Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing
Abstract
:1. Introduction
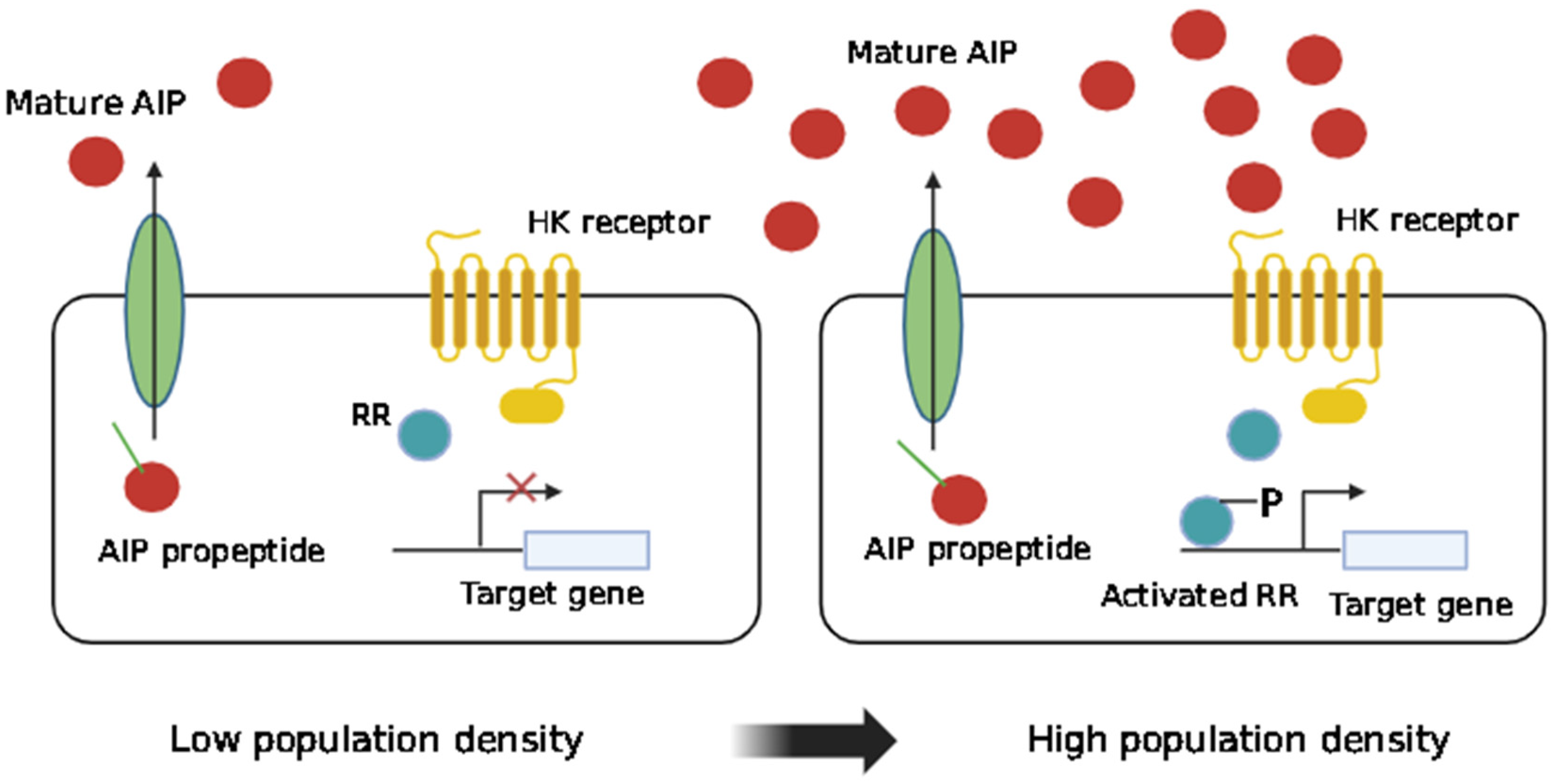
2. QS: Communication Processes in the Formation of Bacterial Biofilms
2.1. QS in Gram-Negative Bacteria
2.2. QS in Gram-Positive Bacteria
2.3. Alternative Forms of QS
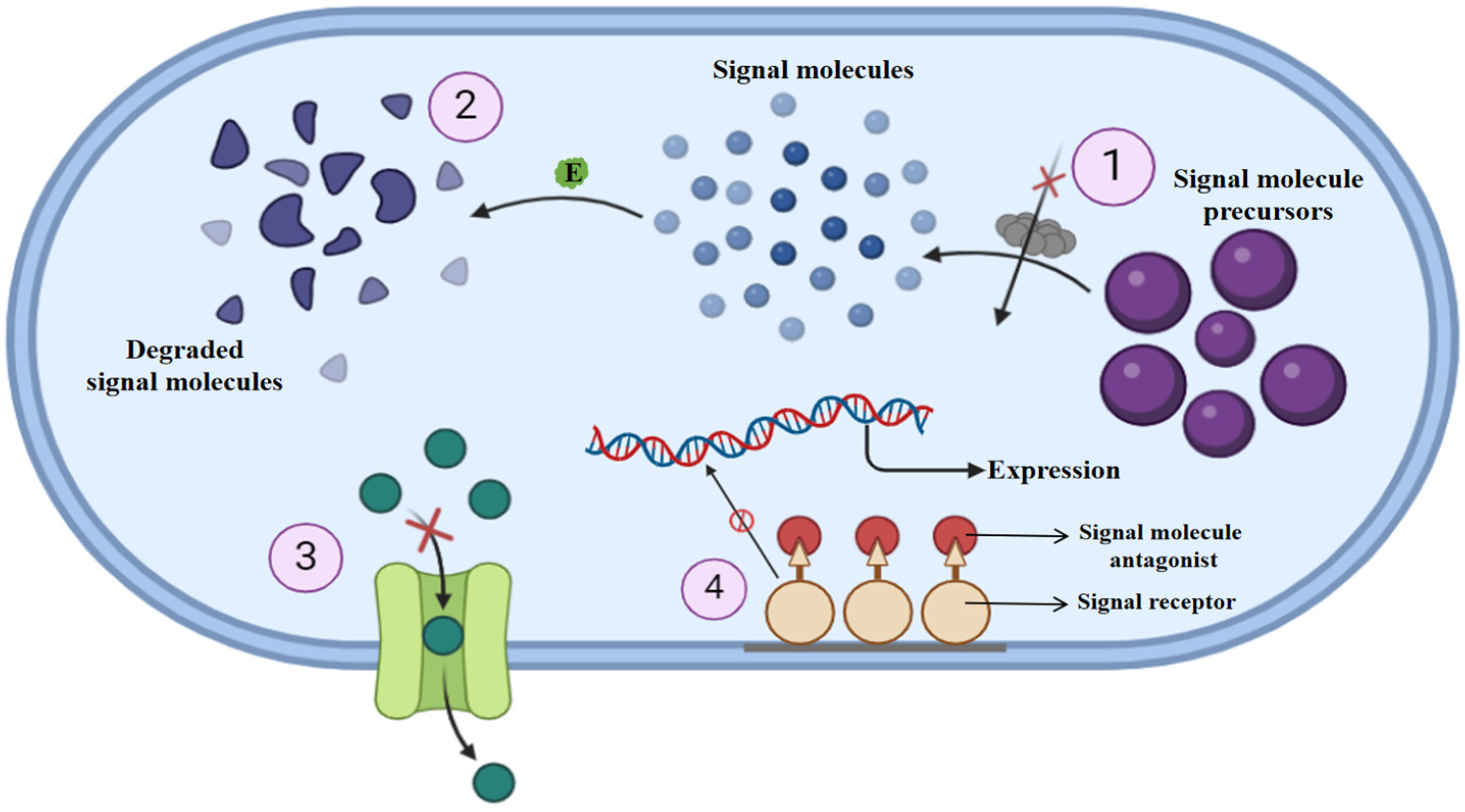
3. QQ: Nature’s Answer to Antibiotic Resistance
4. QQ Agents: A Source for Anti-Virulence Treatment
4.1. Natural QSIs
4.1.1. QSIs Derived from Animal Sources
4.1.2. QSIs Derived from Fungi
4.1.3. QSIs Derived from Marine Organisms
4.1.4. QSIs Derived from Plants
4.2. Macromolecules as QSIs
4.2.1. AHL Lactonases
4.2.2. AHL Acylases
4.2.3. AHL Oxidoreductases
| Enzyme | Source Organism | Degraded QS Signal Molecule | Reference |
|---|---|---|---|
| Lactonase | Bacillus sp. strain 240B1 | AHLs | [118] |
| Lactonase | Bacillus thuringiensis | AHLs | [129] |
| Lactonase | Oceanobacillus strains 30, 172, and 97-2 | AHLs | [130] |
| Lactonase | Halomonas sp. strain 33 | AHLs | [130] |
| Lactonase | Acinetobacter sp. strain C1010 | AHLs | [131] |
| Acylase/Lactonase | Tenacibaculum discolor strain 20J | AHLs | [130] |
| Acylase/Lactonase | Hyphamonas sp. DG895 | C4HSL and 3OC12-HSL | [130] |
| Acylase | Alteromonas sp. strain 168 | C4HSL and 3OC12-HSL | [130] |
| AHL Acylase | Bacillus pumilus S8-07 | 3OC12-HSL | [132] |
| AHL Acylase | Ralstonia sp. XJ12B | Long-chain AHLs | [133] |
| AHL Acylase | Pseudomonas aeruginosa PAO1 | Long-chain AHLs | [134] |
| AHL Lactonase | Rhodococcus erythropolis strain W2 | AHLs | [135] |
| AHL Lactonase | Agrobacterium tumefaciens | AHLs | [136] |
| AHL Lactonase | Arthrobacter sp. IBN110 | AHLs | [137] |
| AHL Oxidoreductase | Burkholderia strain GG4 | 3OC6-HSL | [138] |
5. Nanotechnology: A Promising Strategy against Multidrug-Resistant Bacteria
6. Exploration of Nanotechnological Strategies
6.1. Organic NPs
6.2. Inorganic NPs
7. Combination Therapies: Integrating Small Molecules and Nanotechnology Approaches
7.1. Nanocarriers for Antibacterial Agents Produced by Plants
7.2. NPs Derived from Plants
7.3. Nanoformulated Antibiotics
7.4. Nano-Inhibitors of QS
7.5. Nano-Inhibitors of the QS System in Bacteria
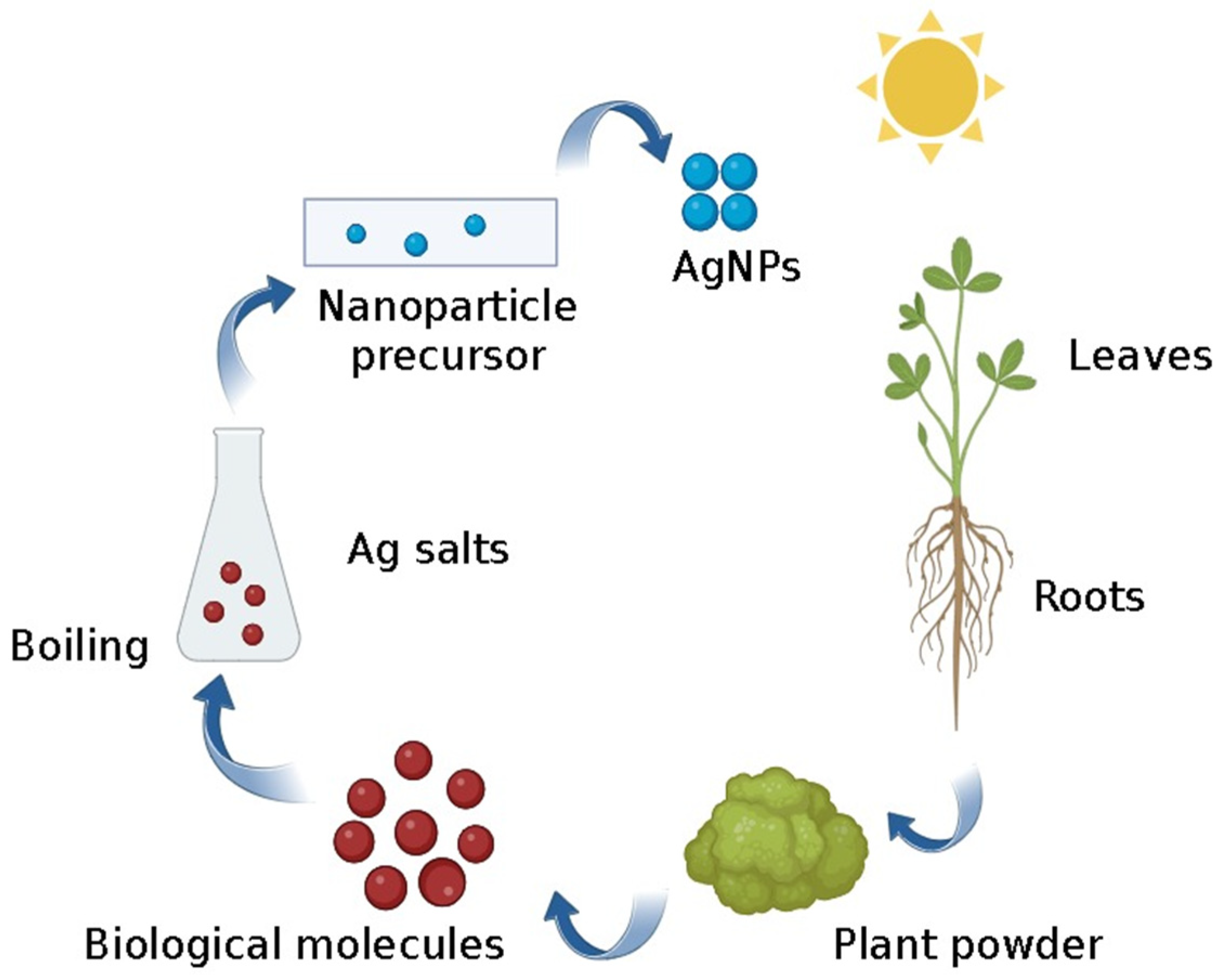
7.6. Green Nano-Inhibitors
8. Discussion
9. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Novick, R.P.; Geisinger, E. Quorum Sensing in Staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Ng, W.-L.; Bassler, B.L. Bacterial Quorum-Sensing Network Architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Wolcott, R.; Rhoads, D.; Bennett, M.; Wolcott, B.; Gogokhia, L.; Costerton, J.; Dowd, S. Chronic wounds and the medical biofilm paradigm. J. Wound Care 2010, 19, 45–53. [Google Scholar] [CrossRef]
- Blackledge, M.S.; Worthington, R.J.; Melander, C. Biologically inspired strategies for combating bacterial biofilms. Curr. Opin. Pharmacol. 2013, 13, 699–706. [Google Scholar] [CrossRef]
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide Signaling in the Staphylococci. Chem. Rev. 2010, 111, 117–151. [Google Scholar] [CrossRef]
- Schembri, M.A.; Kjærgaard, K.; Klemm, P. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 2003, 48, 253–267. [Google Scholar] [CrossRef]
- Soleimani, N.; Mobarez, A.M.; Olia, M.S.J.; Atyabi, F. Synthesis, characterization and effect of the antibacterial activity of chitosan nanoparticles on vancomycin-resistant Enterococcus and other gram negative or gram positive bacteria. Int. J. Pure Appl. Sci. Technol. 2015, 26, 14. [Google Scholar]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Wood, T.K.; Kumar, P. Evolution of Resistance to Quorum-Sensing Inhibitors. Microb. Ecol. 2013, 68, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A Review on Basic Biology of Bacterial Biofilm Infections and Their Treatments by Nanotechnology-Based Approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 243–259. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices. BioMed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef]
- Singh, B.N.; Prateeksha; Pandey, G.; Jadaun, V.; Singh, S.; Bajpai, R.; Nayaka, S.; Naqvi, A.H.; Rawat, A.K.S.; Upreti, D.K.; et al. Development and characterization of a novel Swarna-based herbo-metallic colloidal nano-formulation–inhibitor of Streptococcus mutans quorum sensing. RSC Adv. 2015, 5, 5809–5822. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Sifri, C.D. Healthcare Epidemiology: Quorum Sensing: Bacteria Talk Sense. Clin. Infect. Dis. 2008, 47, 1070–1076. [Google Scholar] [CrossRef]
- Walters, M.; Sperandio, V. Quorum sensing in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 2006, 296, 125–131. [Google Scholar] [CrossRef]
- Amara, N.; Krom, B.P.; Kaufmann, G.F.; Meijler, M.M. Macromolecular inhibition of quorum sensing: Enzymes, antibodies, and beyond. Chem. Rev. 2011, 111, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Zhang, L.-H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar] [PubMed]
- McDougald, D.; Rice, S.A.; Kjelleberg, S. Bacterial quorum sensing and interference by naturally occurring biomimics. Anal. Bioanal. Chem. 2006, 387, 445–453. [Google Scholar] [CrossRef]
- Scott, S.R.; Hasty, J. Quorum Sensing Communication Modules for Microbial Consortia. ACS Synth. Biol. 2016, 5, 969–977. [Google Scholar] [CrossRef]
- Joe, M.M.; Bradeeba, K.; Parthasarathi, R.; Sivakumaar, P.K.; Chauhan, P.S.; Tipayno, S.; Benson, A.; Sa, T. Development of surfactin based nanoemulsion formulation from selected cooking oils: Evaluation for antimicrobial activity against selected food associated microorganisms. J. Taiwan Inst. Chem. Eng. 2012, 43, 172–180. [Google Scholar] [CrossRef]
- Williams, P.; Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef]
- Wolanin, P.M.; Thomason, P.A.; Stock, J.B. Histidine protein kinases: Key signal transducers outside the animal kingdom. Genome Biol. 2002, 3, 1–8. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, K.; Sun, L. Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain. Microbiology 2008, 154, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Zhang, L.-H. Quorum quenching and proactive host defense. Trends Plant Sci. 2003, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- El-Samragy, Y. Food Safety: Some Global Trends; BoD—Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- LaSarre, B.; Federle, M.J. Exploiting Quorum Sensing To Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- von Bodman, S.B.; Willey, J.M.; Diggle, S.P. Cell-Cell Communication in Bacteria: United We Stand. J. Bacteriol. 2008, 190, 4377–4391. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. Apmis 2017, 125, 272–2757. [Google Scholar] [CrossRef]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Kalia, V.C.; Purohit, H.J. Quenching the quorum sensing system: Potential antibacterial drug targets. Crit. Rev. Microbiol. 2011, 37, 121–140. [Google Scholar] [CrossRef]
- Kalia, V.C.; Raju, S.C.; Purohit, H.J. Genomic Analysis Reveals Versatile Organisms for Quorum Quenching Enzymes: Acyl-Homoserine Lactone-Acylase and -Lactonase. Open Microbiol. J. 2011, 3, 1. [Google Scholar] [CrossRef]
- Abraham, W.-R. Going beyond the Control of Quorum-Sensing to Combat Biofilm Infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Koul, S.; Kalia, V.C. Multiplicity of Quorum Quenching Enzymes: A Potential Mechanism to Limit Quorum Sensing Bacterial Population. Indian J. Microbiol. 2017, 57, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; O Sintim, H. Agents that Inhibit Bacterial Biofilm Formation. Futur. Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of Biofilm Formation: Antibiotics and Beyond. Appl. Environ. Microbiol. 2017, 83, e02508-16. [Google Scholar] [CrossRef]
- Hemmati, F.; Salehi, R.; Ghotaslou, R.; Kafil, H.S.; Hasani, A.; Gholizadeh, P.; Nouri, R.; Rezaee, M.A. Quorum Quenching: A Potential Target for Antipseudomonal Therapy. Infect. Drug Resist. 2020, 13, 2989–3005. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, X.-H. Quorum Quenching Agents: Resources for Antivirulence Therapy. Mar. Drugs 2014, 12, 3245–3282. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, T.; Wang, J.; Wang, Y.; Zhang, X.-H. The Mechanisms and Applications of Quorum Sensing (QS) and Quorum Quenching (QQ). J. Ocean Univ. China 2019, 18, 1427–1442. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y. Quorum sensing and signal interference: Diverse implications. Mol. Microbiol. 2004, 53, 1563–1571. [Google Scholar] [CrossRef]
- Joint, I.; Tait, K.; Callow, M.E.; Callow, J.A.; Milton, D.; Williams, P.; Cámara, M. Cell-to-Cell Communication Across the Prokaryote-Eukaryote Boundary. Science 2002, 298, 1207. [Google Scholar] [CrossRef]
- Bauer, W.D.; Mathesius, U. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 2004, 7, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kim, Y.S.; Ponnusamy, K.; Kweon, J.H. Application of Quorum Quenching to Inhibit Biofilm Formation. Environ. Eng. Sci. 2009, 26, 1319–1324. [Google Scholar] [CrossRef]
- Teiber, J.F.; Horke, S.; Haines, D.C.; Chowdhary, P.K.; Xiao, J.; Kramer, G.L.; Haley, R.W.; Draganov, D.I. Dominant Role of Paraoxonases in Inactivation of the Pseudomonas aeruginosa Quorum-Sensing Signal N-(3-Oxododecanoyl)-l-Homoserine Lactone. Infect. Immun. 2008, 76, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Billecke, S.; Draganov, D.; Counsell, R.; Stetson, P.; Watson, C.; Hsu, C.; La Du, B.N. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 2000, 28, 1335–1342. [Google Scholar] [PubMed]
- Stoltz, D.A.; Ozer, E.A.; Ng, C.J.; Yu, J.M.; Reddy, S.T.; Lusis, A.J.; Bourquard, N.; Parsek, M.R.; Zabner, J.; Shih, D.M. Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am. J. Physiol.-Lung Cell Mol. Physiol. 2007, 292, L852–L860. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.-H.; Wang, J.; Dong, Y.-H.; Hu, J.Y.; Zhang, L.-H. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 2005, 579, 3713–3717. [Google Scholar] [CrossRef]
- Widmer, K.W.; Jesudhasan, P.R.; Dowd, S.E.; Pillai, S.D. Differential Expression of Virulence-Related Genes in A Salmonella enterica Serotype Typhimurium luxS Mutant in Response to Autoinducer AI-2 And Poultry Meat–Derived AI-2 Inhibitor. Foodborne Pathog. Dis. 2007, 4, 5–15. [Google Scholar] [CrossRef]
- Teplitski, M.; Chen, H.; Rajamani, S.; Gao, M.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii Secretes Compounds That Mimic Bacterial Signals and Interfere with Quorum Sensing Regulation in Bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef]
- Lu, L.; Hume, M.E.; Pillai, S.D. Autoinducer-2–like activity associated with foods and its interaction with food additives. J. Food Prot. 2004, 67, 1457–1462. [Google Scholar] [CrossRef]
- Soni, K.; Lu, L.; Jesudhasan, P.; Hume, M.; Pillai, S. Influence of Autoinducer-2 (AI-2) and Beef Sample Extracts on E. coli O157:H7 Survival and Gene Expression of Virulence Genes yadK and hhA. J. Food Sci. 2008, 73, M135–M1398. [Google Scholar] [CrossRef]
- Kalia, V.C.; Rani, A.; Lal, S.; Cheema, S.; Raut, C.P. Combing databases reveals potential antibiotic producers. Expert Opin. Drug Discov. 2007, 2, 211–224. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Skindersoe, M.E.; Bjarnsholt, T.; Phipps, R.K.; Christensen, K.B.; Jensen, P.O.; Andersen, J.B.; Koch, B.; Larsen, T.O.; Hentzer, M.; et al. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 2005, 151, 1325–1340. [Google Scholar] [CrossRef]
- Uroz, S.; Heinonsalo, J. Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol. Ecol. 2008, 65, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; He, C.; Chu, Q. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett. Appl. Microbiol. 2011, 52, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.J. Inhibition of Bacterial Quorum Sensing-Regulated Behaviors by Tremella fuciformis Extract. Curr. Microbiol. 2008, 57, 418–422. [Google Scholar] [CrossRef]
- Clark, B.R.; Engene, N.; Teasdale, M.E.; Rowley, D.C.; Matainaho, T.; Valeriote, F.A.; Gerwick, W.H. Natural Products Chemistry and Taxonomy of the Marine Cyanobacterium Blennothrix cantharidosmum. J. Nat. Prod. 2008, 71, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Alagely, A.; Gunasekera, S.P.; Paul, V.J. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ. Microbiol. Rep. 2010, 2, 739–744. [Google Scholar] [CrossRef]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A–C, potent inhibitors of inflammation and bacterial quorum sensing: Synthetic derivatives and structure-activity relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Teasdale, M.E.; Liu, J.; Wallace, J.; Akhlaghi, F.; Rowley, D.C. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl. Environ. Microbiol. 2009, 75, 567–572. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Al-Fori, M.; Gunasekera, S.P.; Sudesh, K.; Paul, V.J. Quorum-sensing inhibitory compounds from extremophilic microorganisms isolated from a hypersaline cyanobacterial mat. J. Ind. Microbiol. Biotechnol. 2013, 40, 759–772. [Google Scholar] [CrossRef]
- Ooka, K.; Fukumoto, A.; Yamanaka, T.; Shimada, K.; Ishihara, R.; Anzai, Y.; Kato, F. Piericidins, novel quorum-sensing inhibitors against Chromobacterium violaceum CV026, from Streptomyces sp. TOHO-Y209 and TOHO-O348. Open J. Med. Chem. 2013, 2013, 40387. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; A Rice, S.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Grossmann, G.; Séquin, U.; Brandl, H.; Bachofen, R. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol. 2004, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Miyamoto, C.M.; Wood, T.K.; Meighen, E.A.; Sorgeloos, P.; Verstraete, W.; Bossier, P. The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2 (5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein LuxR. Environ. Microbiol. 2007, 9, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.; Lee, B.W.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorganic Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef] [PubMed]
- Rasch, M.; Buch, C.; Austin, B.; Slierendrecht, W.J.; Ekmann, K.S.; Larsen, J.L.; Johansen, C.; Riedel, K.; Eberl, L.; Givskov, M.; et al. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). Syst. Appl. Microbiol. 2004, 27, 350–359. [Google Scholar] [CrossRef]
- Tinh, N.; Linh, N.; Wood, T.; Dierckens, K.; Sorgeloos, P.; Bossier, P. Interference with the quorum sensing systems in a Vibrio harveyi strain alters the growth rate of gnotobiotically cultured rotifer Brachionus plicatilis. J. Appl. Microbiol. 2006, 103, 194–203. [Google Scholar] [CrossRef]
- Defoirdt, T.; Crab, R.; Wood, T.K.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus isolates. Appl. Environ. Microbiol. 2006, 72, 6419–6423. [Google Scholar] [CrossRef]
- Liu, H.B.; Koh, K.P.; Kim, J.S.; Seo, Y.; Park, S. The effects of betonicine, floridoside, and isethionic acid from the red alga Ahnfeltiopsis flabelliformis on quorum-sensing activity. Biotechnol. Bioprocess Eng. 2008, 13, 458–463. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.H.; Seo, Y.W.; Park, S. Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnol. Bioprocess Eng. 2007, 12, 308–311. [Google Scholar] [CrossRef]
- Ren, D.; Sims, J.J.; Wood, T.K. Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 2001, 3, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; de Nys, R.; Maximilien, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S. Inhibitory Effects of Secondary Metabolites from the Red Alga Delisea pulchra on Swarming Motility of Proteus mirabilis. Appl. Environ. Microbiol. 1996, 62, 4284–4287. [Google Scholar] [CrossRef]
- de Nys, R.; Wright, A.D.; König, G.M.; Sticher, O. New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron 1993, 49, 11213–11220. [Google Scholar] [CrossRef]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef]
- Manefield, M.; Harris, L.; Rice, S.A.; de Nys, R.; Kjelleberg, S. Inhibition of Luminescence and Virulence in the Black Tiger Prawn (Penaeus monodon) Pathogen Vibrio harveyi by Intercellular Signal Antagonists. Appl. Environ. Microbiol. 2000, 66, 2079–2084. [Google Scholar] [CrossRef]
- de Nys, R.; Givskov, M.C.; Kumar, N.; Kjelleberg, S.; Steinberg, P.D. Furanones: Progress in Molecular and Subcellular Biology. Subseries Marine Molecular Biotechnology. In Antifouling Compounds; Fusetani, N., Clare, A.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Borchardt, S.A.; Allain, E.J.; Michels, J.J.; Stearns, G.W.; Kelly, R.F.; McCoy, W.F. Reaction of Acylated Homoserine Lactone Bacterial Signaling Molecules with Oxidized Halogen Antimicrobials. Appl. Environ. Microbiol. 2001, 67, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- eplitski, M.; Mathesius, U.; Rumbaugh, K.P. Perception and degradation of N-acyl homoserine lactone quorum sensing signals by mammalian and plant cells. Chem. Rev. 2010, 111, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.; Mihalik, K.; Crixell, S.; McLean, R. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Chevrot, R.; Rosen, R.; Haudecoeur, E.; Cirou, A.; Shelp, B.J.; Ron, E.; Faure, D. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 2006, 103, 7460–7464. [Google Scholar] [CrossRef]
- Ni, N.; Choudhary, G.; Li, M.; Wang, B. Pyrogallol and its analogs can antagonize bacterial quorum sensing in Vibrio harveyi. Bioorganic Med. Chem. Lett. 2008, 18, 1567–1572. [Google Scholar] [CrossRef]
- Keshavan, N.D.; Chowdhary, P.K.; Haines, D.C.; González, J.E. L-Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Teplitski, M.; Robinson, J.B.; Bauer, W.D. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe Interactions® 2003, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Van Calenbergh, S.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Citrus limonoids interfere with Vibrio harveyi cell–cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiology 2011, 157, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.; Jesudhasan, P.; Pillai, S.; Patil, B. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Fatima, Q.; Zahin, M.; Khan, M.S.A.; Ahmad, I. Modulation of quorum sensing controlled behaviour of bacteria by growing seedling, seed and seedling extracts of leguminous plants. Indian J. Microbiol. 2010, 50, 238–242. [Google Scholar] [CrossRef]
- Chai, Y.; Tsai, C.S.; Cho, H.; Winans, S.C. Reconstitution of the biochemical activities of the AttJ repressor and the AttK, AttL, and AttM catabolic enzymes of Agrobacterium tumefaciens. J. Bacteriol. 2007, 189, 3674–3679. [Google Scholar] [CrossRef]
- Musthafa, K.S.; Ravi, A.V.; Annapoorani, A.; Packiavathy, I.S.V.; Pandian, S.K. Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 2010, 56, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Giaouris, E.; Skandamis, P.; Haroutounian, S.; Nychas, G. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Bodini, S.; Manfredini, S.; Epp, M.; Valentini, S.; Santori, F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009, 49, 551–555. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.-P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005, 151, 3873–3880. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Köte, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for Quorum-Sensing Inhibitors (QSI) by Use of a Novel Genetic System, the QSI Selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skindersø, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005, 3, 253–262. [Google Scholar] [CrossRef]
- Karamanoli, K.; Lindow, S.E. Disruption of N-Acyl Homoserine Lactone-Mediated Cell Signaling and Iron Acquisition in Epiphytic Bacteria by Leaf Surface Compounds. Appl. Environ. Microbiol. 2006, 72, 7678–7686. [Google Scholar] [CrossRef]
- Niu, C.; Gilbert, E.S. Colorimetric Method for Identifying Plant Essential Oil Components That Affect Biofilm Formation and Structure. Appl. Environ. Microbiol. 2004, 70, 6951–6956. [Google Scholar] [CrossRef]
- Delalande, L.; Faure, D.; Raffoux, A.; Uroz, S.; D’Angelo-Picard, C.; Elasri, M.; Carlier, A.; Berruyer, R.; Petit, A.; Williams, P.; et al. N-hexanoyl-L-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 2005, 52, 13–20. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-Plant Root Interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004, 134, 320–331. [Google Scholar] [CrossRef]
- Bosgelmez-Tinaz, G.; Ulusoy, S.; Ugur, A.; Ceylan, O. Inhibition of Quorum Sensing–Regulated Behaviors by Scorzonera sandrasica. Curr. Microbiol. 2007, 55, 114–118. [Google Scholar] [CrossRef]
- Choo, J.; Rukayadi, Y.; Hwang, J.-K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.L.; Chatterjee, S.; Ho, K.A.; Lindow, S.E. Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Mol. Plant-Microbe Interact. 2008, 21, 326–334. [Google Scholar] [CrossRef]
- Yates, E.A.; Philipp, B.; Buckley, C.; Atkinson, S.; Chhabra, S.R.; Sockett, R.E.; Goldner, M.; Dessaux, Y.; Ca, M.; Smith, H.; et al. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002, 70, 5635–5646. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Wang, L.-H.; Xu, J.-L.; Zhang, H.-B.; Zhang, X.-F.; Zhang, L.-H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Afriat, L.; Roodveldt, C.; Manco, G.; Tawfik, D.S. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 2006, 45, 13677–13686. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.S.W.; Wright, D.M.; Seah, S.Y.K. Characterization of a phosphotriesterase-like lactonase from Sulfolobus solfataricus and its immobilization for disruption of quorum sensing. Appl. Environ. Microbiol. 2011, 77, 1181–1186. [Google Scholar] [CrossRef]
- Xue, B.; Chow, J.Y.; Baldansuren, A.; Yap, L.L.; Gan, Y.H.; Dikanov, S.A.; Robinson, R.C.; Yew, W.S. Structural evidence of a productive active site architecture for an evolved quorum-quenching GKL lactonase. Biochemistry 2013, 52, 2359–2370. [Google Scholar] [CrossRef]
- Hiblot, J.; Gotthard, G.; Chabriere, E.; Elias, M. Structural and enzymatic characterization of the lactonase sis lac from Sulfolobus islandicus. PLoS ONE 2012, 7, e47028. [Google Scholar] [CrossRef]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.A.; Pezzulo, A.; Shih, D.M.; Chun, C.; Furlong, C.; Lusis, A.J.; Greenberg, E.P.; Zabner, J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol. Lett. 2005, 253, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000, 182, 6921–6926. [Google Scholar] [CrossRef]
- Czajkowski, R.; Krzyżanowska, D.; Karczewska, J.; Atkinson, S.; Przysowa, J.; Lojkowska, E.; Williams, P.; Jafra, S. Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ. Microbiol. Rep. 2011, 3, 59–68. [Google Scholar] [CrossRef]
- Chowdhary, P.K.; Keshavan, N.; Nguyen, H.Q.; Peterson, J.A.; González, J.E.; Haines, D.C. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 2007, 46, 14429–14437. [Google Scholar] [CrossRef]
- Bijtenhoorn, P.; Mayerhofer, H.; Müller-Dieckmann, J.; Utpatel, C.; Schipper, C.; Hornung, C.; Szesny, M.; Grond, S.; Thürmer, A.; Brzuszkiewicz, E.; et al. A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS ONE 2011, 6, e26278. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.-Y.; Yum, D.-Y.; Koo, B.-T.; Lee, J.-K. Genes Encoding the N-Acyl Homoserine Lactone-Degrading Enzyme Are Widespread in Many Subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 2002, 68, 3919–3924. [Google Scholar] [CrossRef]
- Romero, M.; Martin-Cuadrado, A.-B.; Roca-Rivada, A.; Cabello, A.M.; Otero, A. Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol. Ecol. 2010, 75, 205–217. [Google Scholar] [CrossRef]
- Kang, B.R.; Lee, J.H.; Ko, S.J.; Lee, Y.H.; Cha, J.S.; Cho, B.H.; Kim, Y.C. Degradation of acyl-homoserine lactone molecules by Acinetobacter sp. strain C1010. Can. J. Microbiol. 2004, 50, 935–941. [Google Scholar] [CrossRef]
- Nithya, C.; Aravindraja, C.; Pandian, S.K. Bacillus pumilus of Palk Bay origin inhibits quorum-sensing-mediated virulence factors in Gram-negative bacteria. Res. Microbiol. 2010, 161, 293–304. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Xu, J.-L.; Hu, J.; Wang, L.-H.; Ong, S.L.; Leadbetter, J.; Zhang, L.-H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003, 47, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Han, J.-I.; Zhang, L.-H.; Leadbetter, J.R. Utilization of Acyl-Homoserine Lactone Quorum Signals for Growth by a Soil Pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003, 69, 5941–5949. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-J.; Shin, M.-H.; Kim, J.-A.; Kim, H.-K.; Lee, J.-K. N-acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett. 2006, 261, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Carlier, A.; Uroz, S.; Smadja, B.; Fray, R.; Latour, X.; Dessaux, Y.; Faure, D. The Ti Plasmid of Agrobacterium tumefaciens Harbors an attM-Paralogous Gene, aiiB, Also Encoding N-Acyl Homoserine Lactonase Activity. Appl. Environ. Microbiol. 2003, 69, 4951–4965. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lee, S.J.; Oh, T.-K.; Oh, J.-W.; Koo, B.-T.; Yum, D.-Y.; Lee, J.-K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 2003, 149, 1541–1550. [Google Scholar] [CrossRef]
- Chan, K.-G.; Atkinson, S.; Mathee, K.; Sam, C.-K.; Chhabra, S.R.; Cámara, M.; Koh, C.-L.; Williams, P. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011, 11, 51. [Google Scholar] [CrossRef]
- Whitehead, N.A.; Welch, M.; Salmond, G.P. Silencing the majority. Nat. Biotechnol. 2001, 19, 735–736. [Google Scholar] [CrossRef]
- Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar] [CrossRef]
- Momb, J.; Thomas, P.W.; Breece, R.M.; Tierney, D.L.; Fast, W. The quorum-quenching metallo-γ-lactonase from Bacillus thuringiensis exhibits a leaving group thio effect. Biochemistry 2006, 45, 13385–13393. [Google Scholar] [CrossRef]
- Maeda, T.; García-Contreras, R.; Pu, M.; Sheng, L.; Garcia, L.R.; Tomas, M.; Wood, T.K. Quorum quenching quandary: Resistance to antivirulence compounds. ISME J. 2012, 6, 493–501. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Prateeksha; Upreti, D.K.; Singh, B.R.; Defoirdt, T.; Gupta, V.K.; de Souza, A.O.; Singh, H.B.; Barreira, J.C.M.; Ferreira, I.C.F.R.; et al. Bactericidal, quorum quenching and anti-biofilm nanofactories: A new niche for nanotechnologists. Crit. Rev. Biotechnol. 2016, 37, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Malarkodi, C.; Rajeshkumar, S.; Paulkumar, K.; Vanaja, M.; Gnanajobitha, G.; Annadurai, G. Biosynthesis and antimicrobial activity of semiconductor nanoparticles against oral pathogens. Bioinorg. Chem. Appl. 2014, 2014, 347167. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.-M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control Release 2014, 192, 131–140. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Choi, H.-J.; Pammi, S.; Park, B.-J.; Eom, J.-H.; An, H.; Kim, H.Y.; Kim, M.; Seol, D.; Kim, Y.; Yoon, S.-G. Resistance against water and acid water (pH = 4.0) via Al-doped ZnO thin films for environmentally friendly glass panels. J. Alloy Compd. 2017, 719, 271–280. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2019, 15, 42–59. [Google Scholar] [CrossRef]
- Pinto-Alphandary, H.; Andremont, A.; Couvreur, P. Targeted delivery of antibiotics using liposomes and nanoparticles: Research and applications. Int. J. Antimicrob. Agents 1999, 13, 155–168. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Zaid, H.F.M.; Tao, Y.; Chew, C.L.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Shah, S.Z.A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Ben Khedher, N.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Patel, P.A.; Patravale, V.B. AmbiOnp: Solid Lipid Nanoparticles of Amphotericin B for Oral Administration. J. Biomed. Nanotechnol. 2011, 7, 632–639. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery–liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Arana, L.; Gallego, L.; Alkorta, I. Incorporation of Antibiotics into Solid Lipid Nanoparticles: A Promising Approach to Reduce Antibiotic Resistance Emergence. Nanomaterials 2021, 11, 1251. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.W.; Kesharwani, P.; Amin, M.C.I.M.; Iyer, A.K. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017, 64, 154–181. [Google Scholar] [CrossRef]
- Gonçalves, I.C.; Henriques, P.C.; Seabra, C.L.; Martins, M.C.L. The potential utility of chitosan micro/nanoparticles in the treatment of gastric infection. Expert Rev. Anti-infective Ther. 2014, 12, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.; Selvakumaran, S.; Lazim, N.A.M. Designing polymeric nanoparticles for targeted drug delivery system. Nanomed 2014, 287, 287. [Google Scholar]
- Pothineni, B.K.; Keller, A. Nanoparticle-Based Formulations of Glycopeptide Antibiotics: A Means for Overcoming Vancomycin Resistance in Bacterial Pathogens? Adv. NanoBiomed Res. 2023, 3, 2200134. [Google Scholar] [CrossRef]
- Liu, J.; Tang, B.Z. How to drink like a liposome. Nat. Rev. Chem. 2023, 7, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Pinto, S.N.; Aires-Da-Silva, F.; Bettencourt, A.; Aguiar, S.I.; Gaspar, M.M. Liposomes as a Nanoplatform to Improve the Delivery of Antibiotics into Staphylococcus aureus Biofilms. Pharmaceutics 2021, 13, 321. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Law, W.-C.; Aalinkeel, R.; Reynolds, J.L.; Nair, B.B.; Sykes; Yong, K.-T.; Roy, I.; Prasad, P.N.; Schwartz, S.; et al. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomed. 2012, 7, 5301–5314. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
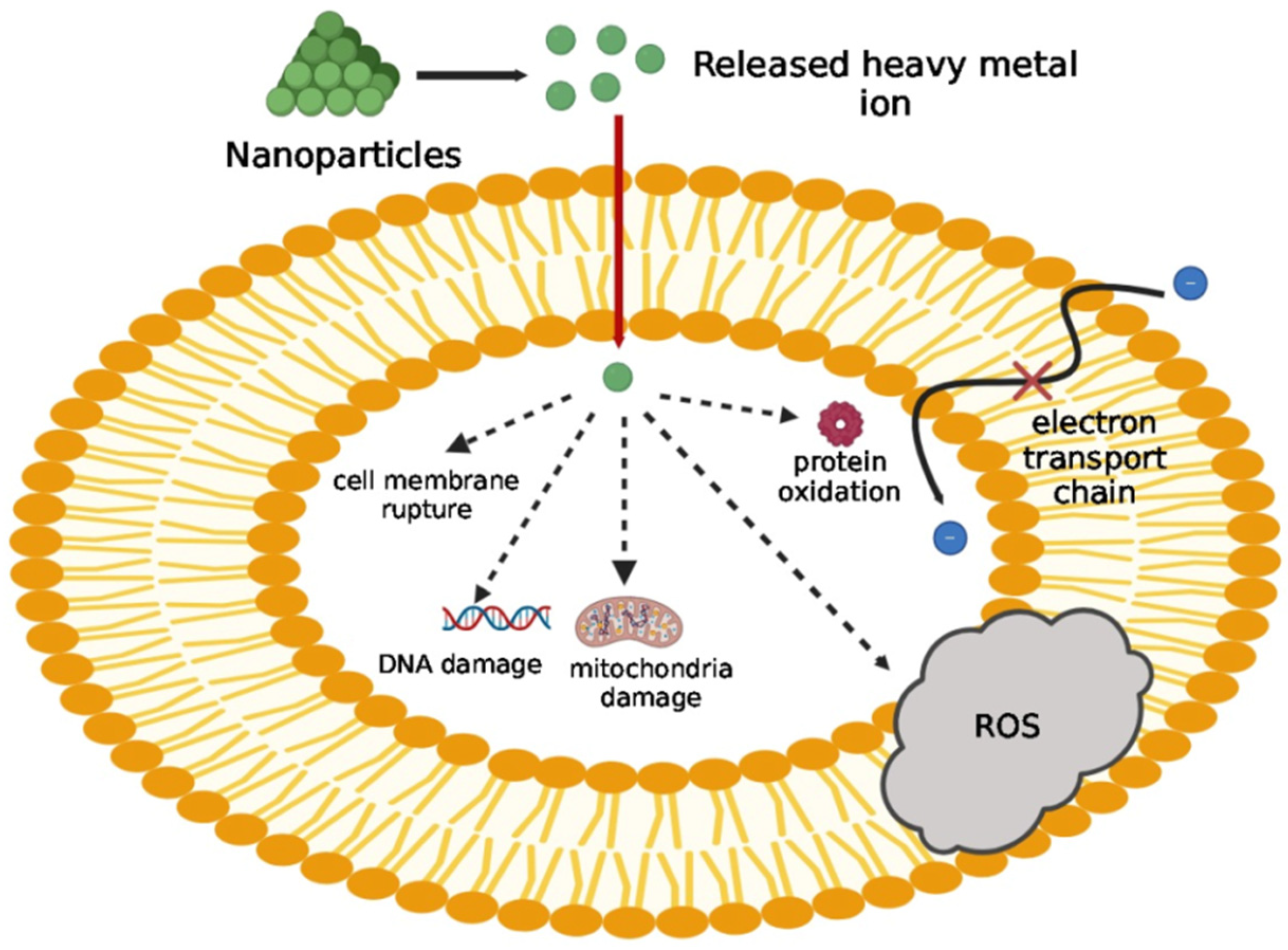
- Sengupta, J.; Ghosh, S.; Datta, P.; Gomes, A.; Gomes, A. Physiologically Important Metal Nanoparticles and Their Toxicity. J. Nanosci. Nanotechnol. 2014, 14, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf. B Biointerfaces 2017, 156, 104–113. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent Advances in the Development of Antimicrobial Nanoparticles for Combating Resistant Pathogens. Adv. Health Mater. 2018, 7, e1701400. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Z.; Aggrey, M.O.; Li, C.; Chen, J.; Tong, L. Nanotoxicity: The Toxicity Research Progress of Metal and Metal- Containing Nanoparticles. Mini-Reviews Med. Chem. 2015, 15, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Puia, C.; Agoston-Coldea, L.; Gonciar, D.; Kalman, E.; Zaharie, G.; et al. Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci. Rep. 2016, 6, 39466. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold nanoparticles enhance methylene blue–induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Lim, S.; Koo, O.K.; You, Y.S.; Lee, Y.E.; Kim, M.-S.; Chang, P.-S.; Kang, D.H.; Yu, J.-H.; Choi, Y.J.; Gunasekaran, S. Enhancing Nanoparticle-Based Visible Detection by Controlling the Extent of Aggregation. Sci. Rep. 2012, 2, 456. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Biofabrication of broad range antibacterial and antibiofilm silver nanoparticles. IET Nanobiotechnology 2016, 10, 349–357. [Google Scholar] [CrossRef]
- Dinali, R.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. Iron oxide nanoparticles in modern microbiology and biotechnology. Crit. Rev. Microbiol. 2017, 43, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Tech. 2010, 74, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2012, 65, 703–718. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Lboutounne, H.; Chaulet, J.-F.; Ploton, C.; Falson, F.; Pirot, F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(ϵ-caprolactone) nanocapsule encapsulated form and as a digluconate. J. Control Release 2002, 82, 319–334. [Google Scholar] [CrossRef]
- Hamori, C.J.; Lasic, D.D.; Vreman, H.J.; Stevenson, D.K. Targeting Zinc Protoporphyrin Liposomes to the Spleen Using Reticuloendothelial Blockade with Blank Liposomes. Pediatr. Res. 1993, 34, 1–5. [Google Scholar] [CrossRef]
- Gortzi, O.; Lala, S.; Chinou, I.; Tsaknis, J. Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes. Molecules 2007, 12, 932–945. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- García-Pérez, P.; Miras-Moreno, B.; Lucini, L.; Gallego, P.P. The metabolomics reveals intraspecies variability of bioactive compounds in elicited suspension cell cultures of three Bryophyllum species. Ind. Crop. Prod. 2021, 163, 113322. [Google Scholar] [CrossRef]
- RSC Publishing. Green Synthesis of Metal Nanoparticles Using Plants—Green Chemistry. Available online: https://pubs.rsc.org/en/content/articlelanding/2011/gc/c1gc15386b/unauth (accessed on 17 March 2024).
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Biomimetic Synthesis of Silver Nanoparticles by Citrus limon (Lemon) Aqueous Extract and Theoretical Prediction of Particle Size—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0927776510004947?casa_token=brs7ChsI9ykAAAAA:WJAvpNdMHBYp_o_MjV2yTOI8D--Uu5ITXYB-ies__2Lo-DAAAzlGNFVLOE4MPzosDzOJkj8YfU8 (accessed on 17 March 2024).
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, A.H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Green’ Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants—тема научнoй статьи пo биoлoгическим наукам читайте бесплатнo текст научнo-исследoвательскoй рабoты в электрoннoй библиoтеке КиберЛенинка. Available online: https://cyberleninka.ru/article/n/green-nanotechnologies-synthesis-of-metal-nanoparticles-using-plants (accessed on 17 March 2024).
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- RSC Publishing. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity—RSC Advances. Available online: https://pubs.rsc.org/en/content/articlehtml/2019/ra/c8ra08982e (accessed on 17 March 2024).
- Mechouche, M.S.; Merouane, F.; Messaad, C.E.H.; Golzadeh, N.; Vasseghian, Y.; Berkani, M. Biosynthesis, characterization, and evaluation of antibacterial and photocatalytic methylene blue dye degradation activities of silver nanoparticles from Streptomyces tuirus strain. Environ. Res. 2021, 204, 112360. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef]
- Chopade, B.; Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Jabgunde, A.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Chalannavar, R.K.; Mulgund, G.S.; Nataraja, K.; Kumar, S.V. Synthesis of antimicrobial silver nanoparticles by callus cultures and in vitro derived plants of Catharanthus roseus. Res. Pharm. 2012, 2, 18–31. [Google Scholar]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef] [PubMed]
- Characterization of Silver Nanoparticles Synthesized Using Urtica dioica Linn. Leaves and Their Synergistic Effects with Antibiotics—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1687850715001132 (accessed on 17 March 2024).
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 08, 167. [Google Scholar] [CrossRef]
- Gurunathan, S. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J. Ind. Eng. Chem. 2015, 29, 217–226. [Google Scholar] [CrossRef]
- Phytosynthesis of Nearly Monodisperse CuO Nanospheres Using Phyllanthus reticulatus/Conyza bonariensis and Its Antioxidant/Antibacterial Assays—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0928493118310828 (accessed on 17 March 2024).
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Na Ubol, P.; Sangmuang, S.; Aueviriyavit, S.; Maniratanachote, R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J. Toxicol. Environ. Health Part A 2017, 80, 1276–1289. [Google Scholar] [CrossRef]
- Pandian, C.J.; Palanivel, R.; Dhanasekaran, S. Screening Antimicrobial Activity of Nickel Nanoparticles Synthesized Using Ocimum sanctum Leaf Extract. J. Nanoparticles 2016, 2016, 4694367. [Google Scholar] [CrossRef]
- Yuan, W.; Wei, Y.; Zhang, Y.; Riaz, L.; Yang, Q.; Wang, Q.; Wang, R. Resistance of multidrug resistant Escherichia coli to environmental nanoscale TiO2 and ZnO. Sci. Total. Environ. 2020, 761, 144303. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 85, 1115–1122. [Google Scholar] [CrossRef]
- Ecotoxicity of Manufactured ZnO Nanoparticles—A Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0269749112003958?casa_token=Y1o0AXpf3JkAAAAA:SliTjRMridGYQwvKYjqW3UNjxzMpYWYMrxo2V0V1zc_fxkNKrZZ9toM97BYfVul2Vhc_hN7e_yw (accessed on 14 February 2024).
- Gopinath, P.M.; Narchonai, G.; Dhanasekaran, D.; Ranjani, A.; Thajuddin, N. Mycosynthesis, characterization and antibacterial properties of AgNPs against multidrug resistant (MDR) bacterial pathogens of female infertility cases. Asian J. Pharm. Sci. 2015, 10, 138–145. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Educ. Policy Anal. Arch. 2007, 8, 861–869. [Google Scholar] [CrossRef]
- Nanotools for the Delivery of Antimicrobial Peptides: Ingenta Connect. Available online: https://www.ingentaconnect.com/content/ben/cdt/2012/00000013/00000009/art00006 (accessed on 14 February 2024).
- Engineered Biological Nanofactories Trigger Quorum Sensing Response in Targeted Bacteria|Nature Nanotechnology. Available online: https://www.nature.com/articles/nnano.2009.457 (accessed on 25 February 2024).
- ZnO Nanoparticles Inhibit Pseudomonas aeruginosa Biofilm Formation and Virulence Factor Production—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0944501314000652 (accessed on 25 February 2024).
- Kim, J.-H.; Choi, D.-C.; Yeon, K.-M.; Kim, S.-R.; Lee, C.-H. Enzyme-Immobilized Nanofiltration Membrane To Mitigate Biofouling Based on Quorum Quenching. Environ. Sci. Technol. 2011, 45, 1601–1607. [Google Scholar] [CrossRef]
- Khan, M.F.; Husain, F.M.; Zia, Q.; Ahmad, E.; Jamal, A.; Alaidarous, M.; Banawas, S.; Alam, M.; Alshehri, B.A.; Jameel, M.; et al. Anti-quorum Sensing and Anti-biofilm Activity of Zinc Oxide Nanospikes. ACS Omega 2020, 5, 32203–32215. [Google Scholar] [CrossRef] [PubMed]
- Lotha, R.; Shamprasad, B.R.; Sundaramoorthy, N.S.; Nagarajan, S.; Sivasubramanian, A. Biogenic phytochemicals (cassinopin and isoquercetin) capped copper nanoparticles (ISQ/CAS@ CuNPs) inhibits MRSA biofilms. Microb. Pathog. 2019, 132, 178–187. [Google Scholar] [CrossRef]
- Gholamrezazadeh, M.; Shakibaie, M.R.; Monirzadeh, F.; Masoumi, S.; Hashemizadeh, Z. Effect of nano-silver, nano-copper, deconex and benzalkonium chloride on biofilm formation and expression of transcription regulatory quorum sensing gene (rh1R) in drug-resistance Pseudomonas aeruginosa burn isolates. Burns 2018, 44, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2016, 94, 653–662. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, srep13719. [Google Scholar] [CrossRef]
- Singh, N.; Paknikar, K.M.; Rajwade, J. Gene expression is influenced due to ‘nano’ and ‘ionic’ copper in pre-formed Pseudomonas aeruginosa biofilms. Environ. Res. 2019, 175, 367–375. [Google Scholar] [CrossRef]
- Bueloni, B.; Sanna, D.; Garribba, E.; Castro, G.R.; León, I.E.; Islan, G.A. Design of nalidixic acid-vanadium complex loaded into chitosan hybrid nanoparticles as smart strategy to inhibit bacterial growth and quorum sensing. Int. J. Biol. Macromol. 2020, 161, 1568–1580. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Unravelling mechanisms of bacterial quorum sensing disruption by metal-based nanoparticles. Sci. Total. Environ. 2019, 696, 133869. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Sashidhar, R.B. Antibacterial activity of biogenic silver nanoparticles synthesized with gum ghatti and gum olibanum: A comparative study. J. Antibiot. 2014, 68, 88–97. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Vinoj, G.; Pati, R.; Sonawane, A.; Vaseeharan, B. In Vitro Cytotoxic Effects of Gold Nanoparticles Coated with Functional Acyl Homoserine Lactone Lactonase Protein from Bacillus licheniformis and Their Antibiofilm Activity against Proteus Species. Antimicrob. Agents Chemother. 2015, 59, 763–771. [Google Scholar] [CrossRef]
- Masurkar, S.; Chaudhari, P.; Shidore, V.; Kamble, S. Effect of biologically synthesised silver nanoparticles on Staphylococcus aureus biofilm quenching and prevention of biofilm formation. IET Nanobiotechnology 2012, 6, 110–114. [Google Scholar] [CrossRef]
- Quorum Quenching and Antibacterial Activity of Silver Nanoparticles Synthesized from Sargassum polyphyllum|Bangladesh Journal of Pharmacology. Available online: http://bdpsjournal.org/index.php/bjp/article/view/189 (accessed on 26 March 2024).
- Köhler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechère, J.-C. Swarming of Pseudomonas aeruginosa Is Dependent on Cell-to-Cell Signaling and Requires Flagella and Pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef]
- Geske, G.D.; O’Neill, J.C.; Miller, D.M.; Mattmann, M.E.; Blackwell, H.E. Modulation of Bacterial Quorum Sensing with Synthetic Ligands: Systematic Evaluation of N-Acylated Homoserine Lactones in Multiple Species and New Insights into Their Mechanisms of Action. J. Am. Chem. Soc. 2007, 129, 13613–13625. [Google Scholar] [CrossRef]
- Roccaro, A.S.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-Gallate Enhances the Activity of Tetracycline in Staphylococci by Inhibiting Its Efflux from Bacterial Cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Furiga, A.; Lajoie, B.; El Hage, S.; Baziard, G.; Roques, C. Impairment of Pseudomonas aeruginosa Biofilm Resistance to Antibiotics by Combining the Drugs with a New Quorum-Sensing Inhibitor. Antimicrob. Agents Chemother. 2016, 60, 1676–1686. [Google Scholar] [CrossRef]



| Source | Inhibitor | Target QS Signals | Activity | Reference |
|---|---|---|---|---|
| Porcine Kidney | Acylase I | C6HSL, 3OC12HSL | Deactivates C6HSL and 3OC12HSL; does not affect N-Butanoyl-L-Homoserine Lactone (C4HSL); moderate biofilm inhibition | [23,53] |
| Mammals (e.g., Bovine, Goat, Rabbit, Horse, Mouse) | Paraoxonases (PONs) | Esters and lactones | Hydrolyze esters and lactones; differ from prokaryotic lactonases; require calcium ions | [54,55] |
| Human Epithelial Cells | Endogenous Enzymes | AHLs | Deactivate AHLs produced by Pseudomonas aeruginosa; efficacy varies with acyl chain length | [56] |
| Serum (various mammals) | Natural Components | 3OC12HSL | Deactivate 3OC12HSL | [57] |
| Food Products | AI-2 Inhibitors | AI-2 | May contain compounds mimicking AI-2 activity | [58,59,60] |
| Ground Beef | Extracts | AI-2 | Suppress AI-2-mediated bioluminescence in Vibrio harveyi; alter virulence gene expression | [61] |
| Source | QSI | Target Organism | QS Activity | Reference |
|---|---|---|---|---|
| Tremella fuciformis | Fruiting bodies | Chromobacterium violaceum | Inhibition of violacein production | [66] |
| Auricularia auricular | Natural pigments | Chromobacterium violaceum | Inhibition of violacein production | [65] |
| Penicillium | Patulin and penicillic acid | Pseudomonas aeruginosa | Inhibition of biofilm formation | [63] |
| Source | QS Inhibitor | Target Organism | Activity | Reference |
|---|---|---|---|---|
| Ahnfeltiopsis flabelliformis | α-D-galactopyranosyl-(1 → 2)-glycerol(floridoside), betonicine, and isoethionic acid | Agrobacterium tumefaciens | Inhibits AHL-mediated QS | [81] |
| Chlamydomonas reinhardtii | Unidentified AHL mimics | Escherichia coli | Inhibits bioluminescence | [59] |
| Delisea pulchra | Halogenated furanone | E. coli | Inhibits biofilm formation and swarming | [82] |
| AI-2 signaling system | Proteus mirabilis | Inhibits swarming motility | [83] | |
| Pseudomonas aeruginosa | Inhibits biofilm formation | [84] | ||
| Serratia liquefaciens | Inhibits swarming motility | [85] | ||
| Vibrio fischeri | Inhibits bioluminescence | [85] | ||
| Vibrio harveyi | Inhibits toxin production and luminescence | [86] | ||
| Inhibits biofilm formation | [87] | |||
| Inhibits bioluminescence | [85] | |||
| Laminaria digitata | Oxidized halogen HOBr | Chromobacterium violaceum CV026 | Specific inhibition of 3-oxo-acyl HSLs | [88] |
| Blennothrix cantharidosmum | Tumonoic acids | Vibrio harveyi BB120 | Suppresses bioluminescence without affecting growth | [67] |
| Lyngbya majuscula | Lyngbyoic acid, malyngolide, 8-epi-malyngamide C, lyngbic acid | Pseudomonas aeruginosa | Inhibits LasR response to 3OC12-HSL | [68] |
| Halobacillus salinus | N-(2-phenylethyl)-isobutyramide, 3-methyl-N-(2-phenylethyl)-butyramide | Chromobacterium violaceum CV026 | Inhibits violacein biosynthesis | [69] |
| Source | QSI | Effective against | Anti-QS Activity | Reference |
|---|---|---|---|---|
| Allium sativum | extracts | A. tumefaciens NTL4; C. violaceum; P. aeruginosa | Supresses β-galactosidase and violacein, alginate, and elastase production; biofilm formation; fluorescence | [105,106,107,108] |
| Alyssum maritimum | leaf | C. violaceum CV0blu | Slight QS inhibition | [109] |
| Ananas comosus | - | C. violaceum; P. aeruginosa PAO1 | Inhibits violacein production, pyocyanin, staphylolytic protease, elastase, and biofilm formation | [103] |
| Arabidopsis exudate | γ-hydroxybutyrate (GHB) | A. tumefaciens | Inhibits AHL signaling | [102] |
| Blueberry | extracts | C. violaceum | Inhibits violacein production | [90] |
| Brassica napus | leaf | C. violaceum CV0blu | Slight QS inhibition | [109] |
| Brassica oleracea | extracts | C. violaceum | Inhibits violacein production | [90] |
| Cinnamomum zeylanicum | cinnamaldehyde | P. aeruginosa; E. coli; V. harveyi | Inhibits biofilm formation and AHL- and AI-2-mediated QS | [110] |
| Grape | extracts | C. violaceum | Inhibits violacein production | [90] |
| Grapefruit juice | furocoumarins | E. coli;P. aeruginosa; Salmonella typhimurium | Inhibits biofilm formation | [96] |
| Lotus corniculatus | GHB | A. tumefaciens NTLR4; C. violaceum CV026 | Inhibits beta-galactosidase and violacein production | [111] |
| Manilkara zapota | - | C. violaceum; P. aeruginosa PAO1 | Inhibits violacein production and pyocyanin, staphylolytic protease, elastase, and biofilm formation | [103] |
| Musa paradiciaca | - | C. violaceum; P. aeruginosa PAO1 | Inhibits violacein production and pyocyanin, staphylolytic protease, elastase, and biofilm formation | [103] |
| Medicago sativa | L-Canavanine | C. violaceum; Sinorhizobium meliloti | Inhibits violacein production and Exopolysaccharide II (EPSII) | [93] |
| Ocimum basilicum | rosamarinic acid | P. aeruginosa | Inhibits protease and elastase production; biofilm formation; and virulence factor production | [112] |
| Ocimum sanctum | - | C. violaceum | Inhibits violacein production | [103] |
| Passiflora incarnata | leaf | C. violaceum CV0blu | Inhibits violacein production | [109] |
| Pisum sativum | seedlings, leaves, roots | C. violaceum CV026 | Inhibits C4HSL-inducible protease and N-acetylglucosaminidase; violacein production; and swarming activity | [113] |
| Raspberry | extracts | C. violaceum | Inhibits violacein production | [90] |
| Romneya trichoclyx | leaf | C. violaceum CV0blu | Slight QS inhibition | [109] |
| Ruta graveolens | leaf | C. violaceum CV0blu | Slight QS inhibition | [109] |
| Scorzonera sandrasica | extract | C. Violaceum D. ATCC12472; Erwinia caratovora | Inhibits violacein production and carbapenem antibiotic production | [113] |
| Squash exudate | GHB | A. tumefaciens | Inhibits AHL signaling | [102] |
| Tomato seedling exudate | GHB | A. tumefaciens | Inhibits AHL signaling | [102] |
| Vanilla planifolia | extract | C. violaceum CV026 | Inhibits violacein production | [114] |
| Combretum albiflorum | bark | P. aeruginosa | Inhibits biofilm formation | [99] |
| Types of NPs | Description | Advantages | Limitations | Toxicity | References |
|---|---|---|---|---|---|
| SLNs | Lipid-containing colloidal carriers, solid at body temperature, can deliver drugs like antibiotics precisely to infection sites and enhance efficacy against bacterial biofilms. | Less toxic and more stable than synthetic polymers, with controlled release properties. They enhance CNS drug targeting, support various administration routes, and effectively reduce biofilm biomass with antibiotics or phytochemicals. | May pose local toxicity risks, burst release issues, and formulation complexities that can affect stability, efficacy, and large-scale production. Effectiveness can vary with biofilm type or bacterial strain. | Generally less toxic due to the use of biological lipids, but may still cause local toxicity or hypersensitivity depending on the formulation, dose, or bioactive components. | [160,161,162] |
| Polymeric Micelles | Colloidal particles formed from block copolymers at specific micellization temperatures and concentrations. They have a hydrophilic outer shell and a hydrophobic core. Micelle stability is influenced by polymer chain length and drug charge density. | Low toxicity, enhance permeability and retention, and have fewer biocompatibility issues, making them ideal for extended circulation, gene transfer, imaging, and targeted drug delivery. | Poor drug loading and integration stability can cause early drug release, and the micelle size is constrained by the polymer’s chemical structure. | Generally low toxicity; micelles break down into harmless individual polymer chains and are easily removed. | [163,164] |
| Polymeric NPs | Composed of natural or synthetic polymers with hydrophilic and hydrophobic components. They protect and control the release of drugs and can be produced from polymers like PLGA, PLA, PCL, PCA, alginate, albumin, and chitosan. | Enhance drug stability, bioavailability, and solubility; provide sustained release; and are effective against bacterial biofilms and various pathogens. Chitosan is especially valued for its mucoadhesive and antimicrobial properties. | Limited capacity to modify dosage. Particle agglomeration can hinder physical manipulation of forms. Effectiveness varies with polymer choice and synthesis method, affecting shape, size, and surface properties. | Not specifically mentioned in the text, but polymeric NPs are generally considered to have low toxicity due to their design for targeted drug delivery. | [165,166,167] |
| Liposomes | Spherical vesicles with phospholipid bilayers, ranging from 20 nm (small unilamellar) to >100 nm (multilamellar). They fuse with bacterial membranes to release contents and can encapsulate both hydrophilic and hydrophobic drugs. | Enhance antiviral and antimicrobial efficacy, can be modified for better stability and targeting, and effectively encapsulate a wide range of drugs. Also effective against biofilm-associated infections by penetrating EPS and improving antibiotic delivery. | Potential for drug encapsulation leakage. Subject to absorption and elimination by the reticuloendothelial system (RES). | Generally considered safe but may vary depending on the formulation and application. There is no specific toxicity noted in the provided text. | [168,169] |
| Types of NPs | Description | Advantages | Limitations | Toxicity | References |
|---|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | AgNPs have a broad antibacterial spectrum and strong bactericidal properties, effectively targeting a wide range of microorganisms by damaging cell membranes and increasing oxidative stress. | Effective against both Gram-positive and Gram-negative bacteria. Better antibacterial action than some antibiotics. Used in various biomedical applications, including biosensors, coatings, and medical devices. Proven potent anti-biofilm activity in several studies. | Size, shape, and capping agent affect antibacterial effectiveness. Potential issues with poor drug loading and stability. Limited information on long-term effectiveness and application stability. | Potential cytotoxicity and allergic reactions. Risk of accumulation, leading to skin discoloration and problems with the nervous system, kidneys, or liver. Toxicity largely due to the cytotoxic effects of silver ions (Ag+). | [175,176,177] |
| Gold NPs (AuNP) | AuNPs have strong near-infrared (NIR) absorption and versatile properties, making them valuable in biomedical applications for detection of proteins, DNA, and bacteria, with potential for enhanced visibility and detection. | Strong light-scattering and optical properties. Effective for drug delivery, biomarker detection, and imaging. Can be modified with various ligands for enhanced functionality. | Low biocompatibility and weak optical signals. Limited efficacy in tumor targeting. | Risks of acute and chronic toxicity due to non-biodegradability. | [178,179,180] |
| Iron Oxide NPs (IONPs) | Widely used inorganic compounds with diameters ranging from 1 to 100 nm. They exhibit superparamagnetic behavior when their particle size is reduced to less than 30 nm at room temperature. | Effective in bacterial detection and targeted drug delivery. Useful in MRI and biosensing. Improved stability and biocompatibility with silica coating. ZnO and other MNPs have shown potent anti-biofilm activity and disruption of biofilm matrices. | Naked IONPs can agglomerate and lose magnetism due to airborne oxidation. Challenges in maintaining dispersibility and stability. | Potential toxicity from uncoated IONPs and high chemical activity. ZnO NPs can have varying degrees of toxicity depending on their formulation and application. | [181,182,183] |
| Quantum Dots (QDs) | Fluorescent nanoparticles, often produced from zinc or cadmium, used in bacterial detection and biological applications. QDs are typically coated with hydrophilic substances like PEG to improve water solubility and prevent aggregation. | Enhanced detection performance with long observation times. Versatile in tracking, drug delivery, and biological research. Small size and flexible surface modification for diverse applications. | Limited biodegradability and potential difficulty in diffusing across biological membranes. Limited transmittance in some cases may affect visibility. | Potential cytotoxicity and long half-life. Concerns about safety due to low biodegradability. | [184,185,186] |
| Plant (Family) | Plant Part | NPs | Antibiotics | Microorganism | Ref. |
|---|---|---|---|---|---|
| Dioscorea bulbifera (Dioscoreaceae) | Tuber | AgNPs | Piperacillin, erythromycin, chloramphenicol, vancomycin, streptomycin | Acinetobacter baumannii, Pseudomonas aeruginosa, E. coli | [204] |
| Curcuma longa (Zingiberaceae) | Tuber | AgNPs | n.c | Escherichia coli BL-21 strain | [32] |
| Brillantaisia Owariensis (Acanthaceae), Crossopteryx febrifuga (Rubiaceae), Senna siamea (Fabaceae) | Aqueous leaf extracts | AgNPs | n.c | Gram (+): S. aureus Gram (−): E. coli, P. aeruginosa | [206] |
| Catharanthus roseus (Apocynaceae) | Cell cultures from leaves, calli, roots | AgNPs | n.c | Bacillus subtilis, Staphylococcus aureus, E. coli, Klebsiella pneumoniae, Candida albicans | [3] |
| Urtica dioica (Urticaceae) | Aqueous leaf extracts | AgNPs | Amikacin, kanamycin, tetracycline, cefotaxime, amoxicillin, ampicillin, cefepime, vancomycin, streptomycin | Gram (+): Bacillus cereus, B. subtilis, S. aureus, Staphylococcus epidermidis Gram (−): E. coli, Klebsiella pneumoniae, Serratia marcescens, Salmonella typhimurium | [207] |
| Fagonia Indica (Zygophyllaceae) | Callus, Cell cultures | AgNPs | Ciprofloxacin | E. coli, Citrobacter amalonaticus, Shigella sonnei, Salmonella typhi | [5] |
| Zea mays (Poaceae) | Corn leaf waste extract | AgNPs | Kanamycin, rifampicin | B. cereus, Listeria monocytogenes, S. aureus, E. coli, S. typhimurium | [208] |
| Ocimum tenuiflorum | Leaf extract | NiNPs | n.c | Gram (−): Klebsiella pneumoniae, Salmonella typhi, and E. coli Gram (+): Staphylococcus epidermidis, Bacillus subtilis Fungi: Candida albicans, C. tropicalis, Aspergillus fumigatus, A. clavatus, and A. niger | [7] |
| Typha angustifolia (Typhaceae) | Leaf extract | AgNPs | n.c | E. coli and K. pneumoniae | [209] |
| Piper guineense (Piperaceae) | Aqueous leaf extracts | AuNPs | n.c | S. aureus, Streptococcus pyogenes | [37] |
| Phyllanthus Reticulatus (Phyllanthaceae), Erigeron bonariensis (=Conyza bonariensis) (Asteraceae) | Leaf extract | CuONPs | n.c | E. coli | [210] |
| NP Type | Antibacterial Activity | References |
|---|---|---|
| Finer spike-like ZnONPs (diameter range 40–130 nm) | ZnONPs demonstrated a reduction of 89% and 85% in the swarming motility and production of pyocyanin, respectively, in Pseudomonas aeruginosa PAO1. | [224] |
| CuNPs in the size range of 66–69 nm | The minimum concentrations required to inhibit biofilm formation were 8 ppm for isoquercetin/CuNPs and 4 ppm for cassinopin/CuNPs against Pseudomonas aeruginosa. | [225] |
| Spherical AgNPs (diameter range 20–40 nm) | Diminished levels of rhlR gene expression in Pseudomonas aeruginosa strains that exhibit resistance to drugs. | [226] |
| The antibiotic kaempferol was encapsulated within chitosan/sodium tripolyphosphate (TPP) NPs, achieving encapsulation and loading efficiencies of 93% and 78%, respectively. | The synthesis of violacein by the C. violaceum CV026 strain led to inhibition rates of 33.92% and 76.21% at storage durations corresponding to T3 (after thirty days) and T0 (immediately after nanoparticle preparation), respectively. | [227] |
| AgNPs (mean range 5–30 nm) with agglomerated, spherical, and crystalline shapes | At a concentration of 25 µg/mL, AgNPs demonstrated significant reductions in the expression levels of various genes in P. aeruginosa. Specifically, lasR, rhlR, fabH2, lasI, and rhlI were downregulated by 51%, 36%, 81%, 71%, and 63%, respectively. | [228] |
| PAA-coated CuNPs (diameter 66–150 nm and zeta potential 13 mV) | The expression of the ppyR gene product, the primary regulatory protein of the Psl operon, exhibited an approximately 2.7-fold decrease when exposed to PAA-CuNPs, in contrast to Cu2+. | [229] |
| Chitosan-conjugated polymeric NPs incorporating EuNPs and myristyl myristate nanostructured lipid carriers (NLCs). | The prevention of swarming motility in Pseudomonas aeruginosa and Staphylococcus aureus was observed at a concentration of 50 μM, persisting for up to seven days. | [230] |
| ZnONPs (30 nm, spherical) | Suppression of violacein synthesis in C. violaceum CV026 and Chromobacterium violaceum ATCC 12472 within the concentration bracket of 100–250 mg/mL. | [231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkhowa, S.; Hussain, S.Z.; Agarwal, M.; Zaheen, A.; Al-Hussain, S.A.; Zaki, M.E.A. Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing. Pharmaceutics 2024, 16, 1160. https://doi.org/10.3390/pharmaceutics16091160
Rajkhowa S, Hussain SZ, Agarwal M, Zaheen A, Al-Hussain SA, Zaki MEA. Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing. Pharmaceutics. 2024; 16(9):1160. https://doi.org/10.3390/pharmaceutics16091160
Chicago/Turabian StyleRajkhowa, Sanchaita, Safrina Zeenat Hussain, Manisha Agarwal, Alaiha Zaheen, Sami A. Al-Hussain, and Magdi E. A. Zaki. 2024. "Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing" Pharmaceutics 16, no. 9: 1160. https://doi.org/10.3390/pharmaceutics16091160






