ||||||||
| GG | 46 | 6.0 (4.8–7.2) | Reference (1) | 0.049 | 49 | 10.6 (8.6–12.5) | Reference (1) | 0.1 |
| GA | 27 | 5.4 (3.8–6.9) | 1.81 (1.08–3.00) | 27 | 7.5 (5.8–9.2) | 1.69 (1.04–2.73) | ||
| AA | 2 | 7.7 (NA-NA) | 0.67 (0.16–2.75) | 2 | 12.4 (NA–NA) | 1.06 (0.26–4.42) | ||
| SNP | Affected, n/ Total, n | (%) | p-Value |
|---|---|---|---|
| Anemia | |||
| ERCC2 rs50872 | |||
| GG | 11/74 | 14.9 | 0.04 |
| GA | 9/58 | 15.5 | |
| AA | 4/8 | 50 | |
| GG-GA b | 20/132 | 15.2 | 0.03 * |
| XRCC1 rs25487 | |||
| CC | 9/52 | 17.3 | 0.12 |
| CT | 15/71 | 21.1 | |
| TT | 0/17 | 0 | |
| CC-CT b | 24/123 | 19.5 | 0.04 * |
| Thrombocytopenia | |||
| ERCC2 rs1799793 | |||
| CC | 6/61 | 9.8 | 0.04 |
| CT | 14/65 | 21.5 | |
| TT | 5/14 | 35.7 | |
| CT-TT a | 19/79 | 24.1 | 0.03 |
| UGT1A1 rs3064744 | |||
| *1/*1 | 12/64 | 18.8 | 0.05 |
| *1/*28 | 5/50 | 10.0 | |
| *28/*28 | 8/24 | 33.3 | |
| *1/*1–*1/*28 b | 17/114 | 14.9 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba, A.; López-Vilaró, L.; Ferre, M.; Majem, M.; Martinez-Recio, S.; Bell, O.; Arranz, M.J.; Salazar, J.; Sullivan, I. ERCC1 and ERCC2 Polymorphisms Predict the Efficacy and Toxicity of Platinum-Based Chemotherapy in Small Cell Lung Cancer. Pharmaceutics 2024, 16, 1121. https://doi.org/10.3390/pharmaceutics16091121
Barba A, López-Vilaró L, Ferre M, Majem M, Martinez-Recio S, Bell O, Arranz MJ, Salazar J, Sullivan I. ERCC1 and ERCC2 Polymorphisms Predict the Efficacy and Toxicity of Platinum-Based Chemotherapy in Small Cell Lung Cancer. Pharmaceutics. 2024; 16(9):1121. https://doi.org/10.3390/pharmaceutics16091121
Chicago/Turabian StyleBarba, Andrés, Laura López-Vilaró, Malena Ferre, Margarita Majem, Sergio Martinez-Recio, Olga Bell, María J. Arranz, Juliana Salazar, and Ivana Sullivan. 2024. "ERCC1 and ERCC2 Polymorphisms Predict the Efficacy and Toxicity of Platinum-Based Chemotherapy in Small Cell Lung Cancer" Pharmaceutics 16, no. 9: 1121. https://doi.org/10.3390/pharmaceutics16091121







