Bioactive Properties and In Vitro Digestive Release of Cannabidiol (CBD) from Tailored Composites Based on Carbon Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cannabidiol (CBD) Identification
2.2.2. Cannabidiol (CBD) Antioxidant Capacity
2.2.3. Cannabidiol (CBD) Antiradical Capacity
2.2.4. Cannabidiol (CBD) Anti-Proliferative Capacity
2.2.5. Cannabidiol (CBD) in Oxidative Stability
2.2.6. Synthesis of Composites Based on Carbon Materials
Synthesis of Carbon Xerogels by Direct Emulsion
Synthesis of Carbon Xerogels by Inverse Emulsion
Synthesis of Carbon Xerogels in Pellets
2.2.7. Surface Modification of Carbon Supports
2.2.8. Characterization of Carbon Supports
2.2.9. Adsorption Isotherms
2.2.10. Complete Factorial Design
2.2.11. Release Assays under Simulated Physiological Conditions
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Cannabidiol (CBD) Bioactivity
3.1.1. Antioxidant and Anti-Radical Capacity
3.1.2. Anti-Proliferative Capacity
3.1.3. Oxidative Stabilization
3.2. Composites Based on Carbon Materials
3.2.1. Carbon Support Properties
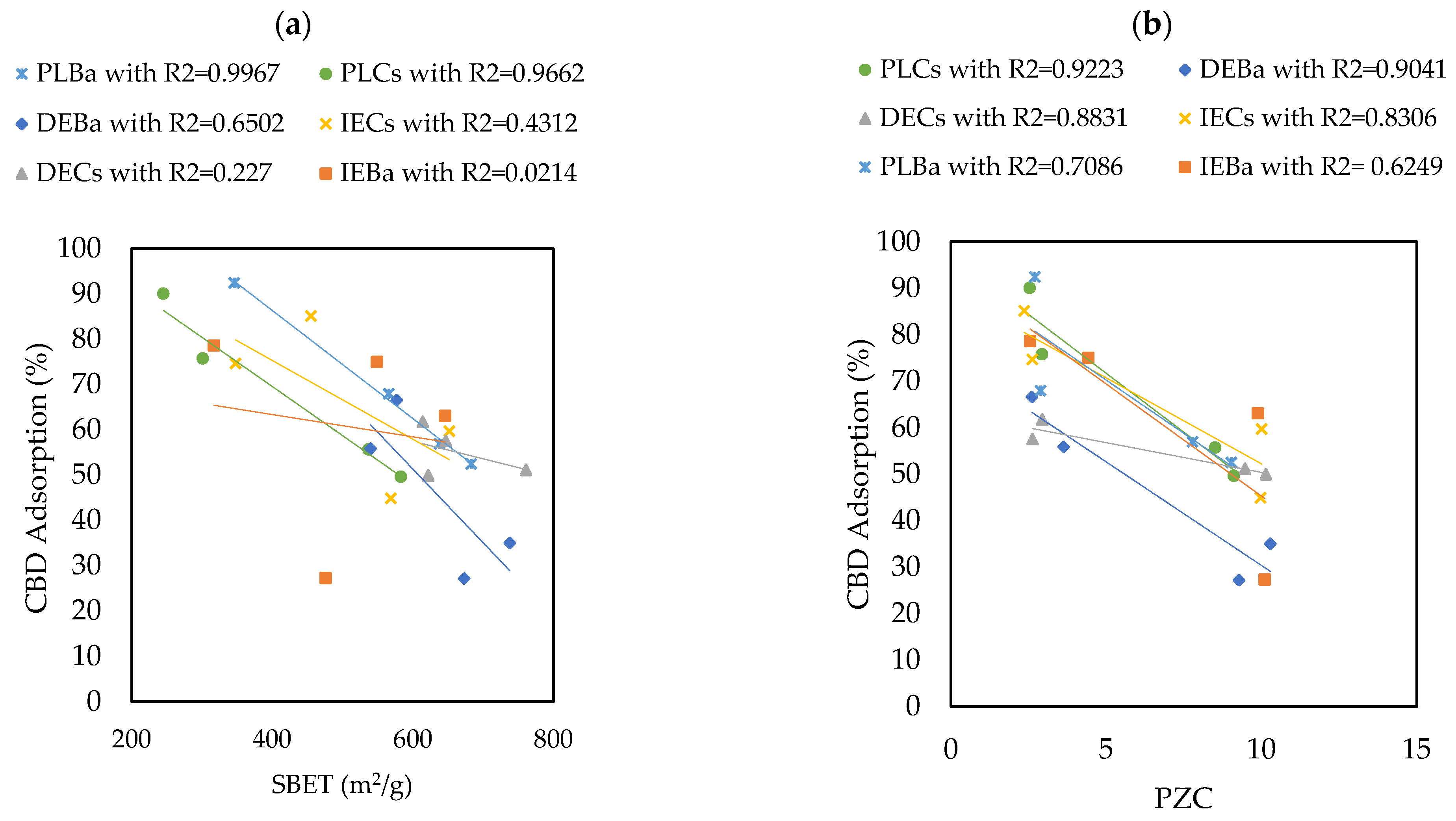
3.2.2. CBD Adsorption on Carbon Supports
3.2.3. CBD Release Assays from Carbon Supports
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stella, N. THC and CBD: Similarities and differences between siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Beers, J.L.; Jackson, K.D.; Zhou, Z. CBD and THC in Special Populations: Pharmacokinetics and Drug–Drug Interactions. Pharmaceutics 2024, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.R.; Alghalayini, A.; Valenzuela, S.M. Current Challenges and Opportunities for Improved Cannabidiol Solubility. Int. J. Mol. Sci. 2023, 24, 14514. [Google Scholar] [CrossRef]
- Su, Y.-T.; Zhang, J. Solubility enhancement and antioxidation maintenance of CBD encapsulated in the P407-RUB nano-micelle system. Curr. Drug Deliv. 2024, 21, 271–282. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Y.; Liu, Y.; Liu, Y.; Pan, J. Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems. Pharmaceutics 2024, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Predic-Atkinson, J.; Nikolic, B.; Pajovic, S.B.; Ivkovic, S.; Adzic, M. Nanocarriers in topical photodynamic therapy. Expert Opin. Drug Deliv. 2024, 21, 279–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, B.; Sun, Y.; Wang, C.; Guo, M. Preparation, stability, antioxidative property and in vitro release of cannabidiol (CBD) in zein-whey protein composite nanoparticles. LWT 2022, 162, 113466. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Zhao, R.; Freeman, K.; McHenry, M.A.; Wang, C.; Guo, M. Impact of carrier oil on interfacial properties, CBD partition and stability of emulsions formulated by whey protein or whey protein-maltodextrin conjugate. LWT 2022, 168, 113933. [Google Scholar] [CrossRef]
- Wang, C.; Dong, C.; Lu, Y.; Freeman, K.; Wang, C.; Guo, M. Digestion behavior, in vitro and in vivo bioavailability of cannabidiol in emulsions stabilized by whey protein-maltodextrin conjugate: Impact of carrier oil. Colloids Surf. B Biointerfaces 2023, 223, 113154. [Google Scholar] [CrossRef]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef]
- Morakul, B.; Junyaprasert, V.B.; Sakchaisri, K.; Teeranachaideekul, V. Cannabidiol-loaded nanostructured lipid carriers (NLCs) for dermal delivery: Enhancement of photostability, cell viability, and anti-inflammatory activity. Pharmaceutics 2023, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Mihailova, L.; Tchekalarova, J.; Shalabalija, D.; Geskovski, N.; Gjorgievska, V.S.; Stefkov, G.; Krasteva, P.; Crcarevska, M.S.; Dodov, M.G. Lipid nano-carriers loaded with Cannabis sativa extract for epilepsy treatment–in vitro characterization and in vivo efficacy studies. J. Pharm. Sci. 2022, 111, 3384–3396. [Google Scholar] [CrossRef]
- Assadpour, E.; Rezaei, A.; Das, S.S.; Krishna Rao, B.V.; Singh, S.K.; Kharazmi, M.S.; Jha, N.K.; Jha, S.K.; Prieto, M.A.; Jafari, S.M. Cannabidiol-loaded nanocarriers and their therapeutic applications. Pharmaceuticals 2023, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Söpper, U.; Hoffmann, A.; Daniels, R. Mucoadhesion and Mucopenetration of cannabidiol (CBD)-loaded mesoporous carrier systems for buccal drug delivery. Sci. Pharm. 2021, 89, 35. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA nanoparticles for the intraperitoneal administration of CBD in the treatment of ovarian cancer: In Vitro and In Ovo assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, N.J.; Wilson, B.K.; Priestley, R.D.; Prud’homme, R.K. Development of an in vitro release assay for low-density cannabidiol nanoparticles prepared by flash nanoprecipitation. Mol. Pharm. 2022, 19, 1515–1525. [Google Scholar] [CrossRef]
- Shreiber-Livne, I.; Sulimani, L.; Shapira, A.; Procaccia, S.; Meiri, D.; Sosnik, A. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) nanoparticles as a platform for the improved oral delivery of cannabidiol. Drug Deliv. Transl. Res. 2023, 13, 3192–3203. [Google Scholar] [CrossRef]
- Freire, N.F.; Cordani, M.; Aparicio-Blanco, J.; Sanchez, A.I.F.; Dutra, L.; Pinto, M.C.C.; Zarrabi, A.; Pinto, J.C.; Velasco, G.; Fialho, R. Preparation and characterization of PBS (Polybutylene Succinate) nanoparticles containing cannabidiol (CBD) for anticancer application. J. Drug Deliv. Sci. Technol. 2024, 97, 105833. [Google Scholar] [CrossRef]
- Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and preliminary in vitro evaluation of 3D-printed alginate films with cannabidiol (CBD) and cannabigerol (CBG) nanoparticles for potential wound-healing applications. Pharmaceutics 2022, 14, 1637. [Google Scholar] [CrossRef]
- Sam, D.K.; Cao, Y. Porous carbon materials for adsorption: A mini-review. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 721–732. [Google Scholar] [CrossRef]
- Su, R.; Xue, R.; Ma, X.; Zeng, Z.; Li, L.; Wang, S. Targeted improvement of narrow micropores in porous carbon for enhancing trace benzene vapor removal: Revealing the adsorption mechanism via experimental and molecular simulation. J. Colloid Interface Sci. 2024, 671, 770–778. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, J.; Heynderickx, P.M.; Arauzo, P.J.; Ronsse, F. Adsorption mechanism of different dyes on chemical activated carbon as quantitative assessment for wastewater treatment: Comparative study between ZnCl2 and its eutectic. Sep. Purif. Technol. 2024, 334, 126002. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Wang, Z.; Gao, Q. An innovative wood derived carbon-carbon nanotubes-pyrolytic carbon composites with excellent electrical conductivity and thermal stability. J. Mater. Sci. Technol. 2024, 175, 22–28. [Google Scholar] [CrossRef]
- Shi, K.; Xu, Z.; Wang, Y.; Fu, W.; Chen, B. Study on regeneration characteristics of granular activated carbon using ultrasonic and thermal methods. Environ. Sci. Pollut. Res. 2024, 31, 26580–26591. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Guo, X.-Q. Fe (II)–EDTA chelate-induced aromatic hydroxylation of terephthalate as a new method for the evaluation of hydroxyl radical-scavenging ability. Analyst 2001, 126, 928–932. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Martín-Romero, F.J.; Miguel-Lasobras, E.M.; Domínguez-Arroyo, J.A.; González-Carrera, E.; Álvarez, I.S. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod. Biomed. Online 2008, 17, 652–661. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. BanglaJOL. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Zapata Acosta, K.; Piedrahita, A.M.; Alzate, A.F.; Cortés, F.B.; Rojano, B.A. Estabilización oxidativa del aceite de Sacha inchi (Plukenetia volubilis Linneo) con suspensiones de mortiño (Vaccinium meridionale SW). Cienc. Desarro. 2015, 6, 141–153. [Google Scholar] [CrossRef]
- Fagan, J.M.; Sleczka, B.G.; Sohar, I. Quantitation of oxidative damage to tissue proteins. Int. J. Biochem. Cell Biol. 1999, 31, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Microspheres of carbon xerogel: An alternative Pt-support for the selective hydrogenation of citral. Appl. Catal. A Gen. 2014, 482, 318–326. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; De Vicente, J.; Moreno-Castilla, C. Carbon xerogel microspheres and monoliths from resorcinol-formaldehyde mixtures with varying dilution ratios: Preparation, surface characteristics, and electrochemical double-layer capacitances. Langmuir 2013, 29, 6166–6173. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Structural characterization of carbon xerogels: From film to monolith. Microporous Mesoporous Mater. 2012, 153, 24–29. [Google Scholar] [CrossRef]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Development of carbon xerogels as alternative Pt-supports for the selective hydrogenation of citral. Catal. Commun. 2015, 58, 64–69. [Google Scholar] [CrossRef]
- Ebadi, A.; Soltan Mohammadzadeh, J.S.; Khudiev, A. What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Zapata Acosta, K.; Carrasco-Marin, F.; Cortés, F.; Franco, C.; Lopera, S.; Rojano, B. Immobilization of P. stutzeri on Activated Carbons for Degradation of Hydrocarbons from Oil-in-Saltwater Emulsions. Nanomaterials 2019, 9, 500. [Google Scholar] [CrossRef]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
- Nassar, N.N.; Montoya, T.; Franco, C.A.; Cortes, F.B.; Pereira-Almao, P. A new model for describing the adsorption of asphaltenes on porous media at a high pressure and temperature under flow conditions. Energy Fuels 2015, 29, 4210–4221. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ 9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Arango-Romero, P. Evaluación Fitoquímica y Actividad Biológica de Cannabis sativa que se Cultiva en el Departamento del Cauca; Universidad del Cauca: Popayan, Colombia, 2022; pp. 1–123. [Google Scholar]
- Rodríguez, Y.; Sánchez-Catalán, F.; Rojano, B.; Durango, D.; Gil, J.; Marín-Loaiza, J. Caracterización fisicoquímica y evaluación de la actividad antioxidante de propóleos recolectados en el departamento del Atlántico, Colombia. Rev. UDCA Actual. Divulg. Científica 2012, 15, 303–311. [Google Scholar]
- Kasote, D.M.; Bhalerao, B.M.; Jagtap, S.D.; Khyade, M.S.; Deshmukh, K.K. Antioxidant and alpha-amylase inhibitory activity of methanol extract of Colocasia esculenta corm. Pharmacologyonline 2011, 2, 715–721. [Google Scholar]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Dantola, M.L.; Vignoni, M.; Serrano, M.P.; Lorente, C.; Thomas, A.H. Oxidation of biomolecules photosensitized by pterin derivatives. An. De La Asoc. Química Argent. 2020, 107, 164–187. [Google Scholar]
- Kang, Y.; Chen, J.; Duan, Z.; Li, Z. Predicting Dissolution of Entecavir Using the Noyes Whitney Equation. Dissolution Technol. 2023, 30, 38–45. [Google Scholar] [CrossRef]
- Esazadeh, K.; Ezzati Nazhad Dolatabadi, J.; Andishmand, H.; Mohammadzadeh-Aghdash, H.; Mahmoudpour, M.; Naemi Kermanshahi, M.; Roosta, Y. Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole and propyl gallate as synthetic food antioxidants. Food Sci. Nutr. 2024, 11, 1–13. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D. Two-stage 3-methylcholanthrene and butylated hydroxytoluene-induced lung carcinogenesis in mice. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 163, pp. 153–173. ISBN 0091-679X. [Google Scholar]
- Shi, Z.; Liang, X.; Zhao, Y.; Liu, W.; Martyniuk, C.J. Neurotoxic effects of synthetic phenolic antioxidants on dopaminergic, serotoninergic, and GABAergic signaling in larval zebrafish (Danio rerio). Sci. Total Environ. 2022, 830, 154688. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Grajeta, H.; Gomułka, K. Hypersensitivity reactions to food additives—Preservatives, antioxidants, flavor enhancers. Int. J. Environ. Res. Public Health 2022, 19, 11493. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, H.-S.; Tamia, G.; Song, H.-J.; Wei, C.-I. Anticancer Activity of Cannabidiol (CBD) in Human Colorectal Cancer Cells: A Mechanistic Study. Curr. Dev. Nutr. 2022, 6, 246. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of cannabidiol (CBD) in cancer treatment: A review. Biology 2022, 11, 817. [Google Scholar] [CrossRef]
- Revol, B.; Bagnolati, J.; Micallef, J.; Jouanjus, E. Cannabidiol (CBD): Confronting consumers’ expectations of therapeutic benefits with pharmacological reality. Therapies 2024, 1, 1–7. [Google Scholar] [CrossRef]
- Hazekawa, M.; Nishinakagawa, T.; Kawakubo-Yasukochi, T.; Nakashima, M. Evaluation of IC50 levels immediately after treatment with anticancer reagents using a real-time cell monitoring device. Exp. Ther. Med. 2019, 18, 3197–3205. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, P.-Y.; Yang, X.-P.; Li, H.; Hu, S.-D.; Xu, Y.-X.; Du, X.-H. Cannabidiol regulates apoptosis and autophagy in inflammation and cancer: A review. Front. Pharmacol. 2023, 14, 1094020. [Google Scholar] [CrossRef]
- Praphasawat, R.; Klajing, W.; Palipoch, S.; Wimuttiyanon, J.; Wutti, J.; Saypeark, N.; Wannarat, A.; Panpimanmas, S.; Rawangkarn, A. Cancer Signaling Pathway and Anti-Cancer Mechanism of Cannabidiol. J. Med. Assoc. Thail. 2023, 106, 219–227. [Google Scholar]
- Gęgotek, A.; Atalay, S.; Wroński, A.; Markowska, A.; Skrzydlewska, E. Cannabidiol Decreases Metalloproteinase Activity and Normalizes Angiogenesis Factor Expression in UVB-Irradiated Keratinocytes from Psoriatic Patients. Oxid. Med. Cell. Longev. 2021, 2021, 7624389. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson III, W.E. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Berto, B.M.; Garcia, R.K.A.; Fernandes, G.D.; Barrera-Arellano, D.; Pereira, G.G. Linseed oil: Characterization and study of its oxidative degradation. Grasas Y Aceites 2020, 71, 337. [Google Scholar] [CrossRef]
- Kilcast, D.; Subramaniam, P. The stability and shelf-life of food. Food Sci. Technol. Nutr. 2000, 5, 575–590. [Google Scholar]
- Corradini, M.G. Shelf life of food products: From open labeling to real-time measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants in food and food antioxidants. Food/nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Ansari, S.A.; Keshava, S.; Pendurthi, U.R.; Rao, L.V.M. Oxidative stress product, 4-hydroxy-2-nonenal, induces the release of tissue factor-positive microvesicles from perivascular cells into circulation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 250–265. [Google Scholar] [CrossRef]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial hypertension—Oxidative stress and inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative stress and beta amyloid in Alzheimer’s disease. Which comes first: The chicken or the egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-synuclein aggregation in Parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide dismutase and neurological disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein oxidation-formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Luder, W.F. The Electronic Theory of Acids and Bases. Chem. Rev. 1940, 27, 547–583. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]







| Technique | TEAC (μmol Trolox/100 g CBD) |
|---|---|
| ABTS | 79,609 ± 2586 |
| DPPH | 3385 ± 63 |
| FRAP | 5259 ± 194 |
| Technique | Radical | Result (per 100 g) | |
|---|---|---|---|
| CBD Sample | BHT Reference | ||
| DCFH assay | Total ROS | 4236 ± 213 μmol equivalents Trolox | 5143 ± 239 μmol equivalents Trolox |
| TEREPHTHALIC assay | ●OH | 1549 ± 77 mmol equivalents DMSO | 227 ± 14 mmol equivalents DMSO |
| ORAC assay | ROO● | 156,472 ± 10,600 μmol equivalents Trolox | 63,719 ± 3580 μmol equivalents Trolox |
| Carbon Support | PZC | SBET m2/g |
|---|---|---|
| DECs | 9.8 ± 0.4 a | 646 ± 3 a |
| DECsN | 10.1 ± 0.3 a | 632 ± 1 b |
| DECsP | 2.5 ± 0.2 b | 317 ± 2 c |
| DECsNP | 4.4 ± 0.4 c | 549 ± 2 d |
| DEBa | 9.3 ± 0.4 a | 673 ± 3 e |
| DEBaN | 10.3 ± 0.3 a | 738 ± 2 f |
| DEBaP | 2.6 ± 0.2 b | 577 ± 1 g |
| DEBaNP | 3.6 ± 0.4 c | 540 ± 3 h |
| IECs | 10.0 ± 0.3 a | 652 ± 4 a |
| IECsN | 9.9 ± 0.2 a | 589 ± 1 j |
| IECsP | 2.4 ± 0.2 b | 455 ± 1 k |
| IECsNP | 2.6 ± 0.2 d | 348 ± 2 l |
| IEBa | 9.5 ± 0.3 a | 761 ± 4 m |
| IEBaN | 10.1 ± 0.2 a | 622 ± 3 n |
| IEBaP | 2.6 ± 0.2 b | 647 ± 4 a |
| IEBaNP | 2.9 ± 0.1 d | 614 ± 3 o |
| PLCs | 9.1 ± 0.4 a | 583 ± 2 g |
| CsPLN | 8.5 ± 0.2 e | 537 ± 3 h |
| PLCsP | 2.5 ± 0.2 b | 245 ± 5 p |
| PLCsNP | 2.9 ± 0.1 d | 301 ± 3 q |
| PLBa | 9.0 ± 0.4 a | 683 ± 2 r |
| PLBaN | 7.8 ± 0.1 f | 638 ± 2 s |
| PLBaP | 2.7 ± 0.2 b | 346 ± 5 l |
| PLBaNP | 2.9 ± 0.2 d | 566 ± 4 t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, K.; Vélez, A.D.; Correa, J.A.; Carrasco-Marín, F.; Rojano, B.A.; Franco, C.A.; Cortés, F.B. Bioactive Properties and In Vitro Digestive Release of Cannabidiol (CBD) from Tailored Composites Based on Carbon Materials. Pharmaceutics 2024, 16, 1132. https://doi.org/10.3390/pharmaceutics16091132
Zapata K, Vélez AD, Correa JA, Carrasco-Marín F, Rojano BA, Franco CA, Cortés FB. Bioactive Properties and In Vitro Digestive Release of Cannabidiol (CBD) from Tailored Composites Based on Carbon Materials. Pharmaceutics. 2024; 16(9):1132. https://doi.org/10.3390/pharmaceutics16091132
Chicago/Turabian StyleZapata, Karol, Angie D. Vélez, Jorge A. Correa, Francisco Carrasco-Marín, Benjamín A. Rojano, Camilo A. Franco, and Farid B. Cortés. 2024. "Bioactive Properties and In Vitro Digestive Release of Cannabidiol (CBD) from Tailored Composites Based on Carbon Materials" Pharmaceutics 16, no. 9: 1132. https://doi.org/10.3390/pharmaceutics16091132
APA StyleZapata, K., Vélez, A. D., Correa, J. A., Carrasco-Marín, F., Rojano, B. A., Franco, C. A., & Cortés, F. B. (2024). Bioactive Properties and In Vitro Digestive Release of Cannabidiol (CBD) from Tailored Composites Based on Carbon Materials. Pharmaceutics, 16(9), 1132. https://doi.org/10.3390/pharmaceutics16091132









