An Antibacterial-Loaded PLA 3D-Printed Model for Temporary Prosthesis in Arthroplasty Infections: Evaluation of the Impact of Layer Thickness on the Mechanical Strength of a Construct and Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design and 3D Printing
2.3. Physical Characterizations
2.4. In Vitro Release Studies
2.5. Microbiological Studies
2.6. Statistical Analysis
3. Results and Discussion
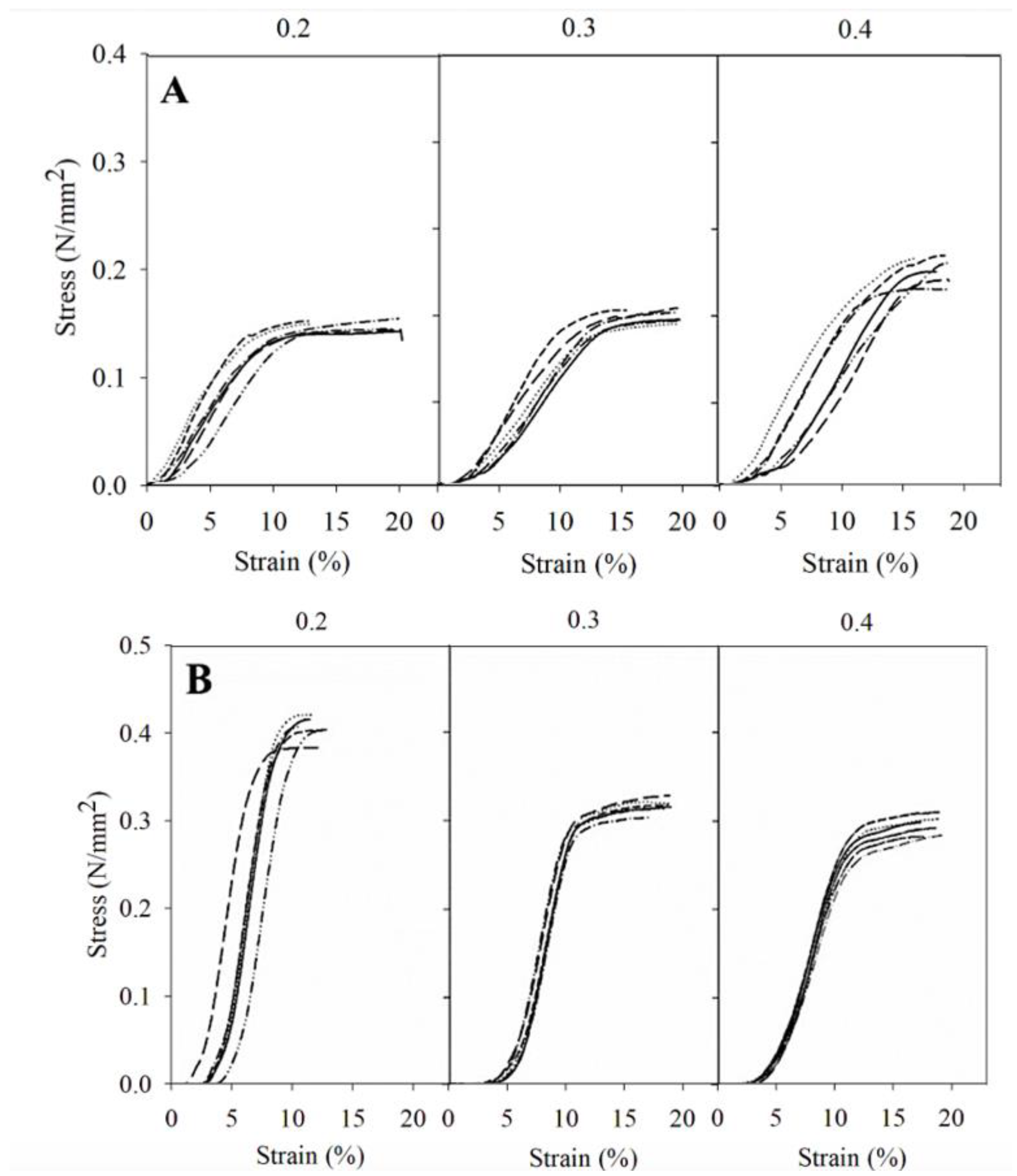
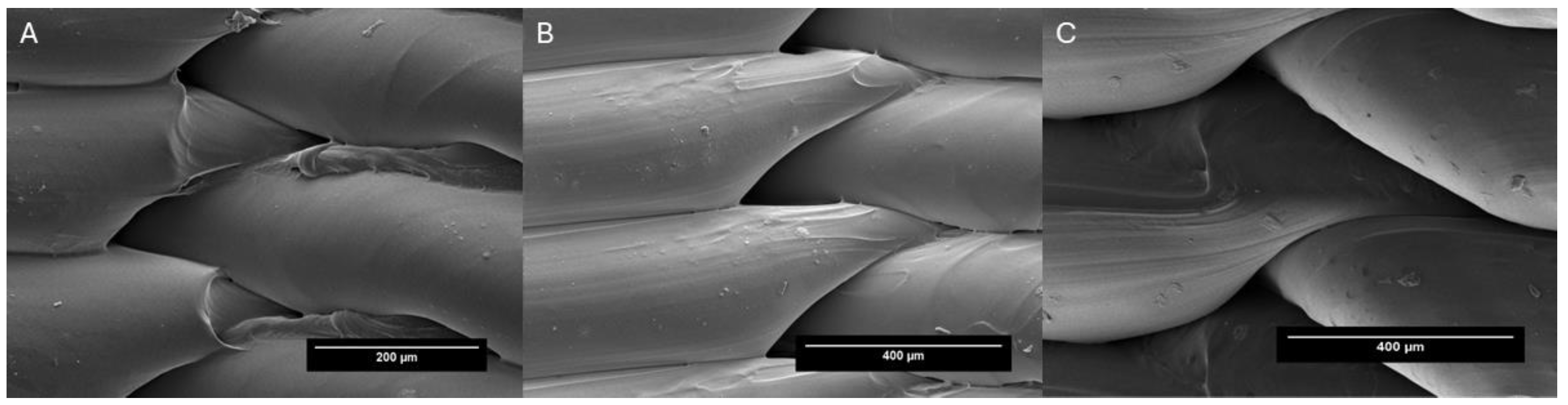
3.1. Physical Characterizations
3.2. Release Studies
3.3. Microbiological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcconoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtlif, M.; Kathju, S.; Stoodley, P. Biofilms in Periprosthetic Orthopedic Infections HHS Public Access. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Tsai, Y.; Chang, C.H.; Lin, Y.C.; Lee, S.H.; Hsieh, P.H.; Chang, Y. Different Microbiological Profiles between Hip and Knee Prosthetic Joint Infections. J. Orthop. Surg. 2019, 27, 2309499019847768. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Senn, L.; Bertelli, C.; Grandbastien, B.; Steinmetz, S.; Boillat-Blanco, N. Prevalence and Factors Associated with Prosthetic Joint Infections in Patients with Staphylococcus aureus Bacteraemia: A 7-Year Retrospective Study. Antibiotics 2022, 11, 1323. [Google Scholar] [CrossRef]
- Zardi, E.M.; Franceschi, F. Prosthetic Joint Infection. A Relevant Public Health Issue. J. Infect. Public Health 2020, 13, 1888–1891. [Google Scholar] [CrossRef]
- Tözün, I.R.; Ozden, V.E.; Dikmen, G.; Karaytuğ, K. Trends in the Treatment of Infected Knee Arthroplasty. EFORT Open Rev. 2020, 5, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; Gallina, M.; Passeri, A.; Gaudio, R.M.; Castelnuovo, N.; Ferrante, P.; Calori, G.M. Prosthetic Joint Infections and Legal Disputes: A Threat to the Future of Prosthetic Orthopedics. J. Orthop. Traumatol. 2021, 22, 44. [Google Scholar] [CrossRef]
- Dunne, N.J.; Hill, J.; McAfee, P.; Kirkpatrick, R.; Patrick, S.; Tunney, M. Incorporation of Large Amounts of Gentamicin Sulphate into Acrylic Bone Cement: Effect on Handling and Mechanical Properties, Antibiotic Release, and Biofilm Formation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 355–365. [Google Scholar] [CrossRef]
- Berberich, C.; Josse, J.; Ruiz, P.S. Patients at a High Risk of PJI: Can We Reduce the Incidence of Infection Using Dual Antibiotic-Loaded Bone Cement? Arthroplasty 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, C.; Cheng, T.; Peng, X.; Zhang, W.; Qin, H.; Zhang, X. A Systematic Review and Meta-Analysis of Antibiotic-Impregnated Bone Cement Use in Primary Total Hip or Knee Arthroplasty. PLoS ONE 2013, 8, e82745. [Google Scholar] [CrossRef]
- Xie, H.; Liu, Y.; An, H.; Yi, J.; Li, C.; Wang, X.; Chai, W. Recent Advances in Prevention, Detection and Treatment in Prosthetic Joint Infections of Bioactive Materials. Front. Bioeng. Biotechnol. 2022, 10, 1053399. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, L.; Rosso, F.; Marmotti, A.; Bonasia, D.E.; Bruzzone, M.; Rossi, R. The Use of Spacers (Static and Mobile) in Infection Knee Arthroplasty. Curr. Rev. Musculoskelet. Med. 2015, 8, 373–382. [Google Scholar] [CrossRef]
- Samelis, P.V.; Papagrigorakis, E.; Sameli, E.; Mavrogenis, A.; Savvidou, O.; Koulouvaris, P. Current Concepts on the Application, Pharmacokinetics and Complications of Antibiotic-Loaded Cement Spacers in the Treatment of Prosthetic Joint Infections. Cureus 2022, 14, e20968. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, S.; Tunç, T.; Pazarci, Ö.; Sümer, Z. Research into Biocompatibility and Cytotoxicity of Daptomycin, Gentamicin, Vancomycin and Teicoplanin Antibiotics at Common Doses Added to Bone Cement. Jt. Dis. Relat. Surg. 2020, 31, 328–334. [Google Scholar] [CrossRef]
- Cara, A.; Ballet, M.; Hemery, C.; Ferry, T.; Laurent, F.; Josse, J. Antibiotics in Bone Cements Used for Prosthesis Fixation: An Efficient Way to Prevent Staphylococcus aureus and Staphylococcus epidermidis Prosthetic Joint Infection. Front. Med. 2021, 7, 576231. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.R.; Killen, C.; Murphy, M.; Perry, M.; Romano, J.; Brown, N. The Impact of Antibiotic-Loaded Bone Cement on Antibiotic Resistance in Periprosthetic Knee Infections. CiOS Clin. Orthop. Surg. 2020, 12, 318–323. [Google Scholar] [CrossRef]
- Holleyman, R.J.; Deehan, D.J.; Walker, L.; Charlett, A.; Samuel, J.; Shirley, M.D.F.; Baker, P.N. Staphylococcal Resistance Profiles in Deep Infection Following Primary Hip and Knee Arthroplasty: A Study Using the NJR Dataset. Arch. Orthop. Trauma. Surg. 2019, 139, 1209–1215. [Google Scholar] [CrossRef]
- Mensah, L.M.; Love, B.J. A Meta-Analysis of Bone Cement Mediated Antibiotic Release: Overkill, but a Viable Approach to Eradicate Osteomyelitis and Other Infections Tied to Open Procedures. Mater. Sci. Eng. C 2021, 123, 111999. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline. Polymers 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Attaeyan, A.; Shahgholi, M.; Khandan, A. Fabrication and Characterization of Novel 3D Porous Titanium-6Al-4V Scaffold for Orthopedic Application Using Selective Laser Melting Technique. Iran. J. Chem. Chem. Eng. 2024, 43, 66–82. [Google Scholar]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D Printing Promotes the Development of Drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef] [PubMed]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization Study and Comparative in Vitro–in Vivo Hydrolysis of PLA Reinforcement Ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and Chemotherapeutic Enhanced Three-Dimensional Printer Filaments and Constructs for Biomedical Applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef]
- Benmassaoud, M.M.; Kohama, C.; Kim, T.W.B.; Kadlowec, J.A.; Foltiny, B.; Mercurio, T.; Ranganathan, S.I. Efficacy of Eluted Antibiotics through 3D Printed Femoral Implants. Biomed. Microdevices 2019, 21, 51. [Google Scholar] [CrossRef]
- Amekyeh, H.; Tarlochan, F.; Billa, N. Practicality of 3D Printed Personalized Medicines in Therapeutics. Front. Pharmacol. 2021, 12, 646836. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D Printing: An Appealing Route for Customized Drug Delivery Systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Muhammad, A.R.; Sakura, R.R.; Dwilaksana, D.; Trifiananto, M. Layer Height, Temperature Nozzle, Infill Geometry and Printing Speed Effect on Accuracy 3D Printing PETG. REM J. 2022, 7, 81–88. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Luu, D.N.; Nai, S.M.L.; Zhu, Z.; Chen, Z.; Wei, J. The Role of Powder Layer Thickness on the Quality of SLM Printed Parts. Arch. Civ. Mech. Eng. 2018, 18, 948–955. [Google Scholar] [CrossRef]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Effect of Layer Thickness and Printing Orientation on Mechanical Properties and Dimensional Accuracy of 3D Printed Porous Samples for Bone Tissue Engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Li, P.l.; Chu, F.t.; Shen, G. Influence of the Three-Dimensional Printing Technique and Printing Layer Thickness on Model Accuracy. J. Orofac. Orthop. 2019, 80, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Elkaseer, A.; Schneider, S.; Scholz, S.G. Experiment-Based Process Modeling and Optimization for High-Quality and Resource-Efficient FFF 3D Printing. Appl. Sci. 2020, 10, 2899. [Google Scholar] [CrossRef]
- Bueno-López, C.; Tamarit-Martínez, C.; Alambiaga-Caravaca, A.M.; Balaguer-Fernández, C.; Merino, V.; López-Castellano, A.; Rodilla, V. 3D Printing of Temporary Prostheses for Controlled-Release of Drugs: Design, Physical Characterization and Preliminary Studies. Pharmaceuticals 2021, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, M.; Kowalski, Ł.; Kosoń-Schab, A. Research on the Influence of Processing Parameters on the Specific Tensile Strength of FDM Additive Manufactured PET-G and PLA Materials. Polymers 2022, 14, 2446. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, M.H.; Lai, C.J.; Wang, S.H.; Zeng, Y.S.; Hsieh, C.H.; Pan, C.Y.; Huang, W.C. Effect of Printing Parameters on the Thermal and Mechanical Properties of 3d-Printed Pla and Petg, Using Fused Deposition Modeling. Polymers 2021, 13, 1758. [Google Scholar] [CrossRef]
- El-Ries, M.A.; Khaled, E.; Zidane, F.I.; Ibrahim, S.A.; Abd-Elmonem, M.S. Catalytic Spectrophotometric Determination of Iodide in Pharmaceutical Preparations and Edible Salt. Drug Test. Anal. 2012, 4, 129–135. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.L.; Yu, D.G.; Bligh, S.W.A. Electrospun Janus Beads-on-a-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef]
- Zhong, Y.; Whittington, C.F.; Zhang, L.; Haynie, D.T. Controlled Loading and Release of a Model Drug from Polypeptide Multilayer Nanofilms. Nanomedicine 2007, 3, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, B.; Li Biomaterials, B. Tunable Drug Loading and Release from Polypeptide Multilayer Nanofi Lms. Int. J. Nanomed. 2009, 4, 37–53. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Martínez-Moreno, J.; Merino, V.; Nácher, A.; Rodrigo, J.L.; Bonet Yuste, B.B.; Merino-Sanjuán, M. Bioactivity of Ceftazidime and Fluconazole Included in Polymethyl Methacrylate Bone Cement for Use in Arthroplasty. J. Arthroplast. 2017, 32, 3126–3133. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Meiabadi, M.S.; Moradi, M.; Karamimoghadam, M.; Ardabili, S.; Bodaghi, M.; Shokri, M.; Mosavi, A.H. Modeling the Producibility of 3d Printing in Polylactic Acid Using Artificial Neural Networks and Fused Filament Fabrication. Polymers 2021, 13, 3219. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of Layer Thickness and Raster Angle on the Mechanical Properties of 3D-Printed PEEK and a Comparative Mechanical Study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef]
- Syrlybayev, D.; Zharylkassyn, B.; Seisekulova, A.; Akhmetov, M.; Perveen, A.; Talamona, D. Optimisation of Strength Properties of FDM Printed Parts—A Critical Review. Polymers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Benli, M.; Eker-Gümüş, B.; Kahraman, Y.; Huck, O.; Özcan, M. Can Polylactic Acid Be a CAD/CAM Material for Provisional Crown Restorations in Terms of Fit and Fracture Strength? Dent. Mater. J. 2021, 40, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, V.S.; Kuentz, L.; Salem, A.; Halbig, M.C.; Salem, J.A.; Singh, M. Additive Manufacturing and Characterization of Metal Particulate Reinforced Polylactic Acid (Pla) Polymer Composites. Polymers 2021, 13, 3545. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, L.; Ma, C.; Niu, Q.; Wang, X. Effect of Layer Thickness on the Physical and Mechanical Properties of Sand Powder 3D Printing Specimens. Front. Earth Sci. 2021, 9, 763202. [Google Scholar] [CrossRef]
- Kuznetsov, V.E.; Solonin, A.N.; Urzhumtsev, O.D.; Schilling, R.; Tavitov, A.G. Strength of PLA Components Fabricated with Fused Deposition Technology Using a Desktop 3D Printer as a Function of Geometrical Parameters of the Process. Polymers 2018, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Bangeas, P.I.; Exadaktylos, A.; Petousis, M. Three-Dimensional Printed Polylactic Acid (PLA) Surgical Retractors with Sonochemically Immobilized Silver Nanoparticles: The Next Generation of Low-Cost Antimicrobial Surgery Equipment. Nanomaterials 2020, 10, 985. [Google Scholar] [CrossRef]
- Long, J.; Nand, A.V.; Ray, S.; Mayhew, S.; White, D.; Bunt, C.R.; Seyfoddin, A. Development of Customised 3D Printed Biodegradable Projectile for Administrating Extended-Release Contraceptive to Wildlife. Int. J. Pharm. 2018, 548, 349–356. [Google Scholar] [CrossRef]
- Ilhan, E.; Ulag, S.; Sahin, A.; Karademir, B.; Ekren, N.; Kilic, O.; Sengor, M.; Kalaskar, D.M.; Nuzhet, F.; Gunduz, O. Journal of the Mechanical Behavior of Biomedical Materials Fabrication of Tissue-Engineered Tympanic Membrane Patches Using 3D-Printing Technology. J. Mech. Behav. Biomed. Mater. 2021, 114, 104219. [Google Scholar] [CrossRef]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef]
- Liaskoni, A.; Wildman, R.D.; Roberts, C.J. 3D Printed Polymeric Drug-Eluting Implants. Int. J. Pharm. 2021, 597, 120330. [Google Scholar] [CrossRef]
- Chunate, H.-T.; Khamwannah, J.; Azeez, A.; Aliyu, A.; Tantavisut, S.; Puncreobutr, C.; Khamkongkaeo, A.; Tongyam, C.; Tumkhanon, K.; Phetrattanarangsi, T.; et al. Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols. Materials 2021, 14, 6576. [Google Scholar] [CrossRef]
- Patel, S.K.; Khoder, M.; Peak, M.; Alhnan, M.A. Controlling Drug Release with Additive Manufacturing-Based Solutions. Adv. Drug Deliv. Rev. 2021, 174, 369–386. [Google Scholar] [CrossRef]
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A Review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2016, 23, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, H.; Han, H.; Shen, Y.; Gu, S.; He, Y.; Guo, S. 3D Printing and Coating to Fabricate a Hollow Bullet-Shaped Implant with Porous Surface for Controlled Cytoxan Release. Int. J. Pharm. 2018, 552, 91–98. [Google Scholar] [CrossRef]
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D Printing of Multi-Compartmental PVA Capsules for Drug Delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686. [Google Scholar] [CrossRef]
- Sharma, V.; Shaik, K.M.; Choudhury, A.; Kumar, P.; Kala, P.; Sultana, Y.; Shukla, R.; Kumar, D. Investigations of Process Parameters during Dissolution Studies of Drug Loaded 3D Printed Tablets. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Bataille, B.; Aubert, A.; Rossi, J.C.; Soulairol, I. A Qbd Approach for Evaluating the Effect of Selective Laser Sintering Parameters on Printability and Properties of Solid Oral Forms. Pharmaceutics 2021, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Mastnak, T.; Mihelič, M.; Maver, U.; Finšgar, M. Clindamycin-Based 3D-Printed and Electrospun Coatings for Treatment of Implant-Related Infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef]
- Aldrich, A.; Kuss, M.A.; Duan, B.; Kielian, T. 3D Bioprinted Scaffolds Containing Viable Macrophages and Antibiotics Promote Clearance of Staphylococcus aureus Craniotomy-Associated Biofilm Infection HHS Public Access. ACS Appl. Mater. Interfaces 2019, 11, 12298–12307. [Google Scholar] [CrossRef]
- Mai, H.N.; Hyun, D.C.; Park, J.H.; Kim, D.Y.; Lee, S.M.; Lee, D.H. Antibacterial Drug-Release Polydimethylsiloxane Coating for 3d-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects. Pharmaceuticals 2020, 13, 304. [Google Scholar] [CrossRef]






| Printing temperature | 200 °C |
| Platform temperature | 60 °C |
| Print speed | 60 mm/s |
| Layer thickness | 0.2, 0.3, and 0.4 mm |
| Infill | 10% |
| Small cylinder | Height: 11.00 mm Diameter: 11.80 mm External wall thickness: 1.35 mm Total volume: 1.20 cm3 Capacity up to fill: 268 µL |
| Large cylinder | Height: 30.14 mm Diameter: 31.26 mm External wall thickness: 1.37 mm Total volume: 23.12 cm3 Total capacity up to fill: 16.5 mL |
| Construct Size | Orientation Test | Layer Thickness (mm) | Breaking Load (Mean ± sd) (Kp) | Compression (Mean ± SD) (mm) |
|---|---|---|---|---|
| 23.12 cm3 | Horizontal | 0.2 | 137.1 ± 5.9 | 3.6 ± 0.9 |
| 0.3 | 140.0 ± 17.7 | 1.8 ± 0.6 | ||
| 0.4 | 244.4 ± 23.2 | 3.2 ±0.9 | ||
| Vertical | 0.2 | 490.6 ± 7.4 | 0.8 ± 0.1 | |
| 0.3 | 500.3 ± 2.3 | 0.9 ± 0.2 | ||
| 0.4 | 472.5 ± 13.9 | 1.2 ± 0.1 | ||
| 1.20 cm3 | Horizontal | 0.2 | 92.6 ± 5.1 | 1.5 ± 0.3 |
| 0.3 | 99.0 ± 3.8 | 1.6 ± 0.1 | ||
| 0.4 | 125.0 ± 7.7 | 1.6 ± 0.1 | ||
| Vertical | 0.2 | 202.5 ± 6.3 | 1.0 ± 0.1 | |
| 0.3 | 158.1 ± 4.1 | 1.5 ± 0.1 | ||
| 0.4 | 147.4 ± 5.4 | 1.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamarit-Martínez, C.; Bernat-Just, L.; Bueno-López, C.; Alambiaga-Caravaca, A.M.; Merino, V.; López-Castellano, A.; Rodilla, V. An Antibacterial-Loaded PLA 3D-Printed Model for Temporary Prosthesis in Arthroplasty Infections: Evaluation of the Impact of Layer Thickness on the Mechanical Strength of a Construct and Drug Release. Pharmaceutics 2024, 16, 1151. https://doi.org/10.3390/pharmaceutics16091151
Tamarit-Martínez C, Bernat-Just L, Bueno-López C, Alambiaga-Caravaca AM, Merino V, López-Castellano A, Rodilla V. An Antibacterial-Loaded PLA 3D-Printed Model for Temporary Prosthesis in Arthroplasty Infections: Evaluation of the Impact of Layer Thickness on the Mechanical Strength of a Construct and Drug Release. Pharmaceutics. 2024; 16(9):1151. https://doi.org/10.3390/pharmaceutics16091151
Chicago/Turabian StyleTamarit-Martínez, Carlos, Lucía Bernat-Just, Carlos Bueno-López, Adrián M. Alambiaga-Caravaca, Virginia Merino, Alicia López-Castellano, and Vicent Rodilla. 2024. "An Antibacterial-Loaded PLA 3D-Printed Model for Temporary Prosthesis in Arthroplasty Infections: Evaluation of the Impact of Layer Thickness on the Mechanical Strength of a Construct and Drug Release" Pharmaceutics 16, no. 9: 1151. https://doi.org/10.3390/pharmaceutics16091151
APA StyleTamarit-Martínez, C., Bernat-Just, L., Bueno-López, C., Alambiaga-Caravaca, A. M., Merino, V., López-Castellano, A., & Rodilla, V. (2024). An Antibacterial-Loaded PLA 3D-Printed Model for Temporary Prosthesis in Arthroplasty Infections: Evaluation of the Impact of Layer Thickness on the Mechanical Strength of a Construct and Drug Release. Pharmaceutics, 16(9), 1151. https://doi.org/10.3390/pharmaceutics16091151









