Lipid-Based Nanocarriers: Bridging Diagnosis and Cancer Therapy
Abstract
:1. Introduction
2. Key Parameters in Lipid Nanoparticles Design for Theranostics
2.1. Structure, Design, and Geometry of Nanoparticles
2.2. Surface Chemistry
2.3. Surface Functionalization Strategies
- (1)
- Direct attachment of the ligand to pre-existing nanocarriers. The direct attachment of ligands to existing nanocarriers is a method in which the ligands are bound to the nanocarrier surface via lipid heads or PEG chains. This technique relies on a series of chemical reactions for covalent bonding, such as the formation of amide bonds, the formation of thioester bonds through the addition of maleimide–thiol, the construction of disulfide bridges, hydrazone linkage, and the utilization of biorthogonal chemistry. This strategy has a major advantage because it does not require the alteration of the nanocarriers’ composition before the conjugation process. However, it requires reactive groups on the nanoparticle surface to establish a covalent bond [73,74].
- (2)
- Incorporating a lipid–PEG–ligand complex during the nanocarrier formulation. In this protocol, first, the lipid–PEG–ligand complex is synthesized. Following this, the conjugate is combined with structural lipids using a one-pot assembly technique in order to produce targeted lipid nanoparticles. This method facilitates the practical adjustment of ligand density by regulating the proportion of targeting ligands incorporated. However, it has been demonstrated that a significant fraction of targeting ligands end up facing the internal cavities of the lipid nanoparticles, making them inaccessible for active targeting purposes [73,74,75,76].
- (3)
- Post-insertion of lipid–PEG–ligand micelles. This strategy for the preparation of targeted lipid nanoparticles is advantageous for its simplicity and the stability it confers to the nanoparticles. It is based on the amphiphilicity of the targeted ligands, whose hydrophobic chains can be incorporated into the lipid bilayers of preformed nanoparticles, while the hydrophilic heads remain exposed to the aqueous environment. The process, commonly referred to as the post-insertion method, involves the incubation of preformed nanoparticles with micelles formed by PEG–lipids, which are amphiphilic compounds that self-assemble into micelles above their critical micelle concentration (CMC). Under controlled conditions, PEG–lipid conjugates can be transferred into the lipid layer of the nanoparticles. After incorporating the PEG–lipid, a chemical reaction can be used to covalently attach the ligands to the nanoparticle surface, ensuring the stability and specificity of the targeting. However, the implementation of such a protocol may result in the existence of residual reactive end groups on the surface that may cross-link and, consequently, facilitate a more expeditious elimination from the blood circulation. Furthermore, unreacted groups located on the internal surface of the particles may undergo undesirable interactions with drug molecules or other lipid components. Hence, an alternative approach involves the pre-attachment of the ligand to the PEG–lipid prior to its incorporation into lipid-based nanoparticles. This approach can prove to be more advantageous as it guarantees the presence of the ligand on the nanoparticle surface. This method is particularly beneficial because it allows for the optimization of ligand insertion conditions separately from drug preparation and loading, leading to high conjugation efficiency and ensuring that ligands are positioned on the external surfaces of lipid-based nanoparticles [73,74,75,77].
- (4)
- Noncovalent adsorption of the ligand onto the surfaces of lipid-based nanocarriers. This method uses techniques such as physical adsorption or ionic bonding to functionalize lipid-based nanocarriers. In physical adsorption, ligands attach to the surface through weak interaction forces, such as electrostatic interactions, hydrogen bonds, hydrophobic interactions, and van der Waals forces. The advantages of noncovalent adsorption encompass rapid functionalization, versatility in ligand types, and reversibility. However, the stability of noncovalent adsorption is a major concern, especially for in vivo applications where lipid-based nanocarriers must maintain functionality in complex and dynamic environments. Conjugation techniques, which generate stronger and more durable bonds between the ligand and the nanoparticle, are favored for long-term applications or when greater stability is required [73,75,78].
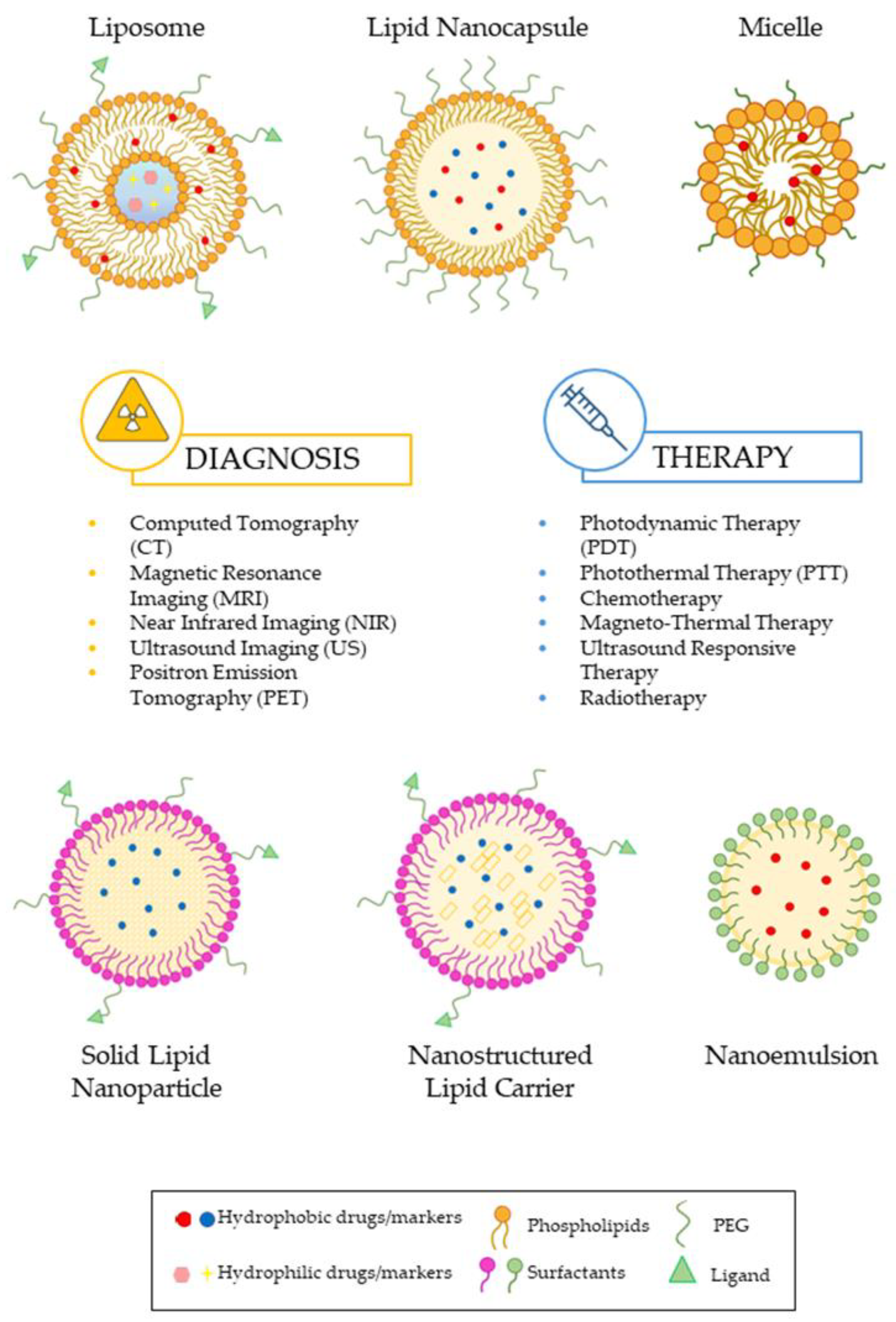
3. Case Studies: Lipid Nanoparticles in Theranostic Field
3.1. Liposomes
3.2. Solid-Lipid Nanoparticles (SLNs)
3.3. Nanostructured Lipid Carriers (NLCs)
3.4. Lipid Nanocapsules (LNCs)
3.5. Lipid Nanoemulsions (NEs)
3.6. Lipid Micelles
4. Clinical Barriers in Lipid-Based Nanotheranostics
Challenges and Strategies in the Development and Clinical Translation of Lipid-Based Nanocarriers
- (1)
- Physicochemical stability and scalability. Ensuring the stability of lipid-based nanocarriers during storage and handling is a critical task. Indeed, lipid-based nanocarriers frequently encounter stability concerns, such as aggregation, fusion, and leakage of encapsulated drugs. These issues can be addressed by optimizing the lipid composition, adjusting the types and ratios of lipids used, and incorporating appropriate stabilizing agents, enhancing the structural stability and integrity of nanocarriers [134,136]. Furthermore, scaling up the production of lipid-based nanocarriers while maintaining quality and consistency is a significant challenge. Some approaches to overcoming these difficulties include developing reproducible processes to ensure consistency during large-scale production [136] and implementing strict quality control measures at each step of the manufacturing process to ensure that the final product meets the required standards [134]. These strategies have the potential to significantly enhance the stability and scalability of lipid-based nanocarriers, making them more feasible for widespread application in theranostics.
- (2)
- High costs. The high costs associated with lipid-based nanocarriers represent a great challenge for clinical translation. Some of the primary factors that contribute to these expenses are associated with manufacturing procedures, scale-up expenses arising from the intricate nature of specialized equipment and processes required for the transition from laboratory to full-scale production, and the cost of raw materials [133,137]. There are several strategies that could be used to overcome these problems. For instance, the implementation of more efficient production techniques can help to distribute expenses and streamline development procedures. In addition, it could be useful to obtain funding from government grants and private investors to provide the necessary financial support for research.
- (3)
- Long-term monitoring of patients. Lipid nanocarriers pose several challenges in clinical translation. For instance, comprehending the distribution of these nanocarriers within the body, their safety profile, and their clearance is of utmost significance [134]. The adoption of personalized approaches based on individual patient characteristics and the use of advanced imaging techniques to monitor the distribution of nanocarriers within the body could be turning points for the widespread and safe application of lipid nanocarriers. Moreover, the utilization of lipid nanocarriers may pose a risk of immune reactions, which may complicate their long-term usage [138]. Therefore, it is essential to conduct extensive preclinical testing to assess the safety of immunological agents.
- (4)
- Complex regulatory pathways. The approval process for nanomedicines is extremely complex. The lack of specific guidelines for nanocarriers can lead to significant delays. However, advanced in vitro and in vivo models can provide more reliable data on the safety and efficacy of nanomedicines, helping to overcome regulatory hurdles. It is essential to develop regulatory guidelines specifically for nanomedicines in order to streamline the approval process [134]. Therefore, establishing appropriate guidelines and regulations will be essential for a safe and effective clinical translation. Collaboration is essential to advance lipid-based nanocarriers in cancer therapy. By combining resources, data, and expertise, academia, industry, and regulatory agencies can tackle common challenges more effectively. This collaborative approach can significantly improve the clinical translation process, ensuring that promising therapies reach patients faster. Joint research initiatives have the potential to yield novel solutions, as well as a deeper understanding of the intricate processes involved in the creation and approval of advanced theranostic products.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Kundu, P.; Singh, D.; Singh, A.; Sahoo, S.K. Cancer nanotheranostics: A nanomedicinal approach for cancer therapy and diagnosis. Anti-Cancer Agents Med. Chem. 2020, 20, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Anderson, R.C.; Lan, X.; Conti, P.S.; Chen, K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med. Res. Rev. 2020, 40, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, G.; Lymperopoulos, P.; Alikari, V.; Dafogianni, C.; Zyga, S.; Margari, N. Application of theranostics in oncology. In GeNeDis 2016: Geriatrics; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 119–128. [Google Scholar]
- Chavda, V.P.; Khadela, A.; Shah, Y.; Postwala, H.; Balar, P.; Vora, L. Current status of Cancer Nanotheranostics: Emerging strategies for cancer management. Nanotheranostics 2023, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Bharti, M.; Malviya, R.; Sundram, S.; Goyal, P. Leveraging Advancement in Robotics in the Treatment of Cancer. In Targeted Cancer Therapy in Biomedical Engineering; Springer Nature: Singapore, 2023; pp. 365–404. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, X.; Guo, R.; Ye, Z.; Fu, H.; Fu, N.; Zhang, J. Organic nanoplatforms for iodinated contrast media in CT imaging. Molecules 2021, 26, 7063. [Google Scholar] [CrossRef]
- Dreifuss, T.; Barnoy, E.; Motiei, M.; Popovtzer, R. Theranostic gold nanoparticles for CT imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Berlin/Heidelberg, Germany, 2017; pp. 403–427. [Google Scholar] [CrossRef]
- Anuar, M.K.; Harun, A.Z.; Razak, K.A.; Rahman, W.N. CT contrast agent of Platinum nanodendrites: Preliminary study. J. Phys. Conf. Ser. 2019, 1248, 012010. [Google Scholar] [CrossRef]
- Tarighatnia, A.; Fouladi, M.R.; Tohidkia, M.R.; Johal, G.; Nader, N.D.; Aghanejad, A.; Ghadiri, H. Engineering and quantification of bismuth nanoparticles as targeted contrast agent for computed tomography imaging in cellular and animal models. J. Drug Deliv. Sci. Technol. 2021, 66, 102895. [Google Scholar] [CrossRef]
- Lambert, J.W.; Sun, Y.; Stillson, C.; Li, Z.; Kumar, R.; Wang, S.; Yeh, B.M. An intravascular tantalum oxide–based CT contrast agent: Preclinical evaluation emulating overweight and obese patient size. Radiology 2018, 289, 103–110. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Ai, K.; Yuan, Q.; Lu, L. Recent advances in ytterbium-based contrast agents for in vivo X-ray computed tomography imaging: Promises and prospects. Contrast Media Mol. Imaging 2014, 9, 26–36. [Google Scholar] [CrossRef]
- Wu, M.; Liao, Y.; Guo, D.; Zhai, M.; Xia, D.; Zhang, Z.; Huang, Y. Manganese-based nanomaterials in diagnostics and chemodynamic therapy of cancers: New development. RSC Adv. 2024, 14, 14722–14741. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron oxide nanoparticles: An alternative for positive contrast in magnetic resonance imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef]
- Chen, X.; Teng, S.; Li, J.; Qiao, X.; Zhao, W.; Xue, Z.; Wang, T. Gadolinium (III)-chelated deformable mesoporous organosilica nanoparticles as magnetic resonance imaging contrast agent. Adv. Mater. 2023, 35, 2211578. [Google Scholar] [CrossRef] [PubMed]
- Langer, O. Use of PET imaging to evaluate transporter-mediated drug-drug interactions. J. Clin. Pharmacol. 2016, 56, S143–S156. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Seo, J.W.; Ferrara, K.; Louie, A. Novel method to label solid lipid nanoparticles with 64Cu for positron emission tomography imaging. Bioconjugate Chem. 2011, 22, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, G.; Fan, X.; Lang, L.; Hou, G.; Chen, L.; Chen, X. PET using a GRPR antagonist 68Ga-RM26 in healthy volunteers and prostate cancer patients. J. Nucl. Med. 2018, 59, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for positron emission tomography: Recent advances in 11C-, 18F-, 13N-, and 15O-labeling reactions. Angew. Chem. Int. Ed. 2019, 58, 2580–2605. [Google Scholar] [CrossRef] [PubMed]
- Cosialls, R.; Simó, C.; Borrós, S.; Gómez-Vallejo, V.; Schmidt, C.; Llop, J.; Casini, A. PET Imaging of Self-Assembled 18F-Labelled Pd2L4 Metallacages for Anticancer Drug Delivery. Chem. A Eur. J. 2023, 29, e202202604. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, X.; Zhao, Y.; Zhang, L.; Yu, Y.; Huang, F.; Huang, H. Tumor targeted nanostructured lipid carrier co-delivering paclitaxel and indocyanine green for laser triggered synergetic therapy of cancer. RSC Adv. 2017, 7, 35086–35095. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, J.Y.; Lee, S.H.; Park, T.G.; Nam, Y.S. Optically traceable solid lipid nanoparticles loaded with siRNA and paclitaxel for synergistic chemotherapy with in situ imaging. Adv. Healthc. Mater. 2013, 2, 576–584. [Google Scholar] [CrossRef]
- Olerile, L.D. Further development of near-infrared mediated quantum dots and paclitaxel Co-loaded nanostructured lipid carrier system for cancer theragnostic. Technol. Cancer Res. Treat. 2020, 19, 1533033820914308. [Google Scholar] [CrossRef]
- Campardelli, R.; Della Porta, G.; Gomez, L.; Irusta, S.; Reverchon, E.; Santamaria, J. Au–PLA nanocomposites for photothermally controlled drug delivery. J. Mater. Chem. B 2014, 2, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A.; Kolios, M.C. Near-infrared absorbing nanoemulsions as nonlinear ultrasound contrast agents for cancer theranostics. J. Mol. Liq. 2019, 287, 110848. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Han, X. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Wang, P. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv. Mater. 2015, 28, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ding, X.F.; Shen, J.Y.; Zhang, X.P.; Ding, X.W.; Xu, B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J. Zhejiang University. Sci. B 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Microfluidic paclitaxel-loaded lipid nanoparticle formulations for chemotherapy. Int. J. Pharm. 2022, 628, 122320. [Google Scholar] [CrossRef]
- Xue, F.; Zhu, S.; Tian, Q.; Qin, R.; Wang, Z.; Huang, G.; Yang, S. Macrophage-mediated delivery of magnetic nanoparticles for enhanced magnetic resonance imaging and magnetothermal therapy of solid tumors. J. Colloid Interface Sci. 2023, 629, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Q.; Li, P.; Cui, X.W.; Dietrich, C.F. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Lett. 2020, 470, 204–219. [Google Scholar] [CrossRef]
- Xie, L.; Ying, X.; Li, X.; Tan, X.; Zhang, T.; Zhang, X.; Han, S. Engineering of gold nanorods as multifunctional theranostic agent for photothermal-enhanced radiotherapy of cancer. Mater. Des. 2023, 225, 111456. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Bukhari, S.Z.; Zeth, K.; Iftikhar, M.; Rehman, M.; Munir, M.U.; Khan, W.S.; Ihsan, A. Supramolecular lipid nanoparticles as delivery carriers for non-invasive cancer theranostics. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Fatima, S.; Taha, M.; Rizwanullah, M.; Firdous, J.; Ahmad, R.; Khan, M.A. Nanomedicines in diagnosis and treatment of cancer: An update. Curr. Pharm. Des. 2020, 26, 1216–1231. [Google Scholar] [CrossRef]
- Nabil, G.; Bhise, K.; Sau, S.; Atef, M.; El-Banna, H.A.; Iyer, A.K. Nano-engineered delivery systems for cancer imaging and therapy: Recent advances, future direction, and patent evaluation. Drug Discov. Today 2019, 24, 462–491. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Clares, B.; E Morales, M.; Gallardo, V.; Ruiz, M.A. Lipid-based drug delivery systems for cancer treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-el-salhi, A.; Elaissari, A. Magnetic nanoparticles: From synthesis to theranostic applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Coene, A.; Leliaert, J. Magnetic nanoparticles in theranostic applications. J. Appl. Phys. 2022, 131, 160902. [Google Scholar] [CrossRef]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold nanoparticles in cancer theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef] [PubMed]
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.T.; Yu, R.S.; Chen, J.C. Progress in polymeric nanomedicines for theranostic cancer treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Patel, M. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Kaur, G.; Khurana, R.K.; Kapoor, S.; Singh, B. Quantum dots and their potential role in cancer theranostics. Crit. Rev. 2015, 32, 461–502. [Google Scholar] [CrossRef]
- Kościk, I.; Jankowski, D.; Jagusiak, A. Carbon nanomaterials for theranostic use. C 2021, 8, 3. [Google Scholar] [CrossRef]
- Masoudi Asil, S.; Guerrero, E.D.; Bugarini, G.; Cayme, J.; De Avila, N.; Garcia, J.; Narayan, M. Theranostic applications of multifunctional carbon nanomaterials. View 2023, 4, 20220056. [Google Scholar] [CrossRef]
- Saluja, V.; Mishra, Y.; Mishra, V.; Giri, N.; Nayak, P. Dendrimers based cancer nanotheranostics: An overview. Int. J. Pharm. 2021, 600, 120485. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.S.; Zahid, N.I.; Madheswaran, T.; Azmi, I.D.M. Recent advances in the development of multifunctional lipid-based nanoparticles for co-delivery, combination treatment strategies, and theranostics in breast and lung cancer. J. Drug Deliv. Sci. Technol. 2022, 71, 103300. [Google Scholar] [CrossRef]
- Mussi, S.V.; Torchilin, V.P. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J. Mater. Chem. B 2013, 1, 5201–5209. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Kwon, S.H.; Choi, J.H.; Lee, A. A promising biocompatible platform: Lipid-based and bio-inspired smart drug delivery systems for cancer therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nanomedicinal approaches in clinics. Int. J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alhasan, A.H. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Simone, E.A.; Dziubla, T.D.; Muzykantov, V.R. Polymeric carriers: Role of geometry in drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1283–1300. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Wang, L.; Tang, J.; Li, J.; Kocaefe, D.; Chen, C. In vivo pharmacokinetic features and biodistribution of star and rod-shaped gold nanoparticles by multispectral optoacoustic tomography. RSC Adv. 2015, 5, 7529–7538. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.F.; Zhou, L.; Liu, Y.; Meng, L.; Zhang, K.; Chen, C. Characterization of gold nanorods in vivo by integrated analytical techniques: Their uptake, retention, and chemical forms. Anal. Bioanal. Chem. 2010, 396, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zheng, C.; Yi, K.; Mintz, R.L.; Lv, S.; Tao, Y.; Li, M. Structural and componential design: New strategies regulating the behavior of lipid-based nanoparticles in vivo. Biomater. Sci. 2023, 11, 4774–4788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ovais, M.; Chen, C. Stimulus-responsive gold nanotheranostic platforms for targeting the tumor microenvironment. Nano Today 2018, 22, 83–99. [Google Scholar] [CrossRef]
- Guyon, L.; Groo, A.C.; Malzert-Freon, A. Relevant physicochemical methods to functionalize, purify, and characterize surface-decorated lipid-based nanocarriers. Mol. Pharm. 2020, 18, 44–64. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Kirpotin, D.; Park, J.W.; Hong, K.; Zalipsky, S.; Li, W.L.; Carter, P. Sterically stabilized anti-HER2 immunoliposomes: Design and targeting to human breast cancer cells in vitro. Biochemistry 1997, 36, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Allen, T.M. Ligand-targeted liposomal anticancer drugs. Prog. Lipid Res. 2003, 42, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, A.; Santhosh, P.B.; Gongadze, E.; Kulkarni, M.; Eleršič, K.; Perutkova, Š.; Iglič, A. Interaction between dipolar lipid headgroups and charged nanoparticles mediated by water dipoles and ions. Int. J. Mol. Sci. 2013, 14, 15312–15329. [Google Scholar] [CrossRef]
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and art of developing lipid nanoparticles for biologics delivery: Focus on development and scale-up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef]
- Paun, R.A.; Jurchuk, S.; Tabrizian, M. A landscape of recent advances in lipid nanoparticles and their translational potential for the treatment of solid tumors. Bioeng. Transl. Med. 2024, 9, e10601. [Google Scholar] [CrossRef]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, transfersomes and niosomes: Production methods and their applications in the vaccinal field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Riccardi, D.; Reverchon, E. Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process. Nanomaterials 2024, 14, 846. [Google Scholar] [CrossRef] [PubMed]
- Low, H.Y.; Yang, C.T.; Xia, B.; He, T.; Lam, W.W.C.; Ng, D.C.E. Radiolabeled Liposomes for Nuclear Imaging Probes. Molecules 2023, 28, 3798. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Ahmady, Z.S.; Beziere, N.S.; Ntziachristos, V.; Kostarelos, K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015, 482, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Moshkelani, D.; Zhang, H. Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: In vivo evaluation for triple-negative breast cancer. Pharm. Res. 2015, 32, 1604–1614. [Google Scholar] [CrossRef]
- Thébault, C.J.; Ramniceanu, G.; Boumati, S.; Michel, A.; Seguin, J.; Larrat, B.; Doan, B.T. Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P. J. Control. Release 2020, 322, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Šimečková, P.; Hubatka, F.; Kotouček, J.; Turánek Knötigová, P.; Mašek, J.; Slavik, J.; Turánek, J. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 4780. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. European. J. Pharm. Sci. 2021, 156, 105576. [Google Scholar]
- Karpuz, M.; Ozgenc, E.; Oner, E.; Atlihan-Gundogdu, E.; Burak, Z. 68Ga-labeled, imatinib encapsulated, theranostic liposomes: Formulation, characterization, and in vitro evaluation of anticancer activity. Drug Dev. Res. 2024, 85, e22136. [Google Scholar] [CrossRef] [PubMed]
- Mirahadi, M.; Ghanbarzadeh, S.; Ghorbani, M.; Gholizadeh, A.; Hamishehkar, H. A review on the role of lipid-based nanoparticles in medical diagnosis and imaging. Ther. Deliv. 2018, 9, 557–569. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization, and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Battaglia, L.; Ugazio, E. Lipid nano-and microparticles: An overview of patent-related research. J. Nanomater. 2019, 1, 2834941. [Google Scholar] [CrossRef]
- Valetti, S.; Mura, S.; Stella, B.; Couvreur, P. Rational design for multifunctional non-liposomal lipid-based nanocarriers for cancer management: Theory to practice. J. Nanobiotechnol. 2013, 11, S6. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Mishra, D.; Dhote, V.; Pradyumna, M.; Chourasia, M.K.; Chaurasia, M.; Jain, N.K. Solid lipid nanoparticles: A promising colloidal carrier. In Novel Carriers for Drug Delivery; PharmaMed Press: Warszawa, Poland, 2014; pp. 278–301. [Google Scholar]
- Jain, V.; Kumar, H.; Chand, P.; Jain, S. Lipid-Based Nanocarriers as Drug Delivery System and Its Applications. In Nanopharmaceutical Advanced Delivery Systems; Wiley: Hoboken, NJ, USA, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Han, Y.; Wang, J.; Yang, J. Preparation and characterization of cisplatin magnetic solid lipid nanoparticles (MSLNs): Effects of loading procedures of Fe3O4 nanoparticles. Pharm. Res. 2015, 32, 482–491. [Google Scholar] [CrossRef] [PubMed]
- De Escalona, M.M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic solid lipid nanoparticles in hyperthermia against colon cancer. Int. J. Pharm. 2016, 504, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Türkkanı, G.; Güngör, B.; Cetin, O.; İçhedef, Ç.; Parlak, Y.; Gümüşer, F.G.; Sayıt Bilgin, B.E.; Teksöz, S. Synthesis, radiolabeling and in vitro evaluation of azathioprine loaded magnetic solid lipid nanoparticles. J. Radioanal. Nucl. Chem. 2023, 332, 4695–4704. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Ucar, E.; Teksoz, S.; Ichedef, C.; Kilcar, A.Y.; Medine, E.I.; Ari, K.; Unak, P. Synthesis, characterization and radiolabeling of folic acid modified nanostructured lipid carriers as a contrast agent and drug delivery system. Appl. Radiat. Isot. 2017, 119, 72–79. [Google Scholar] [CrossRef]
- Elmarzugi, N.; Amara, R.; Eshmela, M.; Eid, A. An overview of nanocapsule and lipid nanocapsule: Recent developments and future prospects. Palest. Med. Pharm. J. 2023, 8, 2. [Google Scholar] [CrossRef]
- Idris, A.H.; Che Abdullah, C.A.; Yusof, N.A.; Asmawi, A.A.; Abdul Rahman, M.B. Nanostructured lipid carrier co-loaded with docetaxel and magnetic nanoparticles: Physicochemical characterization and in vitro evaluation. Pharmaceutics 2023, 15, 1319. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.M.; Yadava, S.K.; Singh, R.; Giri, J. Lipid nanocapsules co-encapsulating paclitaxel and salinomycin for eradicating breast cancer and cancer stem cells. Colloids Surf. B Biointerfaces 2021, 204, 111775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Poudel, B.K.; Regmi, S.; Pathak, S.; Ruttala, H.B.; Gautam, M.; Kim, J.O. Paclitaxel and erlotinib-co-loaded solid lipid core nanocapsules: Assessment of physicochemical characteristics and cytotoxicity in non-small cell lung cancer. Pharm. Res. 2018, 35, 96. [Google Scholar] [CrossRef]
- Gupta, B.; Pathak, S.; Poudel, B.K.; Regmi, S.; Ruttala, H.B.; Gautam, M.; Kim, J.O. Folate receptor-targeted hybrid lipid-core nanocapsules for sequential delivery of doxorubicin and tanespimycin. Colloids Surf. B Biointerfaces 2017, 155, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lollo, G.; Ullio-Gamboa, G.; Fuentes, E.; Matha, K.; Lautram, N.; Benoit, J.P. In vitro anti-cancer activity and pharmacokinetic evaluation of curcumin-loaded lipid nanocapsules. Mater. Sci. Eng. C 2018, 91, 859–867. [Google Scholar] [CrossRef]
- Szwed, M.; Torgersen, M.L.; Kumari, R.V.; Yadava, S.K.; Pust, S.; Iversen, T.G.; Sandvig, K. Biological response and cytotoxicity induced by lipid nanocapsules. J. Nanobiotechnol. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Balzeau, J.; Pinier, M.; Berges, R.; Saulnier, P.; Benoit, J.P.; Eyer, J. The effect of functionalizing lipid nanocapsules with NFL-TBS. 40-63 peptide on their uptake by glioblastoma cells. Biomaterials 2013, 34, 3381–3389. [Google Scholar] [CrossRef]
- Fernandes, D.A. Review on the applications of nanoemulsions in cancer theranostics. J. Mater. Res. 2022, 37, 1953–1977. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; K Mandal, U.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical, and nasal route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in translational research—Opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef]
- Sahu, P.; Das, D.; Mishra, V.K.; Kashaw, V.; Kashaw, S.K. Nanoemulsion: A novel eon in cancer chemotherapy. Mini Rev. Med. Chem. 2017, 17, 1778–1792. [Google Scholar] [CrossRef]
- Upadhyay, T.; Ansari, V.A.; Ahmad, U.; Sultana, N.; Akhtar, J. Exploring nanoemulsion for liver cancer therapy. Curr. Cancer Ther. Rev. 2020, 16, 260–268. [Google Scholar]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Souto, E.B. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Piroyan, A.; Ganta, S.; Morse, A.B.; Candiloro, K.M.; Solon, A.L.; Coleman, T.P. In Vitro and In Vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers. Cancer Biol. Ther. 2018, 19, 554–564. [Google Scholar] [CrossRef]
- Li, B.; Tan, T.; Chu, W.; Zhang, Y.; Ye, Y.; Wang, S.; Cao, X. Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Deliv. 2022, 29, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.; Valic, M.S.; Cheng, M.H.; Zheng, G. Porphyrin-lipid stabilized paclitaxel nanoemulsion for combined photodynamic therapy and chemotherapy. J. Nanobiotechnol. 2021, 19, 154. [Google Scholar] [CrossRef]
- Ho, Y.J.; Yeh, C.K. Theranostic performance of acoustic nanodroplet vaporization-generated bubbles in tumor intertissue. Theranostics 2017, 7, 1477. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. What does the future hold for chemotherapy with the use of lipid-based nanocarriers? Future Oncol. 2019, 16, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Park, Y.S. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumor-targeted theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, S.K.; Chang, N.C.; Mohapatra, A.; Uthaman, S.; Lee, B.I.; Tsai, W.B.; Park, I.K. A lipophilic ir-780 dye-encapsulated zwitterionic polymer-lipid micellar nanoparticle for enhanced photothermal therapy and nir-based fluorescence imaging in a cervical tumor mouse model. Int. J. Mol. Sci. 2018, 19, 1189. [Google Scholar] [CrossRef]
- Choi, M.J.; Kang, S.J.; Lee, Y.K.; Choi, K.C.; Lee, D.H.; Jeong, H.Y.; Park, Y.S. Novel Lipid Nanocomplex Co-Carrying Bcl2 siRNA and Quantum Dots for EGF Receptor-Targeted Anti-Cancer Theranosis. Int. J. Mol. Sci. 2024, 25, 6246. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, L.; Jiang, Z.; Pan, H.; Zhang, Y.; Zhu, G.; Duan, X. Cationic micelle-based siRNA delivery for efficient colon cancer gene therapy. Nanoscale Res. Lett. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor targeted curcumin delivery by folate-modified MPEG-PCL self-assembly micelles for colorectal cancer therapy. Int. J. Nanomed. 2020, 2020, 1239–1252. [Google Scholar] [CrossRef]
- Howell, M.; Mallela, J.; Wang, C.; Ravi, S.; Dixit, S.; Garapati, U.; Mohapatra, S. Manganese-loaded lipid-micellar theranostics for simultaneous drug and gene delivery to lungs. J. Control. Release 2013, 167, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Siram, K.; Rahman, S.H.; Balakumar, K.; Duganath, N.; Chandrasekar, R.; Hariprasad, R. Pharmaceutical nanotechnology: Brief perspective on lipid drug delivery and its current scenario. Biomed. Appl. Nanopart. 2019, 91–115. [Google Scholar] [CrossRef]
- Naziris, N.; Demetzos, C. Lipid nanoparticles as platforms for theranostic purposes: Recent advances in the field. J. Nanotheranostics 2022, 3, 86–101. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent progress in lipid nanoparticles for cancer theranostics: Opportunity and challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef] [PubMed]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-Mediated Drug Delivery Systems for Precision Targeting in Oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Babu, M.A.; Jain, A.; Sharma, D. Lipid-based Nanocarriers for mRNA Delivery: Vital Considerations and Applications. Nanosci. Nanotechnol. -Asia 2024, 14, 49–61. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Application | Features | Therapeutic/Imaging Agents |
|---|---|---|---|
| CT | Diagnostic | 3D imaging Deep tissue penetration Clear view of cross-sectional images High-resolution imaging High dose of ionizing radiation | Iodine [8] Gold [9] Platinum [10] Bismuth [11] Tantalum [12] Ytterbium [13] |
| MRI | Diagnostic | Non-invasive High spatial resolution Evaluation of anatomic details Clear view of the cross-sectional images Good soft-tissue contrast Low sensitivity Expensive | Manganese (Mn2+) [14] Iron (Fe3+) [15] Gadolinium (Gd3+) [16] |
| PET | Diagnostic | Non-invasive Low spatial resolution High sensitivity 3D imagining Unlimited penetration depth Quantitative analysis Use of radioactive probes (toxic potential) | 11C [17] 64Cu [18] 68Ga [19] 13N [20] 18F [21] |
| NIR | Diagnostic | Non-invasive Non-ionizing Deep tissue penetration Low tissue absorption and scattering High fluorescence intensity Low sensitivity | IR-780 [22] ICG [23] Quantum dots [24,25] Gold NPs [26] |
| USI | Diagnostic | Real-time measurement Non-ionizing and non-radio labeling High temporal and spatial resolution Low sensitivity US-frequency-dependent depth penetration | Perfluorocarbon [27] Gold NPs [28] Carbon NPs [29] Polymer NPs [30] |
| PDT | Therapeutic | Minimally invasive Selective targeting of tumors Photo-responsive Oxidative stress due to photodynamic effect | Tetrapyrrole family [31] ICG [32] Quantum dots [33] |
| PTT | Therapeutic | Non-invasive Low toxicity Conversion of photo energy to thermal energy High specificity Low size effect | Carbon dots [34] Graphene, iron oxide, carbon nanotubes, gold, silver [6] |
| Chemotherapy | Therapeutic | Tumor reduction Toxic therapeutic agents Side effects | Doxorubicin [35] Paclitaxel [36] |
| Magneto-Thermal Therapy | Therapeutic | Minimally invasive Deep tumor targeting Safe Side effects | Iron oxide [37] |
| Ultrasound Responsive Therapy | Therapeutic | Non-invasive Efficient drug delivery Side effects | Perfluorocarbon [38] |
| Radiotherapy | Therapeutic | Ionizing radiation Precise targeting Radiotoxicity | Gold nanorods [39] |
| Nanocarriers | Theranostic Agent | Production Technique | Size [nm] | ζ-Potential [mV] | Trial Status | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | DOX + ICG | Lipid film hydration method | 130 | −39 | In vivo | [84] |
| ICG | Thin film/extrusion method | 80 | - | In vivo | [85] | |
| CA4P + IONP | Reverse phase evaporation method | 209 | - | In vivo | [86] | |
| Gd | Lipid film hydration method | 113 | −58 | In vitro | [87] | |
| PCX + VNB | Lipid film hydration method | 190 | −9 | In vivo | [88] | |
| IMT | Lipid film hydration method | 250 | 54 | In vitro | [89] | |
| SLNs | PTX + siRNA + QDs | Emulsification solvent evaporation method | 130 | 36 | In vitro | [24] |
| IR-780 dye | Slightly modified solvent diffusion method | 145 | −3 | In vivo | [98] | |
| Fe3O4 | Double emulsion/solvent evaporation method | 180 | −40/20 | In vitro | [100] | |
| AZA + 99mTc + FeO/Fe3O4 | Solvent diffusion method | 205 | −14 | In vitro | [101] | |
| NLCs | IR-780 dye | Solvent evaporation method | 156 | −48/−12 | In vivo | [22] |
| PTX + 99mTc(CO)3 | Solvent diffusion method | 237 | −34 | In vitro | [106] | |
| PTX + ICG | Solvent diffusion method | 100 | - | In vivo | [23] | |
| PTX + QDs | Oil/water emulsification solvent evaporation technique | 115 | - | In vivo | [25] | |
| IONP + DTX | Solvent injection technique | 110 | - | In vitro | [107] | |
| Lipid Nanocapsules | PTX + SAL | Phase inversion temperature method | 90 | −7 | In vitro | [109] |
| PTX + ERL | Nanoprecipitation/sonication | 196 | −30 | In vivo | [110] | |
| DOX + TNP | Mixing/sonication techniques | 208 | −16 | In vitro | [111] | |
| CCM | Phase inversion technique | 50 | −8 | In vitro | [112] | |
| DiD | Solvent free phase inversion method | 95 | −10 | In vivo | [113] | |
| PTX | Emulsion inversion phase process | 55 | −9 | In vitro | [114] | |
| NEs | DTX + Gd | High-shear homogenization method | 150 | −45 | In vivo | [121] |
| PTX + DHA | Microfluidic technique | 187 | - | In vivo | [122] | |
| PTX + porphyrin | Sonication method | 120 | −2 | In vivo | [123] | |
| PFH | Sonication method | 61 | −72 | In vitro | [27] | |
| Micelles | PTX + QDs | Thin-film method | 41 | −3 | In vivo | [126] |
| IR-780 dye | Sonication method | 318 | −0.2 | In vivo | [127] | |
| QDs + siRNA | Lipid film hydration method | 32 | +2 | In vivo | [128] | |
| siRNA | Lipid film hydration method | 145 | +46 | In vivo | [129] | |
| Cur | Lipid film hydration method | 31 | −4 | In vivo | [130] | |
| MnO | Lipid film hydration method | 100 | +37 | In vivo | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, A.; Provenza, A.C.; Reverchon, G.; Baldino, L.; Reverchon, E. Lipid-Based Nanocarriers: Bridging Diagnosis and Cancer Therapy. Pharmaceutics 2024, 16, 1158. https://doi.org/10.3390/pharmaceutics16091158
Giordano A, Provenza AC, Reverchon G, Baldino L, Reverchon E. Lipid-Based Nanocarriers: Bridging Diagnosis and Cancer Therapy. Pharmaceutics. 2024; 16(9):1158. https://doi.org/10.3390/pharmaceutics16091158
Chicago/Turabian StyleGiordano, Alessandra, Anna Chiara Provenza, Giorgio Reverchon, Lucia Baldino, and Ernesto Reverchon. 2024. "Lipid-Based Nanocarriers: Bridging Diagnosis and Cancer Therapy" Pharmaceutics 16, no. 9: 1158. https://doi.org/10.3390/pharmaceutics16091158
APA StyleGiordano, A., Provenza, A. C., Reverchon, G., Baldino, L., & Reverchon, E. (2024). Lipid-Based Nanocarriers: Bridging Diagnosis and Cancer Therapy. Pharmaceutics, 16(9), 1158. https://doi.org/10.3390/pharmaceutics16091158








