Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ursolic Acid Nanofibers (UANF)
2.2. High-Performance Liquid Chromatography (HPLC) Analysis of UA and UANF
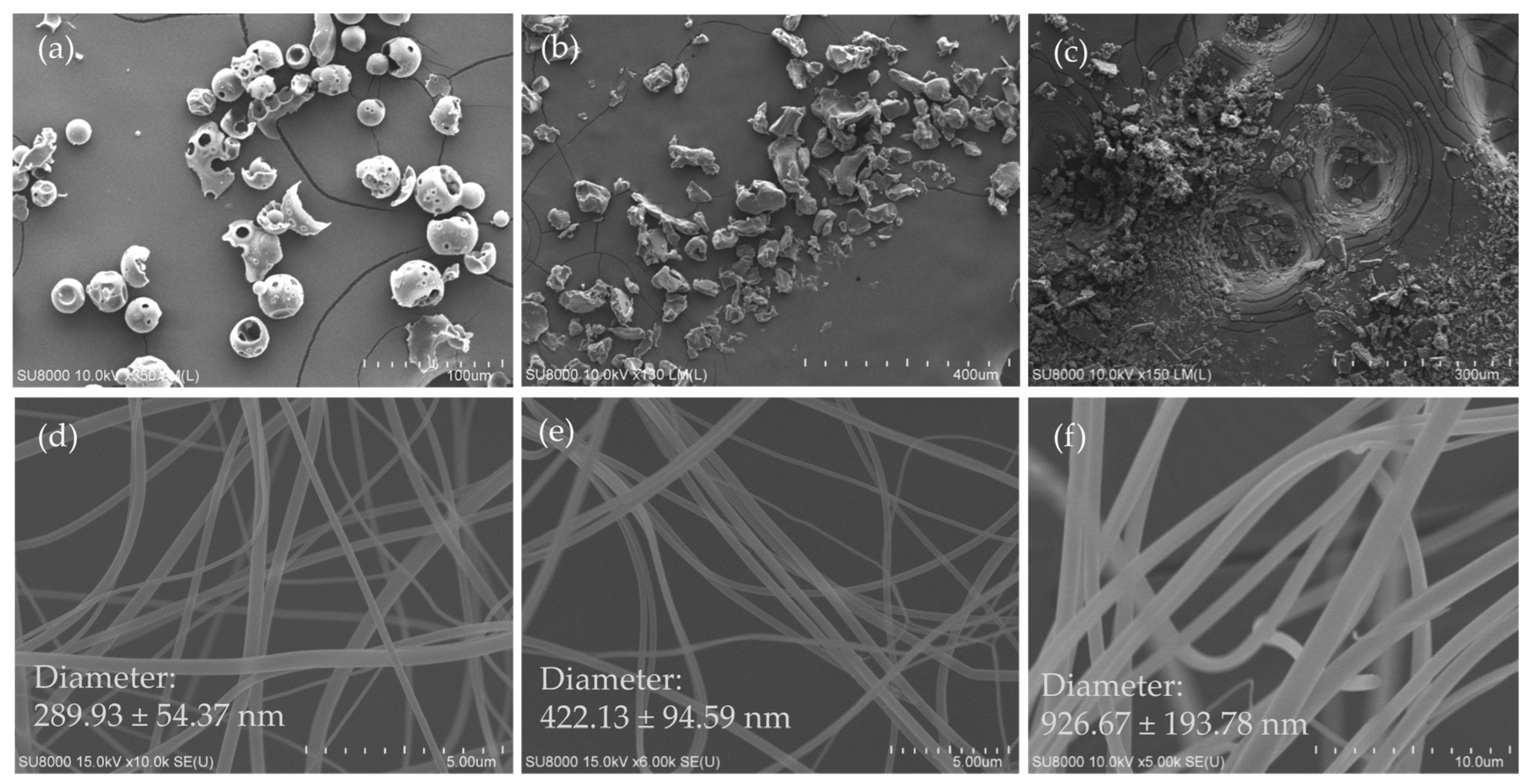
2.3. Observation of Diameter and Morphology of UANF
2.4. Particle Size Measurement of UANFs
2.5. Drug Loading of UANF
2.6. Encapsulation Efficiency of UANFs
2.7. Water Solubility of UANFs
2.8. Determination of Crystalline-to-Amorphous Transformation
2.9. Chemical Structure of UANFs Analyzed by Fourier-Transform Infrared (FTIR) Spectroscopy
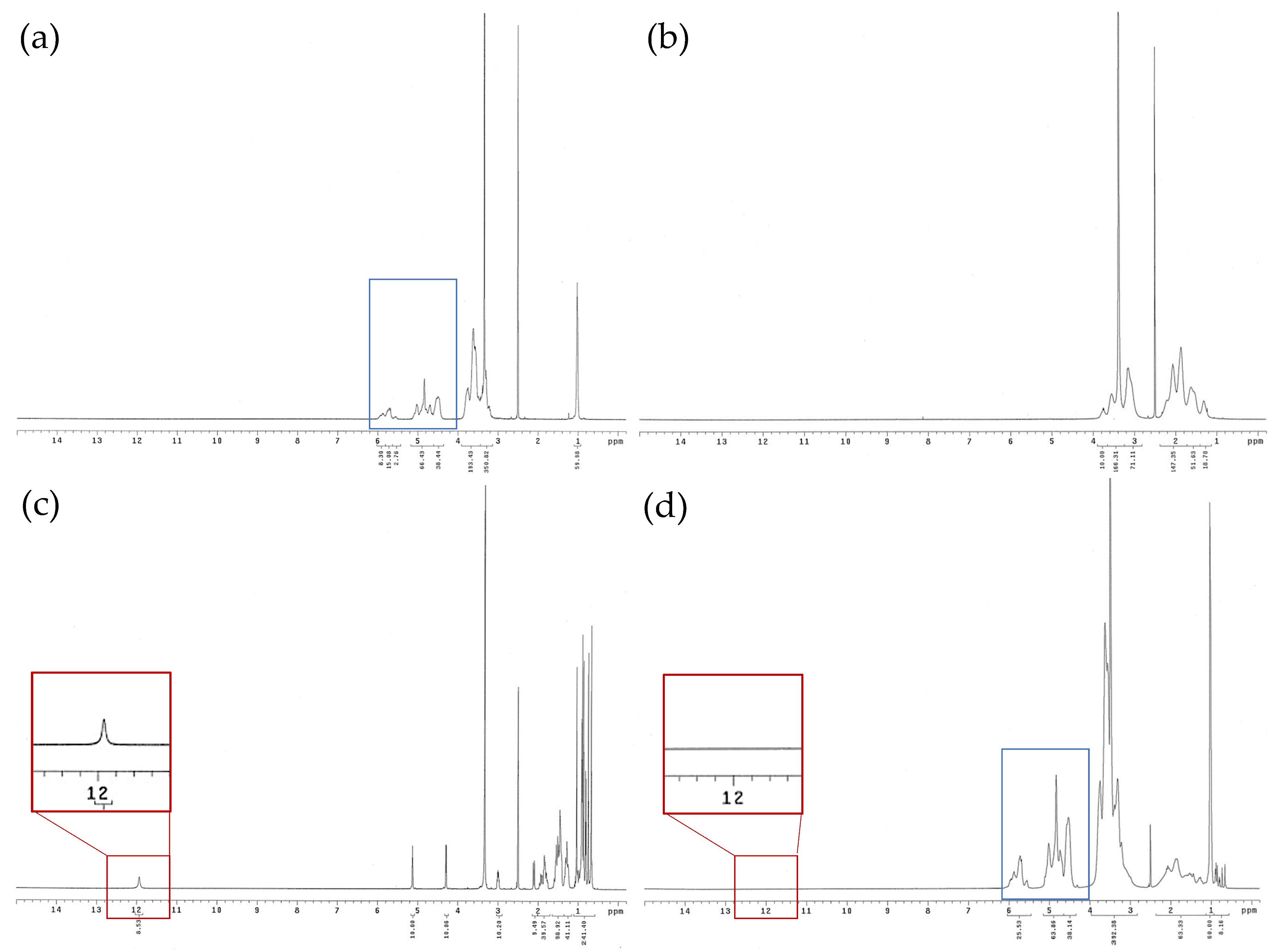
2.10. Chemical Structure of UANF Analyzed by Nuclear Magnetic Resonance (NMR) Spectroscopy
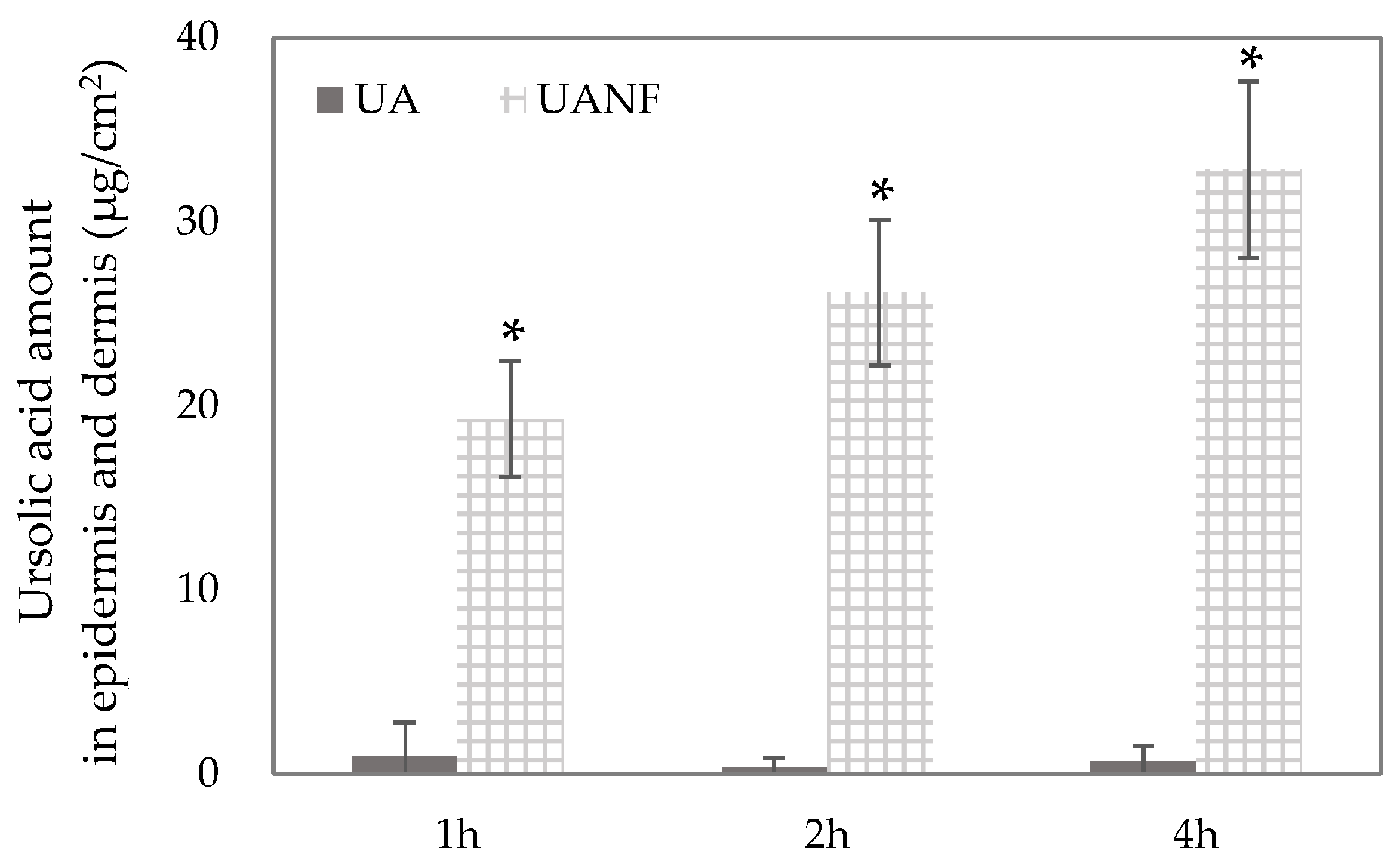
2.11. Ex Vivo Skin Penetration of UA and UANFs
2.12. Cytotoxicity
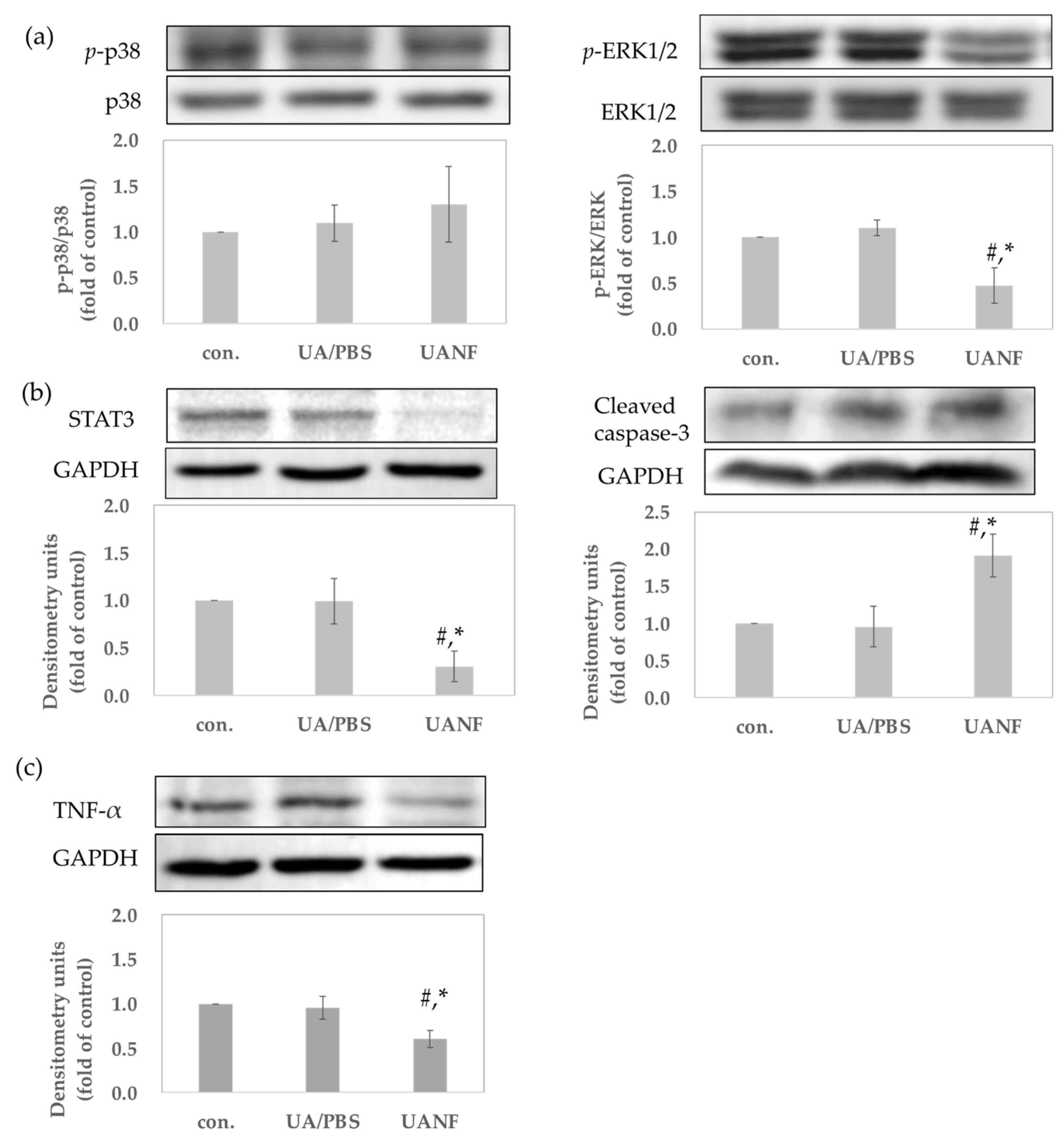
2.13. Anti-Breast Cancer Activity Assay by Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Surface Morphology of Excipients, Ursolic Acid and Its Nanofibers
3.2. Powder X-ray Diffraction Pattern of Ursolic Acid and Its Nanofibers
3.3. FTIR Spectra of Ursolic Acid and Its Nanofibers
3.4. 1H NMR Spectra of Ursolic Acid and Its Nanofibers
3.5. Drug Loading, Water Solubility, Encapsulation Efficiency, and Particle Size of Ursolic Acid Nanofibers
3.6. Particle Size and Morphology of UANFs Reconstituted in Water
3.7. In Vitro Skin Penetration of Ursolic Acid and Its Nanofibers
3.8. Ursolic Acid Nanofibers Showed Better Anti-Breast-Cancer Activity than Raw Ursolic Acid
3.8.1. UANFs Significantly Inhibit Growth of MCF-7 Cells
3.8.2. UANFs Have Anti-Breast Cancer Expression in MCF-7 Cells, Using Western Blot
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 8 July 2024).
- World Health Organization. WHO Launches New Roadmap on Breast Cancer. Available online: https://www.who.int/news/item/03-02-2023-who-launches-new-roadmap-on-breast-cancer (accessed on 8 July 2024).
- Moreno-Aspitia, A.; Hillman, D.W.; Dyar, S.H.; Tenner, K.S.; Gralow, J.; Kaufman, P.A.; Davidson, N.E.; Lafky, J.M.; Reinholz, M.M.; Lingle, W.L.; et al. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: Results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer 2013, 119, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Sood, A.; Daniali, L.N.; Rezzadeh, K.S.; Lee, E.S.; Keith, J. Management and Reconstruction in the Breast Cancer Patient With a Fungating T4b Tumor. Eplasty 2015, 15, e39. [Google Scholar]
- Kondra, K.; Pekcan, A.; Stanton, E.; Cook, A.D.; Jimenez, C.; Aronowitz, A.; Winterhalter, B.A.; Hammoudeh, J.A.; Aronowitz, J.A. Fungating Malignancies: Management of a Distinct Wound Entity. Adv. Ski. Wound Care 2022, 35, 646–652. [Google Scholar] [CrossRef]
- Navin, R.; Kim, S.M. Therapeutic interventions using ursolic acid for cancer treatment. Med. Chem. 2016, 6, 339–344. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic acid: An overview on its cytotoxic activities against breast and colorectal cancer cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Y.L.; Wang, H. Ursolic acid inhibits breast cancer growth by inhibiting proliferation, inducing autophagy and apoptosis, and suppressing inflammatory responses via the PI3K/AKT and NF-κB signaling pathways in vitro. Exp. Ther. Med. 2017, 14, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, X.; Zhang, J.; Li, J.; Wang, N.; Yang, B.; Pan, B.; Zheng, Y.; Wang, X.; Ou, H.; et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Front. Oncol. 2021, 11, 745584. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, W.; Ji, Y.; Liu, Y.; Xue, X. Ursolic Acid Enhances Cytotoxicity of Doxorubicin-Resistant Triple-Negative Breast Cancer Cells via ZEB1-AS1/miR-186-5p/ABCC1 Axis. Cancer Biother. Radiopharm. 2022, 37, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhao, J.; Liu, S.; Xue, Y.; Tang, D.; Yang, J.; Mei, Y.; Li, G.; Xie, Y. Ursolic acid augments the chemosensitivity of drug-resistant breast cancer cells to doxorubicin by AMPK-mediated mitochondrial dysfunction. Biochem. Pharmacol. 2022, 205, 115278. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Renda, G.; İlhan, M.; Bektaş, N.Y. Wound healing acceleration and anti-inflammatory potential of Prunella vulgaris L.: From conventional use to preclinical scientific verification. J. Ethnopharmacol. 2022, 295, 115411. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, Â.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple Strategies to Improve Oral Bioavailability by Fabricating Coamorphous Forms of Ursolic Acid with Piperine: Enhancing Water-Solubility, Permeability, and Inhibiting Cytochrome P450 Isozymes. Mol. Pharm. 2020, 17, 4443–4462. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.G.; Lopes, S.C.; Cassali, G.D.; Ferreira, Ê.; Veloso, E.S.; Leite, E.A.; Braga, F.C.; Ferreira, L.A.; Balvay, D.; Garofalakis, A.; et al. Evaluation of Antitumor Activity of Long-Circulating and pH-Sensitive Liposomes Containing Ursolic Acid in Animal Models of Breast Tumor and Gliosarcoma. Integr. Cancer Ther. 2016, 15, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Song, Y.; Chan, H.; Bi, C.; Yang, X.; Yan, R.; Wang, Y.; Zheng, Y. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. AAPS PharmSciTech 2014, 15, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in vitro and in vivo. Sci. Rep. 2016, 6, 30782. [Google Scholar] [CrossRef]
- Cosmetics_Europe COLIPA Guidelines: Guidelines for Percutaneous Absorption/Penetration. 1997. Available online: https://www.cosmeticseurope.eu/files/8314/6407/9075/Guidelines_for_Percutaneous_Absorption-Penetration_-_1997.pdf (accessed on 15 July 2024).
- Zlobin, A.; Bloodworth, J.C.; Osipo, C. Mitogen-activated protein kinase (MAPK) signaling. In Predictive Biomarkers in Oncology: Applications in Precision Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 213–221. [Google Scholar]
- Malik, S.T.; Naylor, M.S.; East, N.; Oliff, A.; Balkwill, F.R. Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur. J. Cancer 1990, 26, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, T.; Robinson, S.C.; Schulz, M.; Trümper, L.; Balkwill, F.R.; Binder, C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-α dependent up-regulation of matrix metalloproteases. Carcinogenesis 2004, 25, 1543–1549. [Google Scholar] [CrossRef]
- Zafar, S.; Khan, K.; Hafeez, A.; Irfan, M.; Armaghan, M.; Rahman, A.; Sönmez Gürer, E.; Sharifi-Rad, J.; Butnariu, M.; Bagiu, I.; et al. Ursolic acid: A natural modulator of signaling networks in different cancers. Cancer Cell Int. 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Kumar Bid, H.; Rajendran, S.M.; Yadav, P.P.; Konwar, R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: Role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion Venom Causes Upregulation of p53 and Downregulation of Bcl-xL and BID Protein Expression by Modulating Signaling Proteins Erk1/2 and STAT3, and DNA Damage in Breast and Colorectal Cancer Cell Lines. Integr. Cancer Ther. 2017, 17, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guo, R.; You, L.; Abbasi, A.M.; Li, T.; Fu, X.; Liu, R.H. Major triterpenoids in Chinese hawthorn “Crataegus pinnatifida” and their effects on cell proliferation and apoptosis induction in MDA-MB-231 cancer cells. Food Chem. Toxicol. 2017, 100, 149–160. [Google Scholar] [CrossRef]
- Kim, G.D. Ursolic Acid Decreases the Proliferation of MCF-7 Cell-Derived Breast Cancer Stem-Like Cells by Modulating the ERK and PI3K/AKT Signaling Pathways. Prev. Nutr. Food Sci. 2021, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Liu, C.-C.; Li, Y.-T.; Li, M.-Q.; Fu, Y.-T.; Li, X.-C.; Jie, K.; Qian, W.-D. Antifungal and Antibiofilm In Vitro Activities of Ursolic Acid on Cryptococcus neoformans. Curr. Microbiol. 2022, 79, 293. [Google Scholar] [CrossRef]
- Carletto, B.; Koga, A.Y.; Novatski, A.; Mainardes, R.M.; Lipinski, L.C.; Farago, P.V. Ursolic acid-loaded lipid-core nanocapsules reduce damage caused by estrogen deficiency in wound healing. Colloids Surf. B Biointerfaces 2021, 203, 111720. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical attributes of nanofibers: Preparation, drug loading, and tissue regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yen, F.L.; Huang, P.H.; Yang, C.Y.; Yen, C.H. Oleanolic Acid Nanofibers Attenuated Particulate Matter-Induced Oxidative Stress in Keratinocytes. Antioxidants 2021, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Uyar, T. Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int. J. Pharm. 2017, 518, 177–184. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Hu, S.C.; Huang, P.H.; Lin, T.C.; Yen, F.L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef]
- Jasek-Gajda, E.; Jurkowska, H.; JasiŃska, M.; Litwin, J.A.; Lis, G.J. Combination of ERK2 and STAT3 Inhibitors Promotes Anticancer Effects on Acute Lymphoblastic Leukemia Cells. Cancer Genom. Proteom. 2020, 17, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sharma, S.; Teknos, T.N. RhoC regulates cancer stem cells in head and neck squamous cell carcinoma by overexpressing IL-6 and phosphorylation of STAT3. PLoS ONE 2014, 9, e88527. [Google Scholar] [CrossRef]
- Krepler, C.; Xiao, M.; Sproesser, K.; Brafford, P.A.; Shannan, B.; Beqiri, M.; Liu, Q.; Xu, W.; Garman, B.; Nathanson, K.L.; et al. Personalized Preclinical Trials in BRAF Inhibitor-Resistant Patient-Derived Xenograft Models Identify Second-Line Combination Therapies. Clin. Cancer Res. 2016, 22, 1592–1602. [Google Scholar] [CrossRef]
- Shin, M.; Franks, C.E.; Hsu, K.L. Isoform-selective activity-based profiling of ERK signaling. Chem. Sci. 2018, 9, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Yang, Z.; Shen, Y.; Zhou, J.; Shen, Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers 2014, 6, 926–957. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi Sharif, P.; Jabbari, P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine 2020, 130, 155066. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, T.; Wilson, J.; Kulbe, H.; Li, N.F.; Leinster, D.A.; Charles, K.; Klemm, F.; Pukrop, T.; Binder, C.; Balkwill, F.R. Macrophages induce invasiveness of epithelial cancer cells via NF-κB and JNK. J. Immunol. 2005, 175, 1197–1205. [Google Scholar] [CrossRef] [PubMed]



| Ratio (UA:PVP:HPBCD) | Drug Loading (%) | Solubility (µg/mL) | Encapsulation Efficiency (%) | Particle Size (nm) | Polydispersity Index (PDI) |
|---|---|---|---|---|---|
| pure ursolic acid | - | ˂LOD * | - | 3370.00 ± 320.07 | 1.43 ± 0.12 |
| 1:8:10 | 98.88 ± 8.44 | 84.24 ± 10.18 | >99 | - | - |
| 1:8:20 | 90.5 ± 6.81 | 192.17 ± 39.44 | >99 | - | - |
| 1:8:40 | 83.97 ± 0.95 | 606.61 ± 51.91 | >99 | 258.87 ± 14.81 | 0.29 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Wu, T.-H.; Ma, C.-P.; Yen, F.-L. Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity. Pharmaceutics 2024, 16, 1147. https://doi.org/10.3390/pharmaceutics16091147
Fu H, Wu T-H, Ma C-P, Yen F-L. Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity. Pharmaceutics. 2024; 16(9):1147. https://doi.org/10.3390/pharmaceutics16091147
Chicago/Turabian StyleFu, Hsuan, Tzu-Hui Wu, Chih-Peng Ma, and Feng-Lin Yen. 2024. "Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity" Pharmaceutics 16, no. 9: 1147. https://doi.org/10.3390/pharmaceutics16091147
APA StyleFu, H., Wu, T.-H., Ma, C.-P., & Yen, F.-L. (2024). Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity. Pharmaceutics, 16(9), 1147. https://doi.org/10.3390/pharmaceutics16091147







