Passive Immunotherapy of Cynomolgus Monkeys with Anti-Rotavirus IgY
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monkeys
2.2. Rotavirus Inoculum and Specific IgY
2.3. Study Design
2.4. Haematological and Biochemical Analyses
2.5. Histopathological Analysis
2.6. Immunofluorescence
2.7. RVA Detection and Quantification by RT–qPCR
3. Results
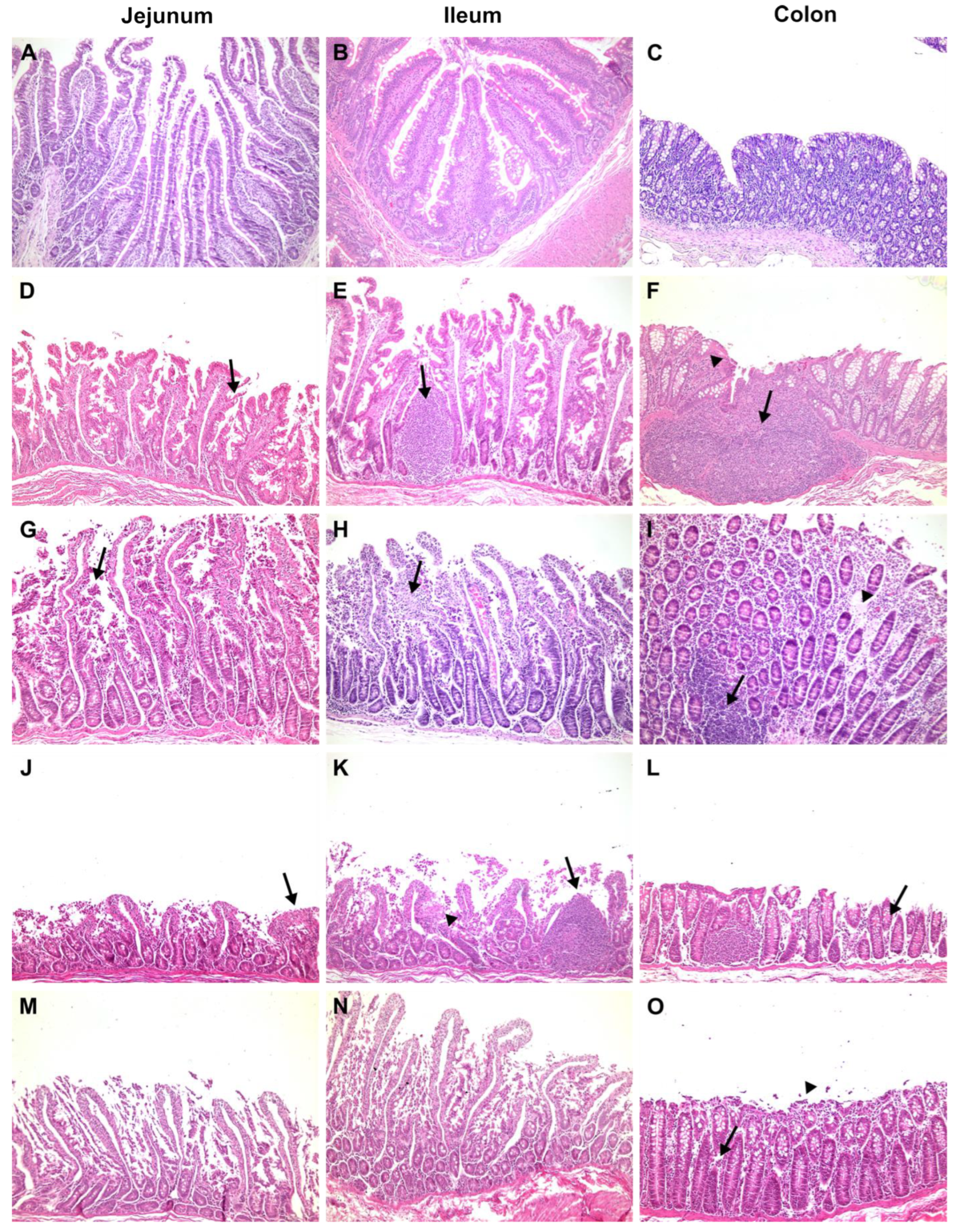
3.1. Clinical, Biochemical, and Histological Features
3.1.1. Negative Control
3.1.2. Positive Control
3.1.3. Proof of Concept
3.1.4. Cynomolgus Monkeys Orally Treated with Anti-RVA IgY
3.1.5. Cynomolgus Monkeys Orally and Intravenously Treated with Anti-RVA IgY
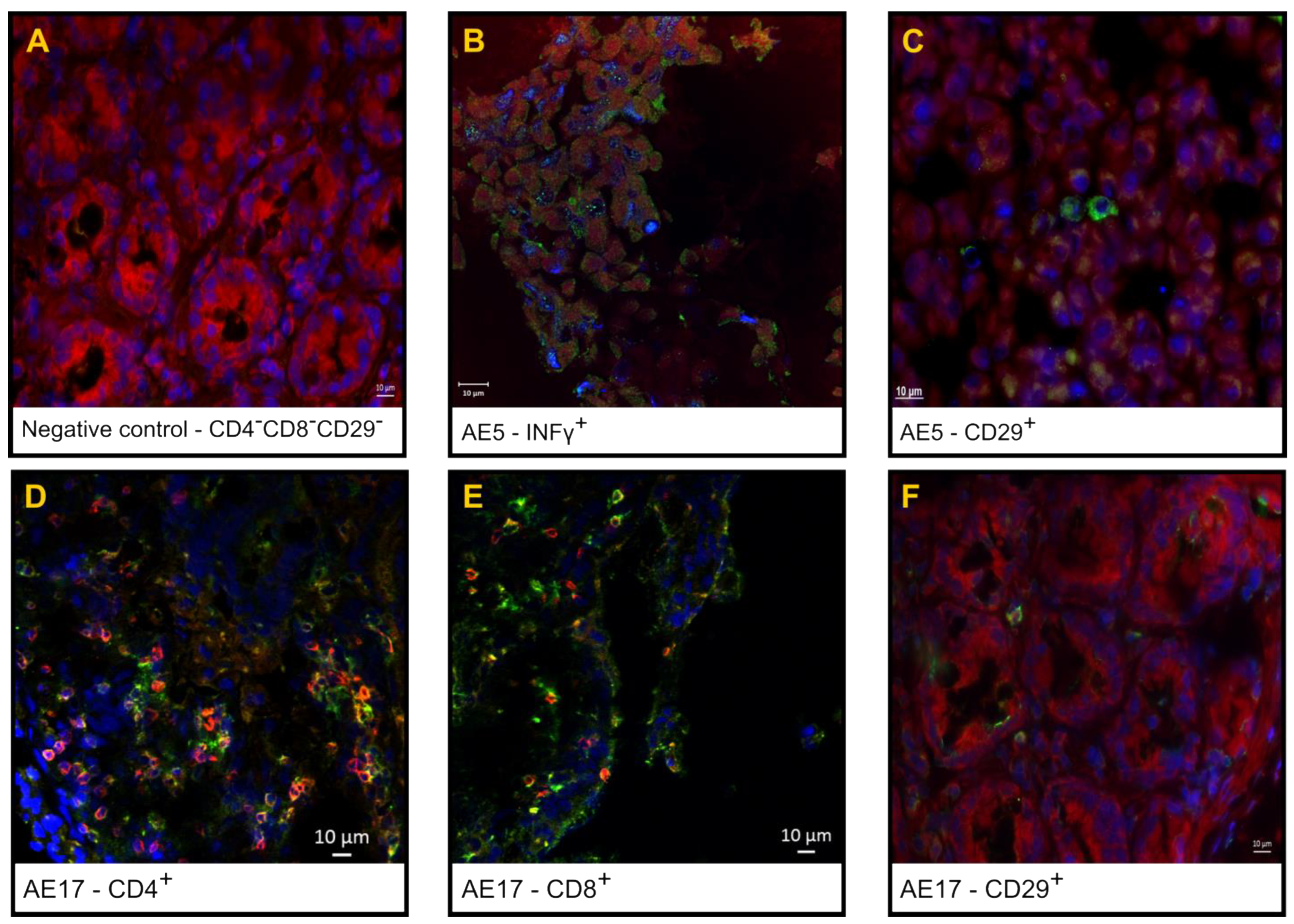
3.2. Phenotypic Analysis of the Inflammatory Infiltrate in the Small Intestine
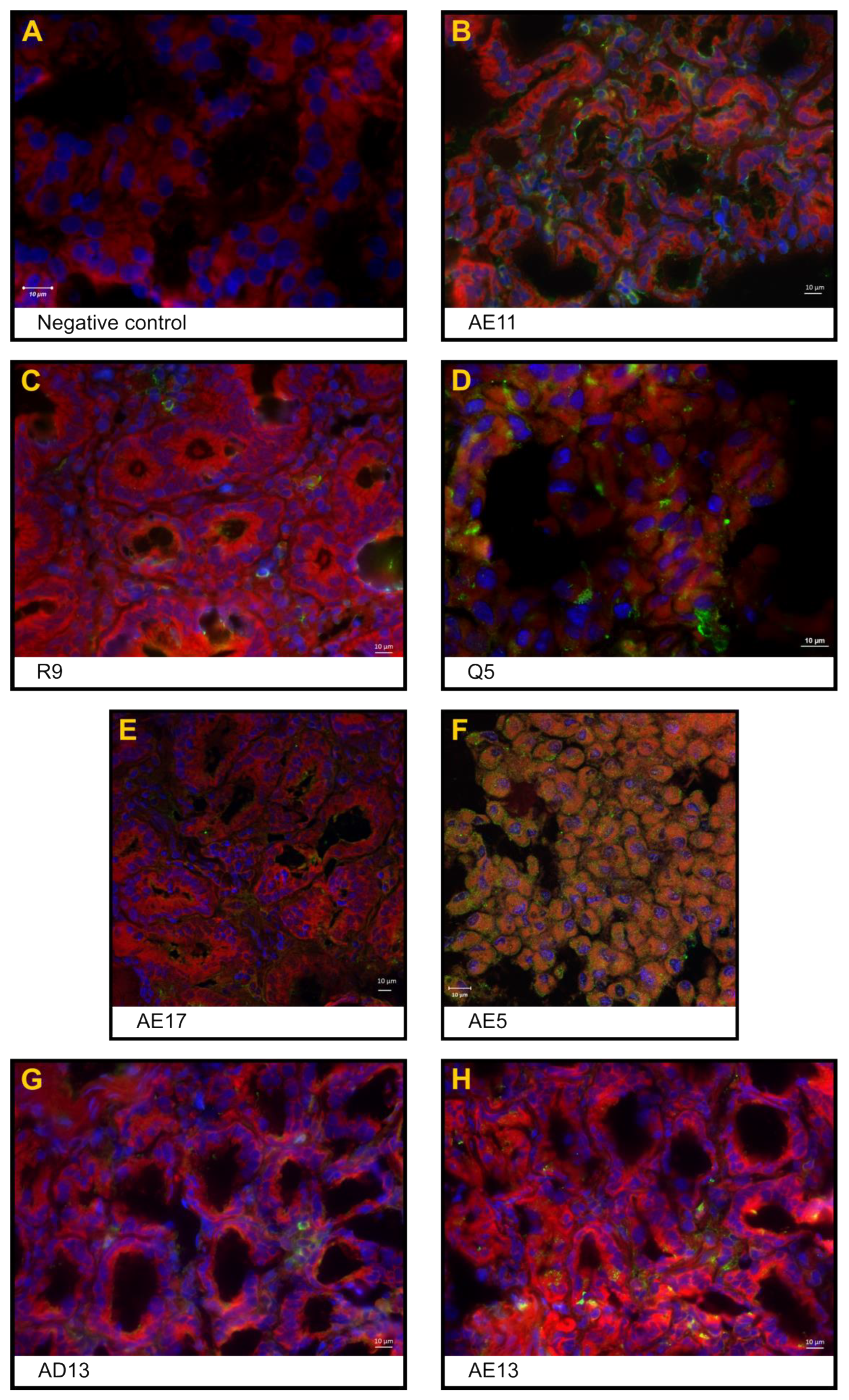
3.3. Expression of the Rotavirus Nonstructural Protein NSP4 in Enterocytes and Inflammatory Cells in the Interstitial Space
3.4. Viral RNA Shedding
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoea in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Rotavirus—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/rotavirus (accessed on 16 February 2024).
- Gutierrez, M.B.; Fialho, A.M.; Maranhão, A.G.; Malta, F.C.; da Silva Ribeiro de Andrade, J.; de Assis, R.M.S.; da Silva e Mouta, S.; Miagostovich, M.P.; Leite, J.P.G.; Fumian, T.M. Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens 2020, 9, 515. [Google Scholar] [CrossRef]
- Das, S.; Varghese, V.; Chaudhuri, S.; Barman, P.; Kojima, K.; Dutta, P.; Bhattacharya, S.K.; Krishnan, T.; Kobayashi, N.; Naik, T.N. Genetic Variability of Human Rotavirus Strains Isolated from Eastern and Northern India. J. Med. Virol. 2004, 72, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Wang, M.; Wang, J.; Ma, Y.; Liu, X.; Wang, M.; Sun, X.; Li, L.; Li, H.; Zhang, Q.; et al. Phylogenetic Analysis of the Viral Proteins VP4/VP7 of Circulating Human Rotavirus Strains in China from 2016 to 2019 and Comparison of Their Antigenic Epitopes with Those of Vaccine Strains. Front. Cell. Infect. Microbiol. 2022, 12, 927490. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Nelson, E.A.S.; Kang, G. Diagnosis, Management, and Prevention of Rotavirus Gastroenteritis in Children. BMJ 2013, 347, f7204. [Google Scholar] [CrossRef]
- Hartling, L.; Bellemare, S.; Wiebe, N.; Russell, K.; Klassen, T.P.; Craig, W. Oral versus Intravenous Rehydration for Treating Dehydration Due to Gastroenteritis in Children. Cochrane Database Syst. Rev. 2006, 2006, CD004390. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.L. Acute Gastroenteritis and Dehydration (Child). In Essential Evidence; Li, S.T., Ebell, M.H., Eds.; 2012; Available online: https://www.essentialevidenceplus.com/Home/Search?keyword=Acute+gastroenteritis+and+dehydration+%28child%29#! (accessed on 26 June 2024).
- Nelson, E.A.S.; Ko, W.K.; Kwan, E.; Leung, S.F.; Poon, K.H.; Chow, C.B.; Sin, W.K.; Wong, Y.K.; Yeung, C.Y. Guidelines for the Management of Acute Diarrhoea in Young Children. HK J. Paediatr. 2003, 8, 203–236. [Google Scholar]
- Guarino, A.; Canani, R.B.; Russo, S.; Albano, F.; Canani, M.B.; Ruggeri, F.M.; Donelli, G.; Rubino, A. Oral Immunoglobulins for Treatment of Acute Rotaviral Gastroenteritis. Pediatrics 1994, 93, 12–16. [Google Scholar] [CrossRef]
- Erhard, M.H.; Schmidt, P.; Zinsmeister, P.; Hofmann, A.; Münster, U.; Kaspers, B.; Wiesmüller, K.H.; Bessler, W.G.; Stangassinger, M. Adjuvant Effects of Various Lipopeptides and Interferon-Gamma on the Humoral Immune Response of Chickens. Poult. Sci. 2000, 79, 1264–1270. [Google Scholar] [CrossRef]
- Nilsson, E.; Hanrieder, J.; Bergquist, J.; Larsson, A. Proteomic Characterization of IgY Preparations Purified with a Water Dilution Method. J. Agric. Food Chem. 2008, 56, 11638–11642. [Google Scholar] [CrossRef]
- Tini, M.; Jewell, U.R.; Camenisch, G.; Chilov, D.; Gassmann, M. Generation and Application of Chicken Egg-Yolk Antibodies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, A.D.; Marcante, M.L.; Poggiali, F.; Hamsøíkovà, E.; Venuti, A. Egg Yolk Antibodies against the E7 Oncogenic Protein of Human Papillomavirus Type 16. Arch. Virol. 2001, 146, 117–125. [Google Scholar] [CrossRef]
- Rollier, C.; Charollois, C.; Jamard, C.; Trepo, C.; Cova, L. Early Life Humoral Response of Ducks to DNA Immunization against Hepadnavirus Large Envelope Protein. Vaccine 2000, 18, 3091–3096. [Google Scholar] [CrossRef]
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreño, V. Egg Yolk IgY: Protection against Rotavirus Induced Diarrhea and Modulatory Effect on the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. [Google Scholar] [CrossRef]
- Vega, C.G.; Bok, M.; Vlasova, A.N.; Chattha, K.S.; Fernández, F.M.; Wigdorovitz, A.; Parreño, V.G.; Saif, L.J. IgY Antibodies Protect against Human Rotavirus Induced Diarrhea in the Neonatal Gnotobiotic Piglet Disease Model. PLoS ONE 2012, 7, e42788. [Google Scholar] [CrossRef] [PubMed]
- Hatta, H.; Tsuda, K.; Ozeki, M.; Kim, M.; Yamamoto, T.; Otake, S.; Hirasawa, M.; Katz, J.; Childers, N.K.; Michalek, S.M. Passive Immunization against Dental Plaque Formation in Humans: Effect of a Mouth Rinse Containing Egg Yolk Antibodies (IgY) Specific to Streptococcus Mutans. Caries Res. 1997, 31, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Horie, N.; Abdou, A.M.; Yang, J.-O.; Yun, S.-S.; Chun, H.-N.; Park, C.-K.; Kim, M.; Hatta, H. Suppressive Effect of Functional Drinking Yogurt Containing Specific Egg Yolk Immunoglobulin on Helicobacter Pylori in Humans. J. Dairy Sci. 2004, 87, 4073–4079. [Google Scholar] [CrossRef]
- Kollberg, H.; Carlander, D.; Olesen, H.; Wejåker, P.-E.; Johannesson, M.; Larsson, A. Oral Administration of Specific Yolk Antibodies (IgY) May Prevent Pseudomonas Aeruginosa Infections in Patients with Cystic Fibrosis: A Phase I Feasibility Study. Pediatr. Pulmonol. 2003, 35, 433–440. [Google Scholar] [CrossRef]
- Mine, Y.; Kovacs-Nolan, J. Chicken Egg Yolk Antibodies as Therapeutics in Enteric Infectious Disease: A Review. J. Med. Food 2002, 5, 159–169. [Google Scholar] [CrossRef]
- Nilsson, E.; Larsson, A.; Olesen, H.V.; Wejåker, P.-E.; Kollberg, H. Good Effect of IgY against Pseudomonas Aeruginosa Infections in Cystic Fibrosis Patients. Pediatr. Pulmonol. 2008, 43, 892–899. [Google Scholar] [CrossRef]
- Shin, J.-H.; Nam, S.-W.; Kim, J.-T.; Yoon, J.-B.; Bang, W.-G.; Roe, I.-H. Identification of Immunodominant Helicobacter Pylori Proteins with Reactivity to H. Pylori-Specific Egg-Yolk Immunoglobulin. J. Med. Microbiol. 2003, 52, 217–222. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yang, M.; Nam, S.W.; Kim, J.T.; Myung, N.H.; Bang, W.-G.; Roe, I.H. Use of Egg Yolk-Derived Immunoglobulin as an Alternative to Antibiotic Treatment for Control of Helicobacter Pylori Infection. Clin. Diagn. Lab. Immunol. 2002, 9, 1061–1066. [Google Scholar] [CrossRef]
- Suzuki, H.; Nomura, S.; Masaoka, T.; Goshima, H.; Kamata, N.; Kodama, Y.; Ishii, H.; Kitajima, M.; Nomoto, K.; Hibi, T. Effect of Dietary Anti-Helicobacter Pylori-Urease Immunoglobulin Y on Helicobacter Pylori Infection. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S1), 185–192. [Google Scholar] [CrossRef] [PubMed]
- LeClaire, R.D.; Hunt, R.E.; Bavari, S. Protection against Bacterial Superantigen Staphylococcal Enterotoxin B by Passive Vaccination. Infect. Immun. 2002, 70, 2278–2281. [Google Scholar] [CrossRef]
- Shimizu, M.; Nagashima, H.; Hashimoto, K. Comparative Studies in Molecular Stability of Immunoglobulin G from Different Species. Comp. Biochem. Physiol. B 1993, 106, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Van Nguyen, S.; Icatlo, F.C.; Umeda, K.; Kodama, Y. Oral Passive IgY-Based Immunotherapeutics: A Novel Solution for Prevention and Treatment of Alimentary Tract Diseases. Hum. Vaccin. Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Bentes, G.A.; Lanzarini, N.M.; Guimarães, J.R.; Heinemann, M.B.; de Volotão, E.M.; da Silva, A.D.S.; Heneine, L.G.D.; de Oliveira, J.M.; Pinto, M.A. Production and Evaluation of Chicken Egg Yolk Immunoglobulin (IgY) against Human and Simian Rotaviruses. Viruses 2022, 14, 1995. [Google Scholar] [CrossRef]
- Andrade, M.R.; Yee, J.; Barry, P.; Spinner, A.; Roberts, J.A.; Cabello, P.H.; Leite, J.P.; Lerche, N.W. Prevalence of Antibodies to Selected Viruses in a Long-Term Closed Breeding Colony of Rhesus Macaques (Macaca Mulatta) in Brazil. Am. J. Primatol. 2003, 59, 123–128. [Google Scholar] [CrossRef]
- Bentes, G.A.; Guimarães, J.R.; de Volotão, E.M.; Fialho, A.M.; Hooper, C.; Ganime, A.C.; Gardinali, N.R.; Lanzarini, N.M.; da Silva, A.D.S.; Pitcovski, J.; et al. Cynomolgus Monkeys (Macaca Fascicularis) as an Experimental Infection Model for Human Group A Rotavirus. Viruses 2018, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Association of Primate Veterinarians’ Humane Endpoint Guidelines for Nonhuman Primates in Biomedical Research. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 6–8.
- MCTI. Available online: https://antigo.mctic.gov.br/mctic/opencms/legislacao/outros_atos/resolucoes/Resolucao_Normativa_Concea_n_60_de_02052023.html (accessed on 7 May 2024).
- Resolucao-Normativa-CONCEA-n-28-de-13.11.2015-D.O.U.-de-16.11.2015-Secao-I-Pag.-44.Pdf—Ministério Da Ciência, Tecnologia e Inovação. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/concea/arquivos/pdf/guia-brasileiro-de-producao-manutencao-ou-utilizacao-de-animais-para-atividades-de-ensino-ou-pesquisa-cientifica/resolucao-normativa-concea-n-28-de-13-11-2015-d-o-u-de-16-11-2015-secao-i-pag-44.pdf/view (accessed on 17 May 2023).
- Rosso, M.C.; Badino, P.; Ferrero, G.; Costa, R.; Cordero, F.; Steidler, S. Biologic Data of Cynomolgus Monkeys Maintained under Laboratory Conditions. PLoS ONE 2016, 11, e0157003. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.G.; Marchevsky, R.S.; dos Santos, D.R.L.; de Oliveira, J.M.; de Paula, V.S.; Lopes, L.M.; Van der Poel, W.H.M.; González, J.E.; Munné, M.S.; Moran, J.; et al. Infection by Brazilian and Dutch Swine Hepatitis E Virus Strains Induces Haematological Changes in Macaca Fascicularis. BMC Infect. Dis. 2013, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; Blakiston Division, McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Iturriza Gómara, M.; Wong, C.; Blome, S.; Desselberger, U.; Gray, J. Molecular Characterization of VP6 Genes of Human Rotavirus Isolates: Correlation of Genogroups with Subgroups and Evidence of Independent Segregation. J. Virol. 2002, 76, 6596–6601. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-Step Quantitative RT-PCR for the Detection of Rotavirus in Acute Gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- Fumian, T.M.; Victoria, M.; Vieira, C.B.; Fioretti, J.M.; Rocha, M.S.; Prado, T.; Guimarães, F.R.; da Gama, N.P.; de Oliveira, J.M.; Mendes, A.C.O.; et al. Enteric Viruses’ Dissemination in a Private Reserve of Natural Heritage. Lett. Appl. Microbiol. 2018, 66, 313–320. [Google Scholar] [CrossRef]
- Freeman, M.M.; Kerin, T.; Hull, J.; McCaustland, K.; Gentsch, J. Enhancement of Detection and Quantification of Rotavirus in Stool Using a Modified Real-Time RT-PCR Assay. J. Med. Virol. 2008, 80, 1489–1496. [Google Scholar] [CrossRef]
- Jiang, B.; McClure, H.M.; Fankhauser, R.L.; Monroe, S.S.; Glass, R.I. Prevalence of Rotavirus and Norovirus Antibodies in Non-Human Primates. J. Med. Primatol. 2004, 33, 30–33. [Google Scholar] [CrossRef]
- Kalter, S.S. Enteric Viruses of Nonhuman Primates. Vet. Pathol. Suppl. 1982, 7, 33–43. [Google Scholar] [CrossRef]
- Maldonado, Y.A.; Yolken, R.H. Rotavirus. Baillieres Clin. Gastroenterol. 1990, 4, 609–625. [Google Scholar] [CrossRef]
- Ciarlet, M.; Conner, M.E.; Finegold, M.J.; Estes, M.K. Group A Rotavirus Infection and Age-Dependent Diarrheal Disease in Rats: A New Animal Model to Study the Pathophysiology of Rotavirus Infection. J. Virol. 2002, 76, 41–57. [Google Scholar] [CrossRef]
- Desselberger, U. Rotavirus Infections: Guidelines for Treatment and Prevention. Drugs 1999, 58, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hossain, M.E.; Haider, N.; Rostal, M.K.; Mukharjee, S.K.; Ferdous, J.; Miah, M.; Rahman, M.; Daszak, P.; Rahman, M.Z.; et al. Molecular Characterization of Group A Rotavirus from Rhesus Macaques (Macaca Mulatta) at Human-Wildlife Interfaces in Bangladesh. Transbound. Emerg. Dis. 2020, 67, 956–966. [Google Scholar] [CrossRef]
- Fenaux, M.; Cuadras, M.A.; Feng, N.; Jaimes, M.; Greenberg, H.B. Extraintestinal Spread and Replication of a Homologous EC Rotavirus Strain and a Heterologous Rhesus Rotavirus in BALB/c Mice. J. Virol. 2006, 80, 5219–5232. [Google Scholar] [CrossRef]
- Horie, Y.; Nakagomi, O.; Koshimura, Y.; Nakagomi, T.; Suzuki, Y.; Oka, T.; Sasaki, S.; Matsuda, Y.; Watanabe, S. Diarrhea Induction by Rotavirus NSP4 in the Homologous Mouse Model System. Virology 1999, 262, 398–407. [Google Scholar] [CrossRef]
- Westerman, L.E.; Xu, J.; Jiang, B.; McClure, H.M.; Glass, R.I. Experimental Infection of Pigtailed Macaques with a Simian Rotavirus, YK-1. J. Med. Virol. 2005, 75, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, C.F.; Marchevsky, R.S.; Stephens, P.R.; Araujo, H.P.; Oliveira, A.V. Murine Experimental Infection with Rotavirus SA-11: Clinical and Immunohistological Characteristics. Exp. Toxicol. Pathol. 1994, 45, 433–438. [Google Scholar] [CrossRef]
- Jain, S.; Haydel, M.J. Child Intussusception. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hashavya, S.; Wilscrhanski, M.; Averbuch, D.; Arbell, D.; Pappo, O.; Shteyer, E. Rotavirus-Associated Colitis in a Six-Month-Old Baby. Pediatr. Int. 2010, 52, e204–e206. [Google Scholar] [CrossRef] [PubMed]
- Tarris, G.; de Rougemont, A.; Charkaoui, M.; Michiels, C.; Martin, L.; Belliot, G. Enteric Viruses and Inflammatory Bowel Disease. Viruses 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The Rotavirus NSP4 Viroporin Domain Is a Calcium-Conducting Ion Channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef]
- Lorrot, M.; Vasseur, M. How Do the Rotavirus NSP4 and Bacterial Enterotoxins Lead Differently to Diarrhea? Virol. J. 2007, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Blutt, S.E.; Ettayebi, K.; Zeng, X.-L.; Broughman, J.R.; Crawford, S.E.; Karandikar, U.C.; Sastri, N.P.; Conner, M.E.; Opekun, A.R.; et al. Human Intestinal Enteroids: A New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J. Virol. 2016, 90, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Majer, M.; Behrens, F.; Weinmann, E.; Mauler, R.; Maass, G.; Baumeister, H.G.; Luthardt, T. Diarrhea in Newborn Cynomologus Monkeys Infected with Human Rotavirus. Infection 1978, 6, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Losonsky, G.A.; Johnson, J.P.; Winkelstein, J.A.; Yolken, R.H. Oral Administration of Human Serum Immunoglobulin in Immunodeficient Patients with Viral Gastroenteritis. A Pharmacokinetic and Functional Analysis. J. Clin. Investig. 1985, 76, 2362–2367. [Google Scholar] [CrossRef]
- Lee, K.A.; Chang, S.K.; Lee, Y.J.; Lee, J.H.; Koo, N.S. Acid Stability of Anti-Helicobacter Pyroli IgY in Aqueous Polyol Solution. J. Biochem. Mol. Biol. 2002, 35, 488–493. [Google Scholar] [CrossRef]
- Chang, H.-M.; Lee, Y.-C.; Chen, C.-C.; Tu, Y.-Y. Microencapsulation Protects Immunoglobulin in Yolk (IgY) Specific against Helicobacter Pylori Urease. J. Food Sci. 2002, 67, 15–20. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Lee, J.-J.; Park, I.-B.; Huh, C.-S.; Baek, Y.-J.; Park, J. Protective Effect of Microencapsulation Consisting of Multiple Emulsification and Heat Gelation Processes on Immunoglobulin in Yolk. J. Food Sci. 2005, 70, E148–E151. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Microencapsulation for the Gastric Passage and Controlled Intestinal Release of Immunoglobulin Y. J. Immunol. Methods 2005, 296, 199–209. [Google Scholar] [CrossRef]
- Shah, R.B.; Khan, M.A. Protection of Salmon Calcitonin Breakdown with Serine Proteases by Various Ovomucoid Species for Oral Drug Delivery. J. Pharm. Sci. 2004, 93, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Pant, N.; Juneja, L.R.; Hammarström, L. Successful Treatment of Rotavirus-Induced Diarrhoea in Suckling Mice with Egg Yolk Immunoglobulin. J. Health Popul. Nutr. 2007, 25, 465–468. [Google Scholar]
| Cynomolgus Code Name | Age (Years/Months) | Weight (kg) | Inoculum Dose (FFU a) | IgY (mg) | |
|---|---|---|---|---|---|
| Negative control (NC) | J6 | 19/01 | 3.380 | Ø | Ø |
| Positive control (PC) | B2 | 27/00 | 3.650 | 3.1 × 107 | Ø |
| AE15 | 0/10 | 1.330 | |||
| AE11 | 1/00 | 1.340 | |||
| Proof of concept (PoC) | R9 | 11/08 | 5.840 | 3.1 × 107 | 2.5 (oral) |
| Q5 | 13/02 | 4.500 | |||
| Oral immunotherapy (OT) | AD15 | 1/10 | 1.600 | 3.1 × 107 | 2.5 (oral) |
| AA3 | 5/03 | 3.970 | |||
| AE5 | 1/02 | 1.250 | |||
| AE17 | 0/10 | 1.130 | |||
| Oral and intravenous immunotherapy (OIVT) | AD13 | 1/11 | 1.150 | 3.1 × 107 | 2.5 (oral) 2.5 (IV b) |
| X5 | 7/03 | 6.650 | |||
| AE1 | 1/03 | 1.550 | |||
| AE13 | 1/00 | 1.200 |
| Monkey | Villi Denudation a | Histological Enteritis | Interstitial Cell Infiltration b | Colitis | RVA Shedding (RNA Copies/mg) c | NSP4 d | Rotavirus-Associated Diarrhoea | Outcome e | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jejunum | Ileum | Jejunum | Ileum | ||||||||
| NC | J6 | Ø | Ø | Ø | Ø | Ø | no | no | ND | no | control |
| PC | B2 | † | ††† | severe | † | † | ND f | no | ND | no | sick |
| AE15 | †† | Ø | moderate | ††† | †† | no | 102 (1 dpi) | ND | no | sick | |
| AE11 | †† | † | moderate | † | † | yes | 103 (1 dpi) | †† | no | sick | |
| PoC | R9 | Ø | Ø | mild | Ø | Ø | no | 103 (2 dpi) | † | no | protected |
| Q5 | †††† | †††† | severe | ††† | ††† | yes | 103 (2 dpi) | ††† | yes | sick/death g | |
| OT | AD15 | Ø | † | mild | † | † | ND | 103 (2 dpi) | ND | no | protected |
| AA3 | † | † | mild | † | † | no | 104 (1 dpi) | ND | no | protected | |
| AE5 | † | ††† | moderate | Ø | Ø | no | 103 (2 dpi) | †† | no | sick | |
| AE17 | †††† | †††† | severe | †† | †† | yes | 103 (2 dpi) | † | no | sick | |
| OIVT | AD13 | † | † | mild | Ø | Ø | no | no | †† | no | protected |
| X5 | ††† | ††† | severe | †† | †† | no | 103 (2 dpi) | ND | yes | sick | |
| AE1 | ††† | ††† | severe | †† | †† | yes | 102 (1 dpi) | ND | no | sick | |
| AE13 | †† | †† | moderate | ††† | †† | yes | 103 (2 dpi) | ††† | no | sick | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentes, G.A.; Guimarães, J.R.; Volotão, E.d.M.; Lanzarini, N.M.; da Silva, A.d.S.; Gardinali, N.R.; Marchevsky, R.S.; Leite, J.P.G.; de Oliveira, J.M.; Pinto, M.A. Passive Immunotherapy of Cynomolgus Monkeys with Anti-Rotavirus IgY. Pharmaceutics 2024, 16, 1149. https://doi.org/10.3390/pharmaceutics16091149
Bentes GA, Guimarães JR, Volotão EdM, Lanzarini NM, da Silva AdS, Gardinali NR, Marchevsky RS, Leite JPG, de Oliveira JM, Pinto MA. Passive Immunotherapy of Cynomolgus Monkeys with Anti-Rotavirus IgY. Pharmaceutics. 2024; 16(9):1149. https://doi.org/10.3390/pharmaceutics16091149
Chicago/Turabian StyleBentes, Gentil Arthur, Juliana Rodrigues Guimarães, Eduardo de Mello Volotão, Natália Maria Lanzarini, Alexandre dos Santos da Silva, Noemi Rovaris Gardinali, Renato Sergio Marchevsky, José Paulo Gagliardi Leite, Jaqueline Mendes de Oliveira, and Marcelo Alves Pinto. 2024. "Passive Immunotherapy of Cynomolgus Monkeys with Anti-Rotavirus IgY" Pharmaceutics 16, no. 9: 1149. https://doi.org/10.3390/pharmaceutics16091149







