Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024
Abstract
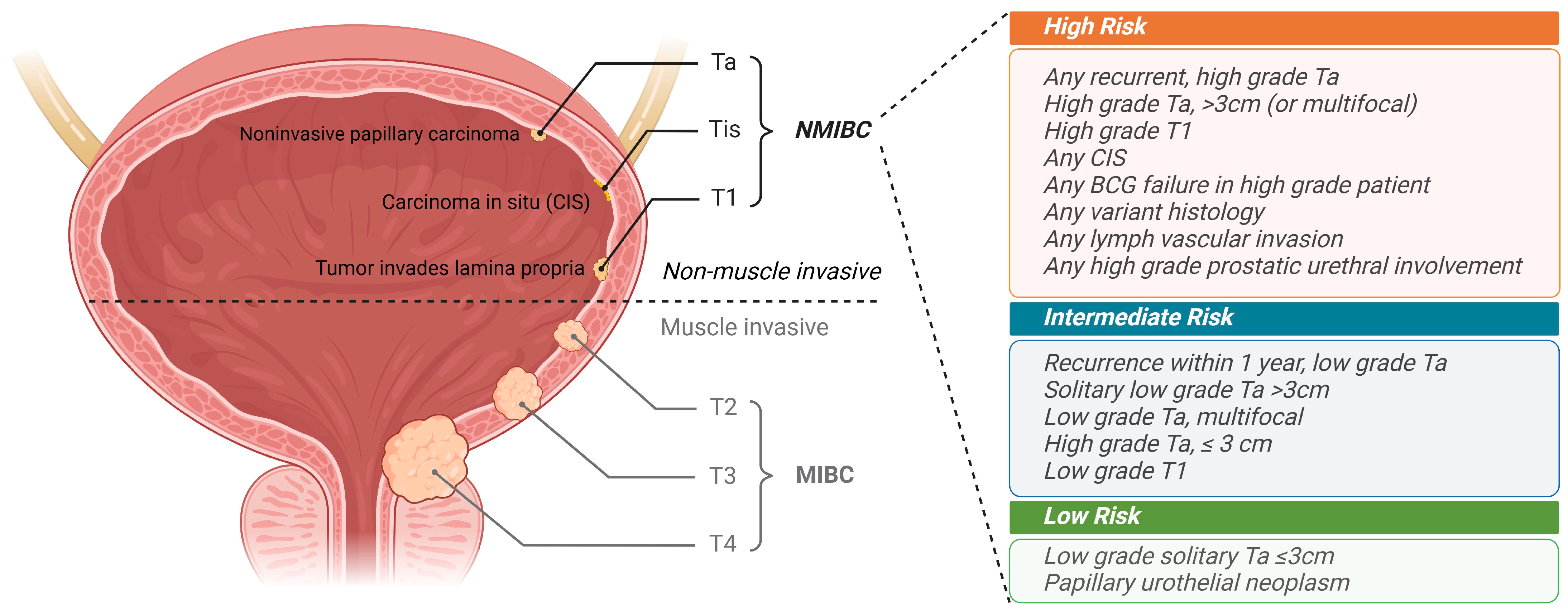
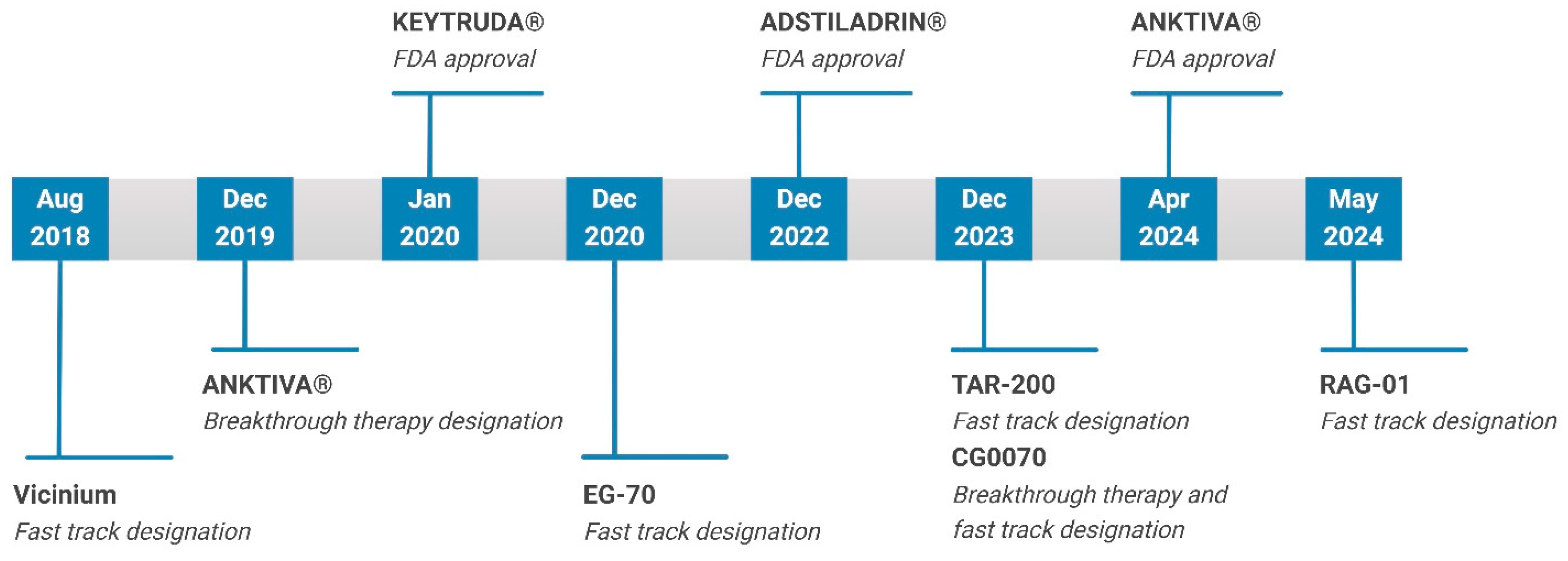
:1. Introduction
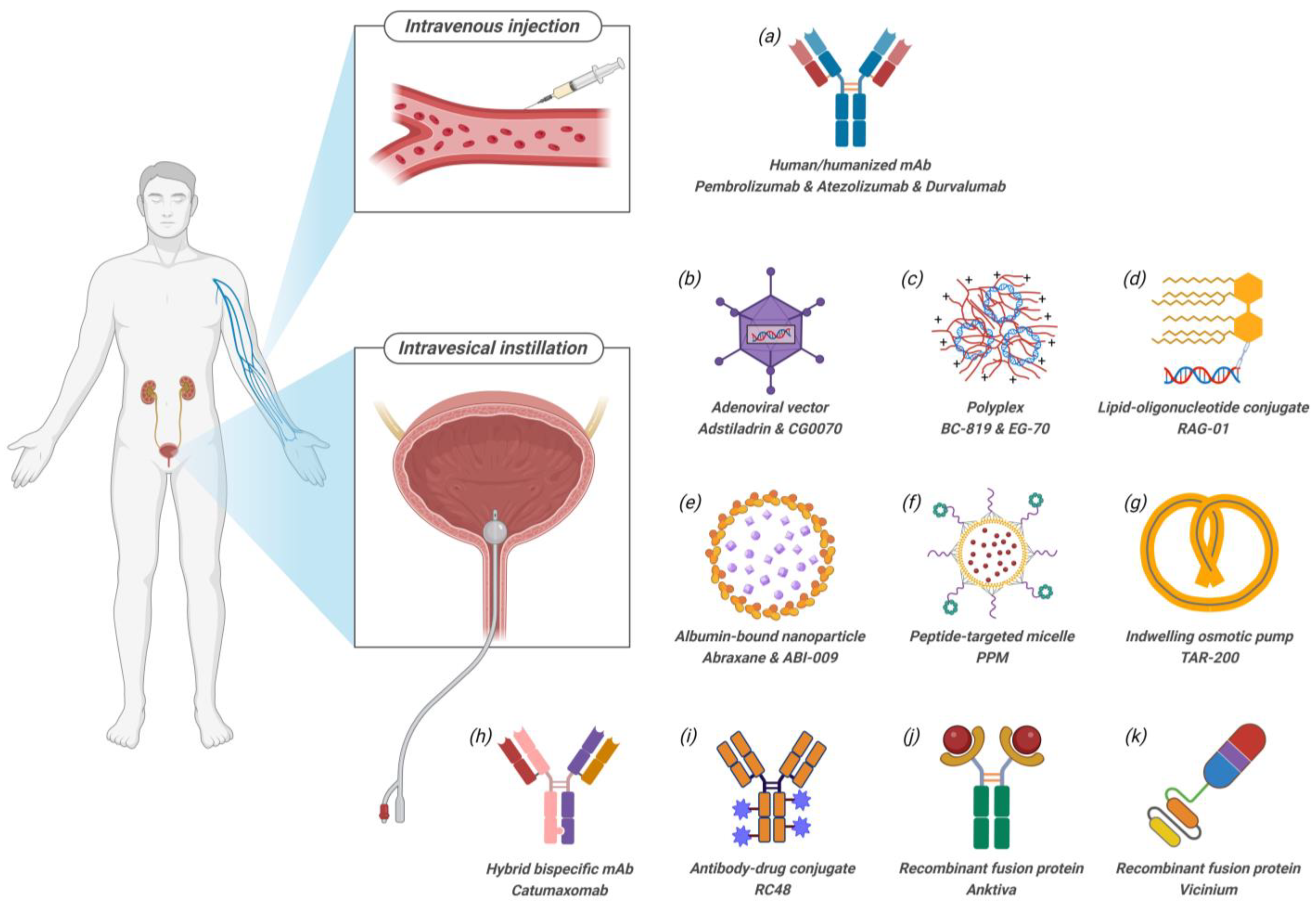
2. Route of Administration (RoA)
3. Small Molecule Drug Formulations
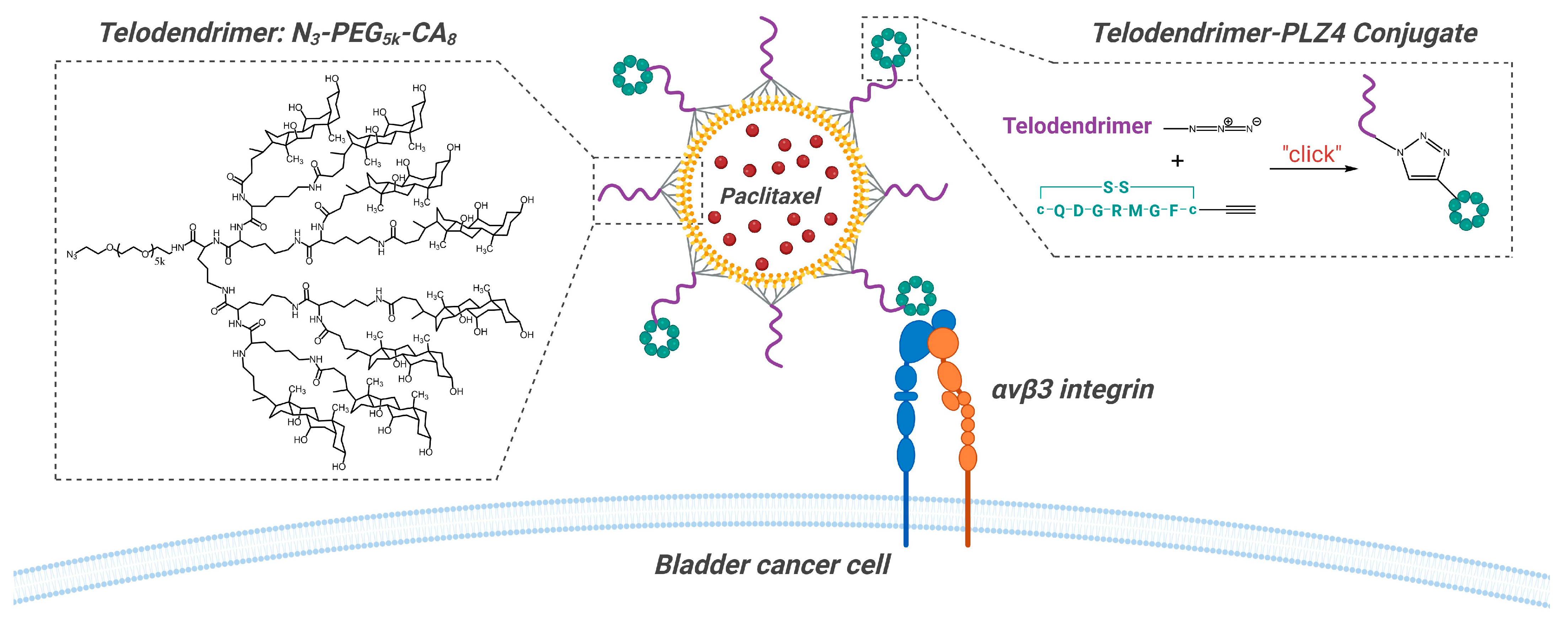
3.1. Peptide-Targeted Micelles
3.2. Albumin-Based Nanoparticles
3.3. Indwelling Device
4. Antibody-Based Therapies and Conjugates
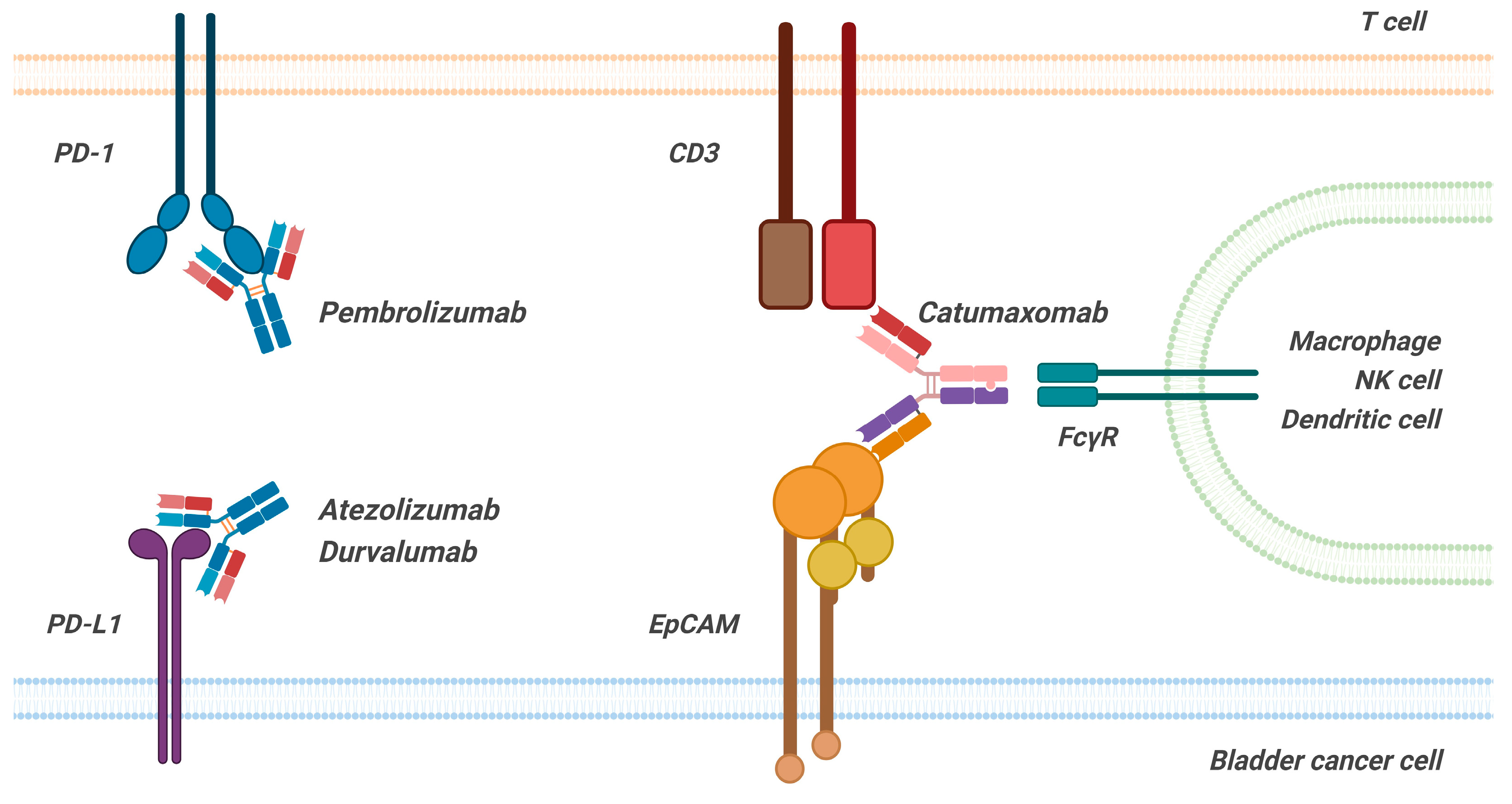
4.1. Human/Humanized Monoclonal Antibodies (mAbs)
4.2. Hybrid Bispecific Monoclonal Antibody
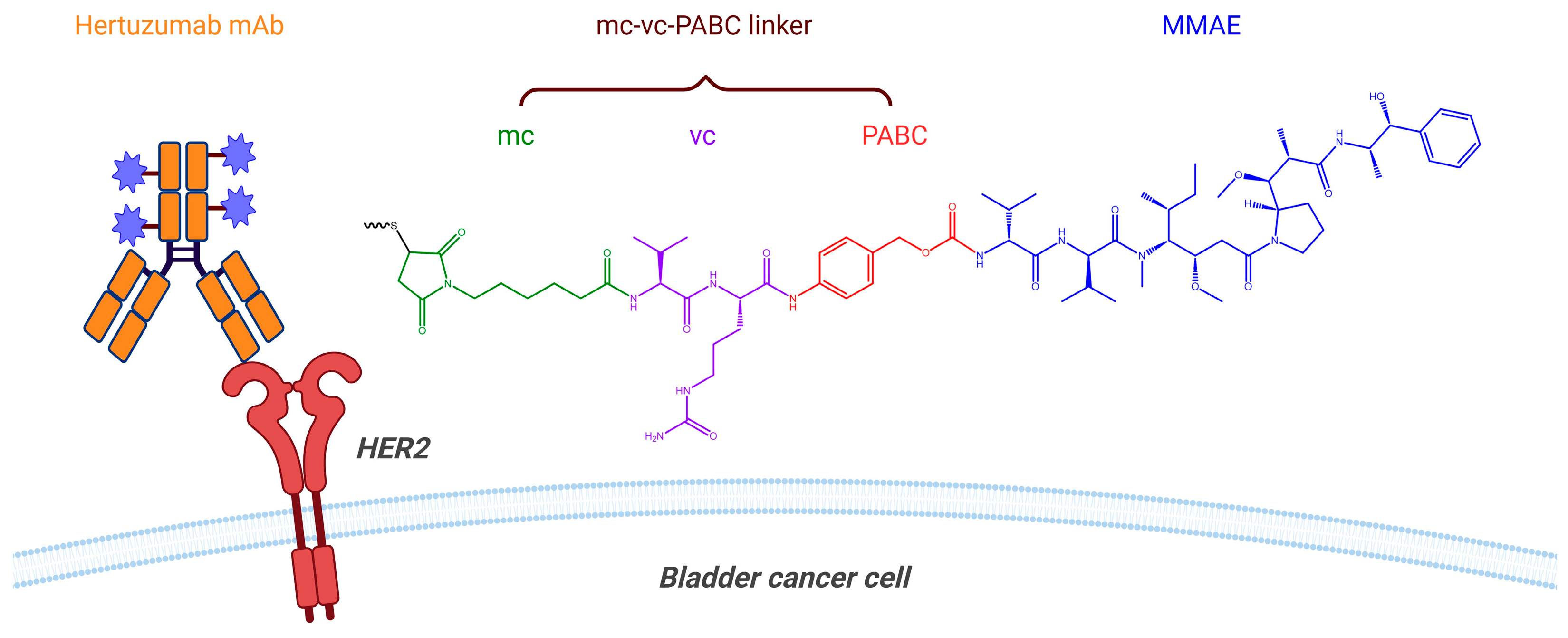
4.3. Antibody–Drug Conjugate (ADC)
5. Recombinant Fusion Protein
5.1. Cytokine–Fusion Protein Complex
5.2. Single-Chain Variable Fragment (scFv)-Targeted Toxin
6. Nucleic Acid Therapeutics
6.1. Adenoviral Vectors
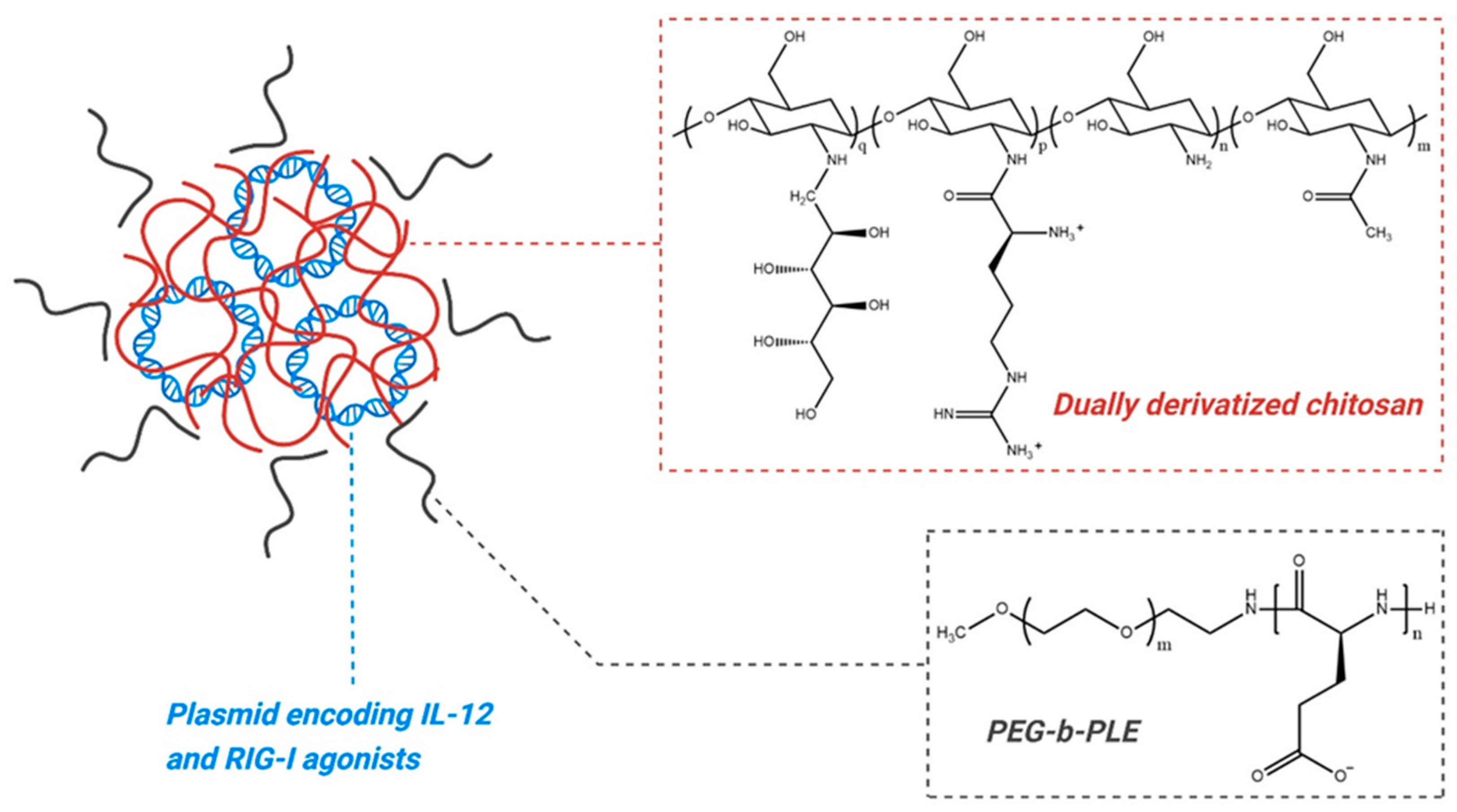
6.2. Plasmid Polyplex
6.3. Oligonucleotide–Lipid Conjugate
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ADC | antibody–drug conjugate |
| Ad5 | serotype 5 adenovirus |
| BCG | Bacillus Calmette–Guérin |
| BsAb | bispecific antibody |
| CA | cholic acid |
| CAR | coxsackie/adenovirus receptor |
| CHO | Chinese hamster ovary |
| CIS | carcinoma in situ |
| CR | complete response |
| DDM | n-Dodecyl β-D-maltoside |
| DDX | dually derivatized oligochitosan |
| DOR | duration of response |
| DTA | diphtheria toxin A |
| EpCAM | epithelial cellular adhesion molecule |
| GAG | glycosaminoglycan |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HG | high grade |
| HGRF | high-grade recurrence-free |
| IL-15 | interleukin-15 |
| LG | low grade |
| LVI | lymph vascular invasion |
| mAb | monoclonal antibody |
| MIBC | muscle-invasive bladder cancer |
| MMAE | monomethyl auristatin E |
| MTD | maximum tolerated dose |
| Nab | nanoparticle albumin-bound |
| NMIBC | non-muscle invasive bladder cancer |
| PABC | para-amino benzyloxycarbonyl |
| PEI | polyethylenimine |
| PEG | polyethylene glycol |
| PEG-b-PLE | polyethylene glycol–polyglutamic acid |
| PPM | PLZ4-coated paclitaxel-loaded micelle |
| PUNLMP | papillary urothelial neoplasm of low malignant potential |
| RC | radical cystectomy |
| RIG-I | retinoic acid-inducible gene I |
| RoA | route of administration |
| RP2D | recommended phase 2 dose |
| saRNA | small activating RNA |
| SAEs | serious adverse events |
| scFv | single-chain variable fragment |
| Tregs | regulatory T cells |
| Syn3 | [N-(3-cholamidopropyl)-N-(3-lactobionamidopropyl)]-cholamide |
| TRAEs | treatment-related adverse events |
| TURBT | transurethral resection of bladder tumor |
| vc | valine-citrulline |
| vp | viral particles |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Tempo, J.; Bolton, D.; O’callaghan, M. Seminal papers in urology: Maintenance Bacillus Calmette-Guerin (BCG) immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study (SWOG-8507). BMC Urol. 2023, 23, 194. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.M.; Brausi, M.; Lamm, D.; O’donnell, M.; Tomita, K.; Woo, H.; Jewett, M.A. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 2005, 66, 108–125. [Google Scholar] [CrossRef]
- Chehroudi, A.C.; Black, P.C. Emerging intravesical therapies for the management of bacillus Calmette-Guerin (BCG)-unresponsive non-muscle-invasive bladder cancer: Charting a path forward. Can. Urol. Assoc. J. 2020, 14, 204–213. [Google Scholar] [CrossRef]
- BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry. Available online: https://www.fda.gov/media/101468/download (accessed on 7 July 2024).
- Nguyen, V.H.; Khan, F.; Shipman, B.M.; Neugent, M.L.; Hulyalkar, N.V.; Cha, N.Y.; Zimmern, P.E.; De Nisco, N.J. A Semi-Quantitative Assay to Measure Glycosaminoglycan Degradation by the Urinary Microbiota. Front. Cell. Infect. Microbiol. 2022, 11, 803409. [Google Scholar] [CrossRef]
- Jafari, N.V.; Rohn, J.L. The urothelium: A multi-faceted barrier against a harsh environment. Mucosal Immunol. 2022, 15, 1127–1142. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Pan, C.-X.; Lin, T.-Y.; Zhang, H.; Luo, J.; Li, Y.; Gao, T.; Lara, P.N., Jr.; White, R.d.V.; Lam, K.S. Multifunctional targeting micelle nanocarriers with both imaging and therapeutic potential for bladder cancer. Int. J. Nanomed. 2012, 7, 2793–2804. [Google Scholar] [CrossRef]
- Lin, T.Y.; Li, Y.P.; Zhang, H.; Luo, J.; Goodwin, N.; Gao, T.; White, R.D.; Lam, K.S.; Pan, C.X. Tumor-Targeting Multi-functional Micelles for Imaging and Chemotherapy of Advanced Bladder Cancer. Nanomedicine 2013, 8, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, H.; Zhu, Z.; Risco, J.G.; Lam, K.S.; Pan, C.-X. A phase I dose-escalation clinical trial with PLZ4-coated paclitaxel-loaded micelles (PPM) in patients with recurrent or refractory non-myoinvasive bladder cancer. J. Clin. Oncol. 2023, 41, TPS4615. [Google Scholar] [CrossRef]
- Loureiro, A.; Azoia, N.G.; Gomes, A.C.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. nab-Paclitaxel mechanisms of action and delivery. J. Control. Release 2013, 170, 365–372. [Google Scholar] [CrossRef] [PubMed]
- ABRAXANE Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf (accessed on 7 July 2024).
- Hu, H.; Niu, Y.; Li, H.Z.; Shen, C.; Huang, S.; Qie, Y.; Wu, Z.; Zhao, G.; Zhang, Z.; Jia, K.; et al. A Novel Approach to the Treatment of High-Risk Non–Muscle-Invasive Bladder Cancer: Combining Immune Checkpoint Inhibitor (ICI) and Micro-tubule Inhibitor (MTI). J. Clin. Oncol. 2023, 41, e16596. [Google Scholar] [CrossRef]
- FARRO Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213312lbl.pdf (accessed on 7 July 2024).
- Phase 1/2 Study of ABI-009 in Nonmuscle Invasive Bladder Cancer. Available online: https://clinicaltrials.gov/study/NCT02009332 (accessed on 23 August 2024).
- Grimberg, D.C.; Shah, A.; Inman, B.A. Overview of Taris GemRIS, a Novel Drug Delivery System for Bladder Cancer. Eur. Urol. Focus 2020, 6, 620–622. [Google Scholar] [CrossRef]
- Tan, W.S.; Kelly, J.D. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2018, 15, 667–685. [Google Scholar] [CrossRef]
- Necchi, A.; Daneshmand, S.; Simone, G.; Xylinas, E.; Morris, D.S.; Spiegelhalder, P.; Zainfeld, D.; Won Kang, T.; Matulay, J.T.; Belkoff, L.H.; et al. TAR-200 in Patients with Bacillus Calmette–Guérin-Unresponsive High-Risk Non–Muscle-Invasive Bladder Cancer: Results from SunRISe-1 Study. J Urol. 2024, 211, e1. [Google Scholar] [CrossRef]
- KEYTRUDA Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf (accessed on 7 July 2024).
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Black, P.C.; Tangen, C.M.; Singh, P.; McConkey, D.J.; Lucia, M.S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.J.; Kassouf, W.; et al. Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin–Unresponsive High-Risk Non–Muscle-Invasive Bladder Cancer: SWOG S1605. Eur. Urol. 2023, 84, 536–544. [Google Scholar] [CrossRef]
- Inman, B.A.; Hahn, N.M.; Stratton, K.; Kopp, R.; Sankin, A.; Skinner, E.; Pohar, K.; Gartrell, B.A.; Pham, S.; Rishipathak, D.; et al. A Phase 1b/2 Study of Atezolizumab with or Without Bacille Calmette-Guérin in Patients with High-risk Non–muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2023, 6, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hahn, N.M.; O’Donnell, M.A.; Efstathiou, J.A.; Zahurak, M.; Rosner, G.L.; Smith, J.; Kates, M.R.; Bivalacqua, T.J.; Tran, P.T.; Song, D.Y. A Phase 1 Trial of Durvalumab in Combination with Bacillus Calmette-Guerin (BCG) or External Beam Radiation Therapy in Patients with BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer: The Hoosier Cancer Research Network GU16-243 ADAPT-BLADDER Study. Eur. Urol. 2023, 83, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Meghani, K.; Cooley, L.F.; Choy, B.; Kocherginsky, M.; Munir, S.S.; Svatek, R.S.; Kuzel, T.; Meeks, J.J. First-in-Human In-travesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur. Urol. 2022, 82, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, D.; Lindhofer, H.; Bokemeyer, C. Development and Approval of the Trifunctional Antibody Catumaxomab (An-ti-EpCAM×anti-CD3) as a Targeted Cancer Immunotherapy. Cancer Treat. Rev. 2010, 36, 458–467. [Google Scholar] [CrossRef]
- Ruf, P.; Bauer, H.W.; Schoberth, A.; Kellermann, C.; Lindhofer, H. First time intravesically administered trifunctional antibody catumaxomab in patients with recurrent non-muscle invasive bladder cancer indicates high tolerability and local immunological activity. Cancer Immunol. Immunother. 2021, 70, 2727–2735. [Google Scholar] [CrossRef]
- Removab Package Insert. Available online: https://ec.europa.eu/health/documents/community-register/2010/2010031575509/anx_75509_en.pdf (accessed on 7 July 2024).
- Albert, F.; Antoniewicz, A.; Grantzow, T.; Fingerle-rowson, G.; Ruf, P. Intravesical Therapy with the Trifunctional Anti-EpCAM/CD3 BsAb Catumaxomab Is Well Tolerated and Shows Encouraging Preliminary Efficacy in Patients with High-Risk NMIBC (CATUNIBLA Phase I Trial). J. Clin. Oncol. 2022, 40, e16555. [Google Scholar] [CrossRef]
- Deeks, E.D. Disitamab Vedotin: First Approval. Drugs 2021, 81, 1929–1935. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC Linker Chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef]
- Hu, H.; Guo, S.; Huang, S.; Jia, K.; Li, P.; Shen, C.; Zhang, Z.; Niu, Y. MP16-09 TRUCE04: A phase II clinical trial of RC48 for high-risk non-muscle-invasive bladder cancer (HR-NMIBC) (NCT05495724). J. Urol. 2024, 211, e243. [Google Scholar] [CrossRef]
- Han, K.-P.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.-A.; Romero, C.A.; et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011, 56, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, N.; Yuan, Y.; Zhu, M.; Hu, X.; Hu, W.; Wang, S.; Wang, C.; Huang, B.; Xing, D. ALT-803 in the treatment of non-muscle-invasive bladder cancer: Preclinical and clinical evidence and translational potential. Front. Immunol. 2022, 13, 1040669. [Google Scholar] [CrossRef] [PubMed]
- ANKTIVA Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761336s000lbl.pdf (accessed on 7 July 2024).
- Di Paolo, C.; Willuda, J.; Kubetzko, S.; Lauffer, I.; Tschudi, D.; Waibel, R.; Plückthun, A.; Stahel, R.A.; Zangemeister-Wittke, U. A Recombinant Immunotoxin Derived from a Humanized Epithelial Cell Adhesion Molecule-Specific Single-Chain Antibody Fragment Has Potent and Selective Antitumor Activity. Clin. Cancer Res. 2003, 9, 2837–2848. [Google Scholar] [PubMed]
- Havaei, S.M.; Aucoin, M.G.; Jahanian-Najafabadi, A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells with the Toxin. Front. Oncol. 2021, 11, 781800. [Google Scholar] [CrossRef]
- O’Donnell, M.; Shore, N.; Keane, T.; Jewett, M.; Dickstein, R.; Wolk, F.; Dillon, R.L.; Cizeau, J.; Kassouf, W. MP16-03 Phase 3 Study of Vicineum in Bcg-Unresponsive Non-Muscle Invasive Bladder Cancer: 24-Month Results. J. Urol. 2021, 206, 296–297. [Google Scholar] [CrossRef]
- Rivera-Marquez, G.; Walter, B.; Dolan, R.; Belfield, S.; Merino, M.; Gurram, S.; Apolo, A.; Niglio, S.; Agarwal, P.; Valera, V.A. Mp16-20 Final Results of a Phase I, Single-Arm Clinical Trial of the Combination of Durvalumab and Vicineum in Bcg Unresponsive, High-Risk Non-Muscle-Invasive Bladder Cancer Patients (Nct03258593). J. Urol. 2024, 211, 248. [Google Scholar] [CrossRef]
- Lee, A. Nadofaragene Firadenovec: First Approval. Drugs 2023, 83, 353–357. [Google Scholar] [CrossRef]
- Narayan, V.M.; Meeks, J.J.; Jakobsen, J.S.; Shore, N.D.; Sant, G.R.; Konety, B.R. Mechanism of action of nadofaragene firadenovec-vncg. Front. Oncol. 2024, 14, 1359725. [Google Scholar] [CrossRef]
- ADSTILADRIN Package Insert. Available online: https://www.fda.gov/media/164029/download (accessed on 7 July 2024).
- Narayan, V.M.; Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; et al. Efficacy of Intravesical Nadofaragene Firadenovec for Patients with BCG-Unresponsive Non–Muscle Invasive Bladder Cancer: 5 Year Follow-Up from a Phase 3 Trial. J. Urol. 2024, 212, 74–86. [Google Scholar] [CrossRef]
- Ramesh, N.; Ge, Y.; Ennist, D.L.; Zhu, M.; Mina, M.; Ganesh, S.; Reddy, P.S.; Yu, D.-C. CG0070, a Conditionally Replicating Granulocyte Macrophage Colony-Stimulating Factor–Armed Oncolytic Adenovirus for the Treatment of Bladder Cancer. Clin. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef]
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A First in Human Phase 1 Study of CG0070, a GM-CSF Expressing Oncolytic Adenovirus, for the Treatment of Nonmuscle Invasive Bladder Cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shah, P.H.; Stewart, T.F.; Nam, J.K.; Bivalacqua, T.J.; Lamm, D.L.; Uchio, E.M.; Geynisman, D.M.; Jacob, J.M.; Meeks, J.J.; et al. Oncolytic Adenoviral Therapy plus Pembrolizumab in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer: The Phase 2 CORE-001 Trial. Nat. Med. 2024, 30, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Tyson, M.D.; Uchio, E.; Nam, J.-K.; Lamm, D.L.; Bivalacqua, T.J.; Shore, N.D.; Kassouf, W.; Steinberg, G.D.; Black, P.C.; Kamat, A.M.; et al. P2-02 pivotal results from BOND-003: A phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for the treatment of high risk, BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 2024, 211, e1. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Codex: Study of Inodiftagene Vixteplasmid (BC-819) in Unresponsive NMIBC. Available online: https://clinicaltrials.gov/study/NCT03719300?tab=results (accessed on 7 July 2024).
- Panicker, R.K.G.; Veilleux, D.; Ma, P.L.; Tam, N.C.M.; Fleet, C.; Cheung, A.; Dauphinee, S.; Chen, X.; Lora, J. Chitosan Polyplex-Based Localized Expression of IL-12 Alone or in Combination with Type-I IFN Inducers for Treatment of Mucosal Cancers. Patent No. WO2020183239A1, 17 September 2020. [Google Scholar]
- Kalota, S.; Joshi, S.; Bui, M.; Dickstein, R.; Liu, J.J.; Lotan, Y.; Johnson, S.; Taylor, J.; Bryce, R.; Tosone, C.; et al. P2-08 Legend: A Phase 1/2 Study of Eg-70 (Detalimogene Voraplasmid), a Novel, Non-Viral Intravesical Gene Therapy for Patients with Bcg-Unresponsive Non-Muscle Invasive Bladder Cancer with Carcinoma in Situ (Cis). J. Urol. 2024, 211, e5. [Google Scholar] [CrossRef]
- Kingwell, K. Small activating RNAs lead the charge to turn up gene expression. Nat. Rev. Drug Discov. 2021, 20, 573–574. [Google Scholar] [CrossRef]
- A Study of RAG-01 in Patients with Non-Muscle-Invasive Bladder Cancer (NMIBC) Who Have Failed Bacillus Calmette Guérin (BCG) Therapy. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06351904 (accessed on 7 July 2024).

| Drug | Trial ID | CR | DOR | TRAEs |
|---|---|---|---|---|
| Pembrolizumab * [24] | KEYNOTE-057 NCT02625961 Phase 2 | 3-Month CR rate = 41% (39 of 96) | Median DOR = 16.2 Mo DOR ≥ 12 Mo = 46% (18 of 39) | Grade 3–4 = 13% (13 of 101) Serious TRAEs = 8% (8 of 101) |
| Atezolizumab [25] | SWOG S1605 NCT02844816 Phase 2 | 6-Month CR rate = 27% (20 of 74) | Median DOR = 17 Mo DOR ≥ 12 Mo = 56% (11 of 20) | Grade 3–5 = 16% (26 of 166) |
| Atezolizumab [26] | NCT02792192 Phase 1b/2 | 6-Month CR rate = 33% (4 of 12) | Median DOR = 6.8 Mo | Grade 3 = 25% (3 of 12) |
| Durvalumab [27] | ADAPT-BLADDER NCT03317158 Phase 1/2 | 3-Month CR rate = 33% (1 of 3) | DOR ≥ 12 Mo = 0% (0 of 1) | Not reported |
| KEYTRUDA | ADSTILADRIN | ANKTIVA | |
|---|---|---|---|
| Approval Year | 2020 | 2022 | 2024 |
| Drug Substance | mAb | DNA Plasmid | Recombinant Fusion Protein |
| Mechanism | PD-1 Checkpoint Inhibitor | Secreting IFNα2b | IL-15 superagonist |
| RoA | Intravenous Injection | Intravesical Instillation | Intravesical Instillation |
| Delivery System | N/A | Adenoviral Vector | Fusion Protein Complex |
| Clinical Trial | NCT02625961 (KEYNOTE-057) | NCT02273849 (CS-003) | NCT03022825 (QUILT-3.032) |
| CR Any Time | 41% (39/96) | 51% (50/98) | 62% (48/77) |
| DOR ≥ 12 Month | 46% (18/39) | 46% (23/50) | 58% (28/48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Darji, S.; Thompson, D.H. Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024. Pharmaceutics 2024, 16, 1154. https://doi.org/10.3390/pharmaceutics16091154
Qu F, Darji S, Thompson DH. Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024. Pharmaceutics. 2024; 16(9):1154. https://doi.org/10.3390/pharmaceutics16091154
Chicago/Turabian StyleQu, Feng, Saloni Darji, and David H. Thompson. 2024. "Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024" Pharmaceutics 16, no. 9: 1154. https://doi.org/10.3390/pharmaceutics16091154
APA StyleQu, F., Darji, S., & Thompson, D. H. (2024). Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024. Pharmaceutics, 16(9), 1154. https://doi.org/10.3390/pharmaceutics16091154







