Platinum Group Metals Nanoparticles in Breast Cancer Therapy
Abstract
:1. Introduction
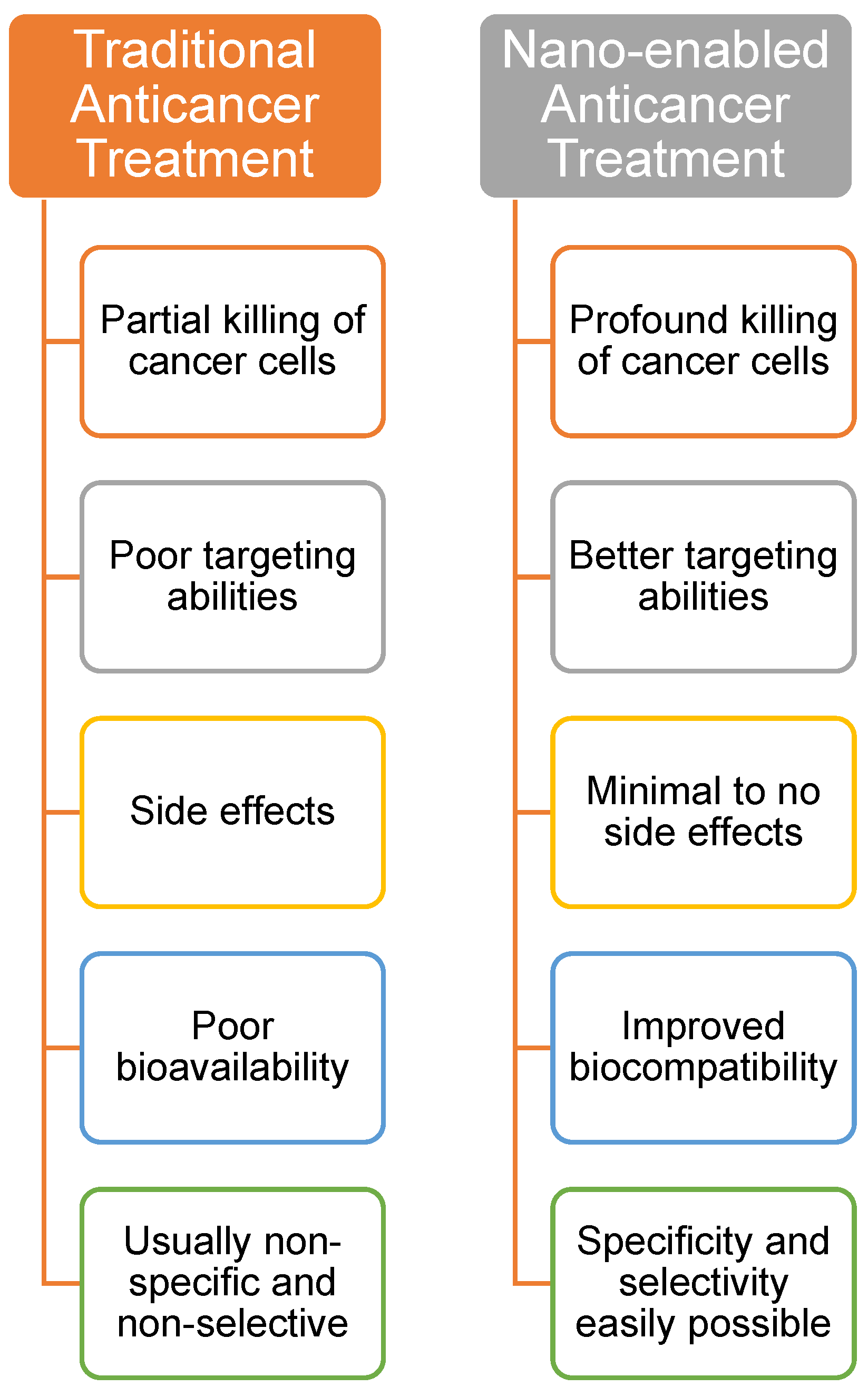
2. Nanotechnology in Breast Cancer Therapy
3. Platinum Group Metal Nanoparticles in Breast Cancer Therapy
3.1. Platinum-Based Nanoparticles
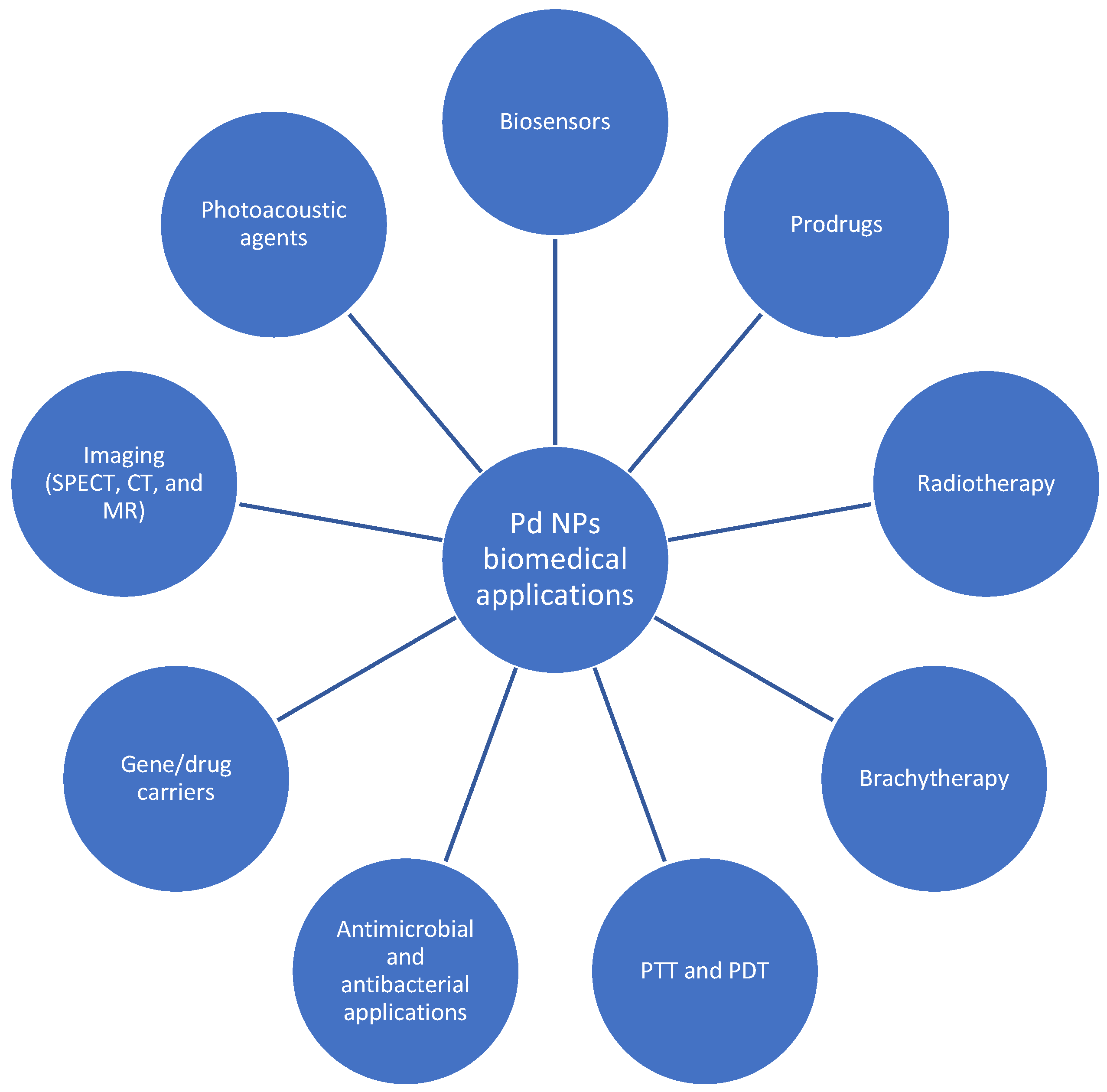
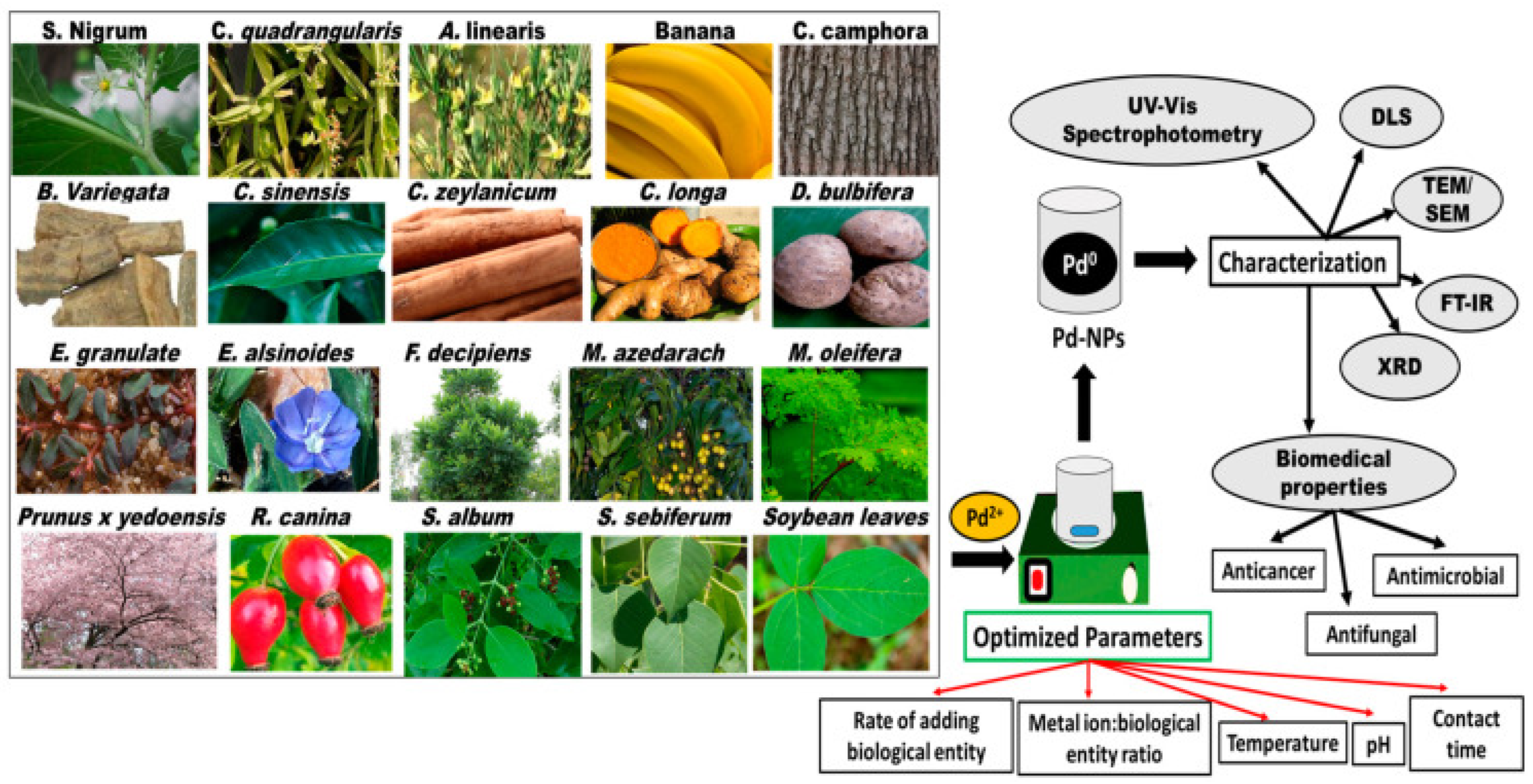
3.2. Palladium-Based Nanoparticles
3.3. Ruthenium-Based Nanoparticles
3.4. Rhodium-Based Nanoparticles
3.5. Osmium-Based Nanoparticles
3.6. Iridium-Based Nanoparticles
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Zhang, P.; Xiao, Y.; Sun, X.; Lin, X.; Koo, S.; Yaremenko, A.V.; Qin, D.; Kong, N.; Farokhzad, O.C.; Tao, W. Cancer Nanomedicine toward Clinical Translation: Obstacles, Opportunities, and Future Prospects. Med 2023, 4, 147–167. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Al-Saigul, A.M.; Abdelgadir, M.H. Breast Cancer: A Review of Risk Factors and Diagnosis. Medicine 2016, 103, e36905. [Google Scholar] [CrossRef]
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.; Ullah, O.; Balant, S.; Rahman, M.H. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int. J. Mol. Sci. 2022, 23, 2856. [Google Scholar] [CrossRef]
- Muhammad, N.; Hanif, M.; Yang, P. Beyond Cisplatin: New Frontiers in Metallodrugs for Hard-to-Treat Triple Negative Breast Cancer. Coord. Chem. Rev. 2024, 499, 215507. [Google Scholar] [CrossRef]
- Thoidingjam, S.; Tiku, A.B. New Developments in Breast Cancer Therapy: Role of Iron Oxide Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 023002. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Nur Khatijah, M.Z.; Al-Sowayan, B.S. Nanotechnology: A Revolutionary Approach to Prevent Breast Cancer Recurrence. Asian J. Surg. 2023, 46, 13–17. [Google Scholar] [CrossRef]
- Omidi, Y.; Mobasher, M.; Castejon, A.M.; Mahmoudi, M. Recent Advances in Nanoscale Targeted Therapy of HER2-Positive Breast Cancer. J. Drug Target. 2022, 30, 687–708. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-Induced Cardiotoxicity: Mechanisms of Action, Incidence, Risk Factors, Prevention, and Treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wang, X.; Marsch, S.; Hunziker, P. Intelligent Nanomaterials for Medicine: Carrier Platforms and Targeting Strategies in the Context of Clinical Application. Nanomedicine 2013, 9, 742–757. [Google Scholar] [CrossRef]
- Gandidzanwa, S.; Beukes, N.; Joseph, S.V.; Janse van Vuuren, A.; Britton, J.; Mashazi, P.; Kilian, G.; Roux, S.; Nyokong, T.; Lee, M.E.; et al. Folate-Functionalised Palladium Nanoparticles for Folate Receptor Targeting in Breast Cancer Cells. Nanotechnology 2023, 34, 465705. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, Q.; Dai, X.; Zhang, X.; Song, D. The Potential Role of Nanomedicine in the Treatment of Breast Cancer to Overcome the Obstacles of Current Therapies. Front. Pharmacol. 2023, 14, 1143102. [Google Scholar] [CrossRef]
- Huang, X.; He, T.; Liang, X.; Xiang, Z.; Liu, C.; Zhou, S.; Luo, R.; Bai, L.; Kou, X.; Li, X.; et al. Advances and Applications of Nanoparticles in Cancer Therapy. MedComm–Oncol. 2024, 3, e67. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Kumar Jha, N.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Klingberg, H.; Kjær, A.; Bendix, P.M.; Oddershede, L.B. Platinum Nanoparticles: A Non-Toxic, Effective and Thermally Stable Alternative Plasmonic Material for Cancer Therapy and Bioengineering. Nanoscale 2018, 10, 9097–9107. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.-A.; Han, H.; Santos, H.A. Nanoparticles-Based Phototherapy Systems for Cancer Treatment: Current Status and Clinical Potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Niculescu, A.G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Neophytou, S.; Nicodemou, E.; Giuffrida, S.G.; Ge, H.; Pascu, S.I. Nano-Theranostics for the Sensing, Imaging and Therapy of Prostate Cancers. Front. Chem. 2022, 10, 830133. [Google Scholar] [CrossRef]
- Liu, C.H.; Grodzinski, P. Nanotechnology for Cancer Imaging: Advances, Challenges, and Clinical Opportunities. Radiol. Imaging Cancer 2021, 3, e200052. [Google Scholar] [CrossRef]
- Sethi, S.; Jonwal, H.; Parihar, R. Nanoparticles in Cancer Treatment: A Review. J. Anal. Pharm. Res. 2023, 12, 88–93. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S. Advantages of Nanomedicine in Cancer Therapy: A Review. ACS Appl. Nano Mater. 2023, 6, 22594–22610. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The Use of Nanoparticulates to Treat Breast Cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New Challenges in the Use of Nanomedicine in Cancer Therapy. Bioengineered 2022, 13, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mutreja, I.; Kaushik, A. Recent Advances in Noble Metal Nanoparticles for Cancer Nanotheranostics. J. Nanotheranostics 2023, 4, 150–170. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef]
- Markowska, A.; Kasprzak, B.; Jaszczyńska-Nowinka, K.; Lubin, J.; Markowska, J. Noble Metals in Oncology. Contemp. Oncol. 2015, 19, 271–275. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured Carriers as Innovative Tools for Cancer Diagnosis and Therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef]
- Ntsimango, S.; Gandidzanwa, S.; Joseph, S.V.; Hosten, E.C.; Randall, M.; Edkins, A.L.; Khene, S.M.; Mashazi, P.; Nyokong, T.; Abrahams, A.; et al. Reaction of Perrhenate with Phthalocyanine Derivatives in the Presence of Reducing Agents and Rhenium Oxide Nanoparticles in Biomedical Applications. Chem. Open 2022, 11, e202200037. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Tong, F.; Wang, Y.; Gao, H. Progress and Challenges in the Translation of Cancer Nanomedicines. Curr. Opin. Biotechnol. 2024, 85, 103045. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Kataoka, K. Progress of Drug-Loaded Polymeric Micelles into Clinical Studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter Phase II Trial of Genexol-PM, a Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Parker, L.M.; Come, S.E.; Burstein, H.J.; Haldoupis, M.; Ryabin, N.; Gelman, R.; Winer, E.P.; Shulman, L.N. Phase II Study of CT-2103 as First- or Second-Line Chemotherapy in Patients with Metastatic Breast Cancer: Unexpected Incidence of Hypersensitivity Reactions. Invest. New Drugs 2007, 25, 369–375. [Google Scholar] [CrossRef]
- Baselga, J.; Manikhas, A.; Cortés, J.; Llombart, A.; Roman, L.; Semiglazov, V.; Byakhov, M.; Lokanatha, D.; Forenza, S.; Goldfarb, R.; et al. Phase III Trial of Nonpegylated Liposomal Doxorubicin in Combination with Trastuzumab and Paclitaxel in HER2-Positive Metastatic Breast Cancer. Ann. Oncol. 2014, 25, 592–598. [Google Scholar] [CrossRef]
- Rugo, H.; Pabbathi, H.; Shrestha, S.; Aithal, S.; Borys, N.; Musso, L.Z.I. Lyso-Thermosensitive Liposomal Doxorubicin Shows Efficacy with Minimal Adverse Events in Patients with Breast Cancer Recurrence at the Chest Wall. Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium. Cancer Res. 2015, 76, P6-13-15. [Google Scholar]
- Rosenbaum, P.; Artaud, C.; Bay, S.; Ganneau, C.; Campone, M.; Delaloge, S.; Gourmelon, C.; Loirat, D.; Medioni, J.; Pein, F.; et al. The Fully Synthetic Glycopeptide MAG-Tn3 Therapeutic Vaccine Induces Tumor-Specific Cytotoxic Antibodies in Breast Cancer Patients. Cancer Immunol. Immunother. 2020, 69, 703–716. [Google Scholar] [CrossRef]
- Ramalingam, V.; Raja, S.; Harshavardhan, M. In situ One-Step Synthesis of Polymer-Functionalized Palladium Nanoparticles: An Efficient Anticancer Agent against Breast Cancer. Dalton Trans. 2020, 49, 3510–3518. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. [Google Scholar] [CrossRef]
- Paca, A.M.; Benson, S.O.; Fatokun, A.A.; Ajibade, P.A. Synthesis, Spectroscopic Characterization and Anticancer Potential Studies of Organoruthenium(II) Arene Dithiocarbamate Complexes. J. Sulfur. Chem. 2023, 45, 12–23. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.S. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules 2020, 25, 4981. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, Y. Multifunctional Platinum-Based Nanoparticles for Biomedical Applications. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1410. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.; Kumar, R.; Thomas, S.; Kumar, M. Surface Functionalized Nanoparticles: A Boon to Biomedical Science. Chem. Biol. Interact. 2023, 380, 110537. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Siddiqui, F. Nanomedicine and Drug Delivery: A Mini Review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Pawar, A.A.; Sahoo, J.; Verma, A.; Lodh, A.; Lakkakula, J. Usage of Platinum Nanoparticles for Anticancer Therapy over Last Decade: A Review. Part. Part. Syst. Charact. 2021, 38, 2100115. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green Synthesis of Platinum Nanoparticles Using Saudi’s Dates Extract and Their Usage on the Cancer Cell Treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Şahin, B.; Aygün, A.; Gündüz, H.; Şahin, K.; Demir, E.; Akocak, S.; Şen, F. Cytotoxic Effects of Platinum Nanoparticles Obtained from Pomegranate Extract by the Green Synthesis Method on the MCF-7 Cell Line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Rao, M.; Chattopadhyay, A.; Bandyopadhyay, A.; Prasad, K.; Jha, A.K. Punica granatum Fabricated Platinum Nanoparticles: A Therapeutic Pill for Breast Cancer. AIP Conf. Proc. 2018, 1953, 8–12. [Google Scholar] [CrossRef]
- Abed, A.S.; Mishaal Mohammed, A.; Khalaf, Y.H. Novel Photothermal Therapy Using Platinum Nanoparticles in Synergy with Near-Infrared Radiation (NIR) against Human Breast Cancer MCF-7 Cell Line. Results Chem. 2022, 4, 100591. [Google Scholar] [CrossRef]
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Manimuthu, T.R. Cytotoxic Potentials of Biologically Fabricated Platinum Nanoparticles from Streptomyces sp. on MCF-7 Breast Cancer Cells. IET Nanobiotechnol. 2017, 11, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rokade, S.S.; Joshi, K.A.; Mahajan, K.; Patil, S.; Tomar, G.; Dubal, D.S.; Parihar, V.S.; Kitture, R.; Bellare, J.R.; Ghosh, S. Gloriosa superba Mediated Synthesis of Platinum and Palladium Nanoparticles for Induction of Apoptosis in Breast Cancer. Bioinorg. Chem. Appl. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Manzoor, S.; Bashir, D.J.; Imtiyaz, K.; Rizvi, M.M.A.; Ahamad, I.; Fatma, T.; Agarwal, N.B.; Arora, I.; Samim, M. Biofabricated Platinum Nanoparticles: Therapeutic Evaluation as a Potential Nanodrug against Breast Cancer Cells and Drug-Resistant Bacteria. RSC Adv. 2021, 11, 24900–24916. [Google Scholar] [CrossRef]
- Puja, P.; Vinita, N.M.; Devan, U.; Velangani, A.J.; Srinivasan, P.; Yuvakkumar, R.; Arul Prakash, P.; Kumar, P. Fluorescence Microscopy-Based Analysis of Apoptosis Induced by Platinum Nanoparticles against Breast Cancer Cells. Appl. Organomet. Chem. 2020, 34, e5740. [Google Scholar] [CrossRef]
- López Ruiz, A.; Villaseco Arribas, E.; McEnnis, K. Poly(Lactic-Co-Glycolic Acid) Encapsulated Platinum Nanoparticles for Cancer Treatment. Mater. Adv. 2022, 3, 2858. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous Platinum Nanoparticle-Based Nanoplatforms for Combined Chemo-Photothermal Breast Cancer Therapy. J. Colloid. Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Gong, X.; Zhang, H.; Ma, K.; Sui, Y.; Chen, B.; Du, Y.; Chen, T.; Yang, D.; et al. Inhibitory Effects of Platinum Nanoparticles Coated with Polyethylene Glycol and Conjugated with Rutin on the MCF-7 Breast Cancer Cell Line. Arab. J. Chem. 2024, 17, 105567. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Seidi, F.; Ghaffarlou, M.; Salehiabar, M.; Charmi, J.; Yaray, K.; Nosrati, H.; Ertas, Y.N. Preparation of Alginate Coated Pt Nanoparticle for Radiosensitization of Breast Cancer Tumor. Int. J. Biol. Macromol. 2023, 233, 123273. [Google Scholar] [CrossRef]
- Ramanathan, E.; Ponnuchamy, K.; Muthusamy, G.; Varatharajan, N.; Sabapathi, D.; Selvaraj, A. Chitosan-Stabilized Platinum Nanoparticles Induce Apoptotic Cell Death in Breast Cancer Cells. Appl. Nanosci. 2023, 13, 3867–3873. [Google Scholar] [CrossRef]
- Mohammadi, H.; Abedi, A.; Akbarzadeh, A.; Mokhtari, M.J.; Shahmabadi, H.E.; Mehrabi, M.R.; Javadian, S.; Chiani, M. Evaluation of Synthesized Platinum Nanoparticles on the MCF-7 and HepG-2 Cancer Cell Lines. Int. Nano Lett. 2013, 3, 28. [Google Scholar] [CrossRef]
- Wang, D.-P.; Shen, J.; Qin, C.Y.; Li, Y.M.; Gao, L.J.; Zheng, J.; Feng, Y.L.; Yan, Z.; Zhou, X.; Cao, J.-M. Platinum Nanoparticles Promote Breast Cancer Cell Metastasis by Disrupting Endothelial Barrier and Inducing Intravasation and Extravasation. Nano Res. 2022, 15, 7366–7377. [Google Scholar] [CrossRef]
- Lee, J.-W.; Son, J.; Yoo, K.M.; Lo, Y.M.; Moon, B. Characterization of the Antioxidant Activity of Gold@platinum Nanoparticles. RSC Adv. 2014, 4, 19824. [Google Scholar] [CrossRef]
- Patel, P.; Nadar, V.M.; Umapathy, D.; Manivannan, S.; Venkatesan, R.; Joseph Arokiyam, V.A.; Pappu, S.; Prakash, P.A.; Mohamed Jabir, M.S.; Gulyás, B.; et al. Doxorubicin-Conjugated Platinum Theranostic Nanoparticles Induce Apoptosis via Inhibition of a Cell Survival (PI3K/AKT) Signaling Pathway in Human Breast Cancer Cells. ACS Appl. Nano Mater. 2021, 4, 198–210. [Google Scholar] [CrossRef]
- Dutta, S.; Hazra, R.; Kar, A.; Ghosh, P.; Patra, P. Platinum Nanoparticles: Tiny Titans in Therapy. Discov. Mater. 2024, 4, 16. [Google Scholar] [CrossRef]
- Samhitha, S.S.; Surabhi, S.; Saireddy, S.K.; Santhosh, G. Platinum Nanoparticles in Biomedical Applications: Antibacterial and Antiviral Perspectives. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Kokkarachedu, V., Sadiku, R., Eds.; Springer International Publishing: Cham, Switherland, 2024; pp. 47–64. ISBN 978-3-031-50093-0. [Google Scholar]
- Shariatzadeh, S.; Moghimi, N.; Khala, F.; Sha, S. Metallic Nanoparticles for the Modulation of Tumor Microenvironment: A New Horizon. Front. Bioeng. Biotechnol. 2022, 10, 847433. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Bangde, P.; Pant, T.; Gaikwad, G.; Jain, R.; Dandekar, P. Trimethyl Chitosan Coated Palladium Nanoparticles as a Photothermal Agent and Its In Vitro Evaluation in 2D and 3D Model of Breast Cancer Cells. Colloids Surf. B Biointerfaces 2022, 211, 112287. [Google Scholar] [CrossRef]
- Alkhalidi, H.M.; Zaman, U.; Khan, D.; ur Rehman, K.; Omar, K.I.; Alissa, M.; Rizg, W.Y.; Bukhary, D.M.; Abdelrahman, E.A.; Refat, M.S.; et al. Microwave Assisted Eco-Benign Synthesis of Novel Palladium Nanoparticles (ACPs-PdNPs): A New Insight into Photocatalytic and Biomedical Applications. J. Mol. Liq. 2023, 392, 123469. [Google Scholar] [CrossRef]
- Chu, S.; Stochaj, U. Exploring Near-Infrared Absorbing Nanocarriers to Overcome Cancer Drug Resistance. Cancer Drug Resist. 2020, 3, 302–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Chen, M.; Chen, X.; Zheng, N. Palladium-Based Nanomaterials for Cancer Imaging and Therapy. Theranostics 2020, 10, 10057–10077. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Peng, Y.; Lu, L.; Peng, S.; Chen, T.; Zhan, M. Near-Infrared Light-Triggered Nano-Prodrug for Cancer Gas Therapy. J. Nanobiotechnol. 2021, 19, 443. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal Tumor-Homing Chitosan Oligosaccharide-Coated Biocompatible Palladium Nanoparticles for Photo-Based Imaging and Therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Ggurunathan, S. Combination of Palladium Nanoparticles and Tubastatin-A Potentiates Apoptosis in Human Breast Cancer Cells: A Novel Therapeutic Approach for Cancer. Int. J. Nanomed. 2017, 12, 6503–6520. [Google Scholar] [CrossRef]
- Shahriari, M.; Sedigh, M.A.; Mahdavian, Y.; Mahdigholizad, S.; Pirhayati, M.; Karmakar, B.; Veisi, H. In Situ Supported Pd NPs on Biodegradable Chitosan/Agarose Modified Magnetic Nanoparticles as an Effective Catalyst for the Ultrasound Assisted Oxidation of Alcohols and Activities against Human Breast Cancer. Int. J. Biol. Macromol. 2021, 172, 55–65. [Google Scholar] [CrossRef]
- Sitia, L.; Sevieri, M.; Signati, L.; Bonizzi, A.; Chesi, A.; Mainini, F.; Corsi, F.; Mazzucchelli, S. HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers 2022, 14, 2424. [Google Scholar] [CrossRef]
- Wu, J.; Wang, M.; Pan, Y.; Pang, Y.; Tang, Y.; Song, C.; Zhu, J.; Zhang, X.; Huang, Q. Synthesis of Manganese-Oxide and Palladium Nanoparticles Co-Decorated Polypyrrole/Graphene Oxide (MnO2@Pd@PPy/GO) Nanocomposites for Anti-Cancer Treatment. RSC Adv. 2022, 12, 23786–23795. [Google Scholar] [CrossRef]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel Platinum–Palladium Bimetallic Nanoparticles Synthesized by Dioscorea bulbifera: Anticancer and Antioxidant Activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.S. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Rokade, S.S.; Joshi, K.A.; Mahajan, K.; Tomar, G.; Dubal, D.; Parihar, V.S.; Kitture, R.; Bellare, J.; Ghosh, S. Novel Anticancer Platinum and Palladium Nanoparticles from Barleria prionitis. Glob. J. Nanomed. 2017, 2, 555600. [Google Scholar] [CrossRef]
- Abdel-Fattah, W.I.; Eid, M.M.; Abd El-Moez, S.I.; Mohamed, E.; Ali, G.W. Synthesis of Biogenic Ag@Pd Core-Shell Nanoparticles Having Anti-Cancer/Anti-Microbial Functions. Life Sci. 2017, 183, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Azzazy, H.M.E.-S.; Bakowsky, U.; Preis, E. Palladium Nanoparticles Fabricated by Green Chemistry: Promising Chemotherapeutic, Antioxidant and Antimicrobial Agents. Materials 2020, 13, 3661. [Google Scholar] [CrossRef]
- Safarkhani, M.; Moghaddam, S.S.; Taghavimandi, F.; Bagherzadeh, M.; Fatahi, Y.; Park, U.; Radmanesh, F.; Huh, Y.S.; Rabiee, N. Bioengineered Smart Nanocarriers for Breast Cancer Treatment: Adorned Carbon-Based Nanocomposites with Silver and Palladium Complexes for Efficient Drug Delivery. ACS Omega 2024, 9, 1183–1195. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, J.; Wang, B.; Chen, L.; Tan, S. Recent Advances in the Development of Noble Metal NPs for Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, 2444516. [Google Scholar] [CrossRef]
- Klebowski, B.; Stec, M.; Depciuch, J.; Gałuszka, A.; Pajor-Swierzy, A.; Baran, J.; Parlinska-Wojtan, M. Gold-Decorated Platinum and Palladium Nanoparticles as Modern Nanocomplexes to Improve the Effectiveness of Simulated Anticancer Proton Therapy. Pharmaceutics 2021, 13, 1726. [Google Scholar] [CrossRef]
- MubarakAli, D.; Kim, H.; Venkatesh, P.S.; Kim, J.-W.; Lee, S.-Y. A Systemic Review on the Synthesis, Characterization, and Applications of Palladium Nanoparticles in Biomedicine. Appl. Biochem. Biotechnol. 2023, 195, 3699–3718. [Google Scholar] [CrossRef]
- Alaqarbeh, M.; Adil, S.F.; Ghrear, T.; Khan, M.; Bouachrine, M.; Al-Warthan, A. Recent Progress in the Application of Palladium Nanoparticles: A Review. Catalysts 2023, 13, 1343. [Google Scholar] [CrossRef]
- Golestannezhad, N.; Divsalar, A.; Badalkhani-Khamseh, F.; Rasouli, M.; Seyedarabi, A.; Ghalandari, B.; Ding, X.; Goli, F.; Bekeschus, S.; Movahedi, A.A.M.; et al. Oxali-Palladium Nanoparticle Synthesis, Characterization, Protein Binding, and Apoptosis Induction in Colorectal Cancer Cells. J. Mater. Sci. Mater. Med. 2024, 35, 4. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Kang, K.; Choi, M.H.; Kim, Y.J.; Kim, Y.K.; Min, D.H.; Jang, H. Revisiting of Pd Nanoparticles in Cancer Treatment: All-Round Excellence of Porous Pd Nanoplates in Gene-Thermo Combinational Therapy. ACS Appl. Mater. Interfaces 2018, 10, 13819–13828. [Google Scholar] [CrossRef]
- Song, M.; Liu, N.; He, L.; Liu, G.; Ling, D.; Su, X.; Sun, X. Porous Hollow Palladium Nanoplatform for Imaging-Guided Trimodal Chemo-, Photothermal-, and Radiotherapy. Nano Res. 2018, 11, 2796–2808. [Google Scholar] [CrossRef]
- Gil, Y.-G.; Kang, S.; Chae, A.; Kim, Y.-K.; Min, D.-H.; Jang, H. Synthesis of Porous Pd Nanoparticles by Therapeutic Chaga Extract for Highly Efficient Tri-Modal Cancer Treatment†. Nanoscale 2018, 10, 19810–19817. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Mishra, K.; Lee, R.Y.; Kim, S.H. Antioxidant and Anti-Tyrosinase Activities of Palladium Nanoparticles Synthesised Using Saururus chinesis. J. Clust. Sci. 2016, 27, 733–744. [Google Scholar] [CrossRef]
- Arif, M. A Critical Review of Palladium Nanoparticles Decorated in Microgels. Polymers 2023, 15, 3600. [Google Scholar] [CrossRef]
- Bugwandeen, A.; Singh, K.; Daniels, A.; Singh, D.; David, L.L.; Singh, M. In Vitro Cytotoxicity Profiles of Some Polymers and Inorganic Nanoparticles Commonly Used in Nanomedicine. Curr. Top. Toxicol. 2023, 19, 1–11. [Google Scholar]
- Yin, X.; Fan, T.; Zheng, N.; Yang, J.; Yan, L.; He, S.; Ai, F.; Hu, J. Palladium Nanoparticle Based Smart Hydrogels for NIR Light-Triggered Photothermal/Photodynamic Therapy and Drug Release with Wound Healing Capability. Nanoscale Adv. 2023, 5, 1729–1739. [Google Scholar] [CrossRef]
- Li, D.; Feng, J.; Zhang, X.; Zhao, P.; Xing, L.; Chen, B.; Fan, L. Theoretical and Experimental Study on the Photothermal Effect of Palladium Nanoparticles Based on a Finite Element Model. Lasers Med. Sci. 2024, 39, 3. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Abbas, Z.; Rojanasakul, Y. Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors 2021, 11, 364. [Google Scholar] [CrossRef]
- Weiss, J.T.; Dawson, J.C.; Macleod, K.G.; Rybski, W.; Fraser, C.; Torres-Sánchez, C.; Patton, E.E.; Bradley, M.; Carragher, N.O.; Unciti-Broceta, A. Extracellular Palladium-Catalysed Dealkylation of 5-Fluoro-1-Propargyl-Uracil as a Bioorthogonally Activated Prodrug Approach. Nat. Commun. 2014, 5, 3277. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-to-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2020, 10, 66. [Google Scholar] [CrossRef]
- Chlumsky, O.; Purkrtova, S.; Michova, H.; Sykorova, H.; Slepicka, P.; Fajstavr, D.; Ulbrich, P.; Viktorova, J.; Demnerova, K. Antimicrobial Properties of Palladium and Platinum Nanoparticles: A New Tool for Combating Food-Borne Pathogens. Int. J. Mol. Sci. 2021, 22, 7892. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, M.; Mokarram, P.; Ahmadi, M.; Bozorg-ghalati, F. Biosynthesis of Palladium, Platinum, and Their Bimetallic Nanoparticles Using Rosemary and Ginseng Herbal Plants: Evaluation of Anticancer Activity Moloud. Sci. Rep. 2024, 14, 5798. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Nguyen, Q.V.; Huynh, T.-C. Simple, Green, and Low-Temperature Method for Preparation of Palladium Nanoparticles with Controllable Sizes and Their Characterizations. J. Nanopartic. Res. 2020, 22, 73. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Zhu, X.; Chen, X.; Zhou, Y.; Yuan, G.; Gong, Y.; Liu, J. Iridium/Ruthenium Nanozyme Reactors with Cascade Catalytic Ability for Synergistic Oxidation Therapy and Starvation Therapy in the Treatment of Breast Cancer. Biomaterials 2020, 238, 119848. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, 2101467. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jia, F.; Cui, X.; Pan, Z.; Wu, Y. Boosting Nutrient Starvation-Dominated Cancer Therapy through Curcumin-Augmented Mitochondrial Ca2+ Overload and Obatoclax-Mediated Autophagy Inhibition as Supported by a Novel Nano-Modulator GO-Alg@CaP/CO. J. Nanobiotechnol. 2022, 20, 225. [Google Scholar] [CrossRef]
- Li, J.; Tong, D.; Lin, J. Current Status of Cancer Starvation Therapy. J. Zhejiang Univ. Med. Sci. 2022, 51, 241–250. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Y.; Yu, Q.; Zhou, Y.; Zhang, R.; Chen, X.; Hong, A.; Liu, J. The Effects of Luminescent Ruthenium(II) Polypyridyl Functionalized Selenium Nanoparticles on BFGF-Induced Angiogenesis and AKT/ERK Signaling. Biomaterials 2013, 34, 171–180. [Google Scholar] [CrossRef]
- Mfengwana, P.M.A.H.; Sone, B.T. Green Synthesis and Characterization of Ruthenium Oxide Nanoparticles Using Gunnera Perpensa for Potential Anticancer Activity against MCF7 Cancer Cells. Sci. Rep. 2023, 13, 22638. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Sech, C.L.; Lacombe, S. Platinum Nanoparticles: A Promising Material for Future Cancer Therapy? Nanotechnology 2010, 21, 085103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, X.; Cao, C.; Sun, J.; Liu, J. Transferrin Modified Ruthenium Nanoparticles with Good Biocompatibility for Photothermal Tumor Therapy. J. Colloid. Interface Sci. 2018, 511, 325–334. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Chelora, J.; Xiong, Y.V.; Kershaw, S.; Fai, L.K.; Lo, P.-K.; Wai Cheah, K.L.; Rogach, A.; Antonio Zapien, J.; et al. Ruthenium(II) Complex Incorporated UiO-67 Metal–Organic Framework Nanoparticles for Enhanced Two-Photon Fluorescence Imaging and Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, D.; Gui, L.; Li, Y.; Wang, W.; Liu, J.; Wang, Y. A Dual-Targeting Ruthenium Nanodrug That Inhibits Primary Tumor Growth and Lung Metastasis via the PARP/ATM Pathway. J. Nanobiotechnol. 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A. Polymeric Nanoparticles Containing Ruthenium Complexes for Biomedical Applications: A Mini-Review on Recent Developments. ACS Appl. Polym. Mater. 2024. [Google Scholar] [CrossRef]
- Vogt, C.; Saladino, G.M.; Shaker, K.; Arsenian-Henriksson, M.; Hertz, H.M.; Toprak, M.S.; Brodin, B.A. Organ Uptake, Toxicity and Skin Clearance of Ruthenium Contrast Agents Monitored IN VIVO by X-ray Fluorescence. Nanomedicine 2023, 18, 1161–1173. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-Based Antitumor Drugs and Delivery Systems from Monotherapy to Combination Therapy. Nanoscale 2022, 14, 16339–16375. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in Medicine: Current Clinical Uses and Future Prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar] [CrossRef]
- Kshatriya, V.V.; Kumbhare, M.R.; Jadhav, S.V.; Thorat, P.J.; Bhambarge, R.G. Properties and Emerging Applications of Ruthenium Nanoclusters. BIO Intergration 2024, 5, 990. [Google Scholar] [CrossRef]
- El-Sayed, W.N.; Alkabli, J.; Elshaarawy, R.F.M.; Hassan, Y.A. Improving Ruthenium Nanoparticle Physicochemical Properties and Chemotherapeutic Efficacy by Dual-Encapsulating with New Amphiphilic Chitosan and Imidazolium Ionic Liquid. Arab. J. Chem. 2024, 17, 105655. [Google Scholar] [CrossRef]
- Gopinath, K.; Karthika, V.; Gowri, S.; Senthilkumar, V.; Kumaresan, S.; Arumugam, A. Antibacterial Activity of Ruthenium Nanoparticles Synthesized Using Gloriosa superba L. Leaf Extract. J. Nanostruct. Chem. 2014, 4, 83. [Google Scholar] [CrossRef]
- Munteanu, A.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef]
- Blunden, B.M.; Stenzel, M.H. Incorporating Ruthenium into Advanced Drug Delivery Carriers—An Innovative Generation of Chemotherapeutics. J. Chem. Technol. Biotechnol. 2014, 90, 1177–1195. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Spratlin, J.L.; Kavan, P.; Goodwin, R.A.; McWhirter, E.; Thompson, D.; Jones, M.; McAllister, E.R.; Machado, A.; Lemmerick, Y.; et al. BOLD-100-001 (TRIO039): A Phase Ib Dose-Escalation Study of BOLD-100 in Combination with FOLFOX Chemotherapy in Patients with Advanced Gastrointestinal Solid Tumors. J. Clin. Oncol. 2021, 39, TPS145. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Srivastava, S.; Singh, R.; Mustafa, G. Recent Advances and Application of Ruthenium Complexes in Tumor Malignancy. Mater. Today Proc. 2023, 72, 2822–2827. [Google Scholar] [CrossRef]
- Dehaen, G.; Verwilst, P.; Eliseeva, S.V.; Laurent, S.; Elst, L.V.; Muller, R.N.; Borggraeve, W.M.D.; Binnemans, K.; Parac-Vogt, T.N. A Heterobimetallic Ruthenium—Gadolinium Complex as a Potential Agent for Bimodal Imaging. Inorg. Chem. 2011, 50, 10005–10014. [Google Scholar] [CrossRef] [PubMed]
- Prado, T.M.; Cincotto, F.H.; Moraes, F.C.; Machado, S.A.S. Electrochemical Sensor-Based Ruthenium Nanoparticles on Reduced Graphene Oxide for the Simultaneous Determination of Ethinylestradiol and Amoxicillin. Electroanalysis 2017, 29, 1278–1285. [Google Scholar] [CrossRef]
- Peixoto, R.C.A.; Miranda-Vilela, A.L.; Filho, J.d.S.; Carneiro, M.L.B.; Oliveira, R.G.S.; da Silva, M.O.; de Souza, A.R.; Báo, S.N. Antitumor Effect of Free Rhodium (II) Citrate and Rhodium (II) Citrate-Loaded Maghemite Nanoparticles on Mice Bearing Breast Cancer: A Systemic Toxicity Assay. Tumor Biol. 2015, 36, 3325–3336. [Google Scholar] [CrossRef]
- Chaves, N.L.; Amorim, D.A.; Lopes, C.A.P.; Estrela-Lopis, I.; Böttner, J.; De Souza, A.R.; Báo, S.N. Comparison of the Effect of Rhodium Citrate-Associated Iron Oxide Nanoparticles on Metastatic and Non-Metastatic Breast Cancer Cells. Cancer Nanotechnol. 2019, 10, 7. [Google Scholar] [CrossRef]
- Machuca, A.; Garcia-Calvo, E.; Anunciação, D.S.; Luque-Garcia, J.L. Rhodium Nanoparticles as a Novel Photosensitizing Agent in Photodynamic Therapy against Cancer. Chem. A Eur. J. 2020, 26, 7685–7691. [Google Scholar] [CrossRef]
- Machuca, A.; Garcia-Calvo, E.; Anunciação, D.S.; Luque-Garcia, J.L. Integration of Transcriptomics and Metabolomics to Reveal the Molecular Mechanisms Underlying Rhodium Nanoparticles-Based Photodynamic Cancer Therapy. Pharmaceutics 2021, 13, 1629. [Google Scholar] [CrossRef]
- Sohrabi, M.; Bikhof Torbati, M.; Lutz, M.; Meghdadi, S.; Farrokhpour, H.; Amiri, A.; Amirnasr, M. Application of Cyclometalated Rhodium(III) Complexes as Therapeutic Agents in Biomedical and Luminescent Cellular Imaging. J. Photochem. Photobiol. A Chem. 2022, 423, 113573. [Google Scholar] [CrossRef]
- Yang, G.J.; Wang, W.; Mok, S.W.F.; Wu, C.; Law, B.Y.K.; Miao, X.M.; Wu, K.J.; Zhong, H.J.; Wong, C.Y.; Wong, V.K.W.; et al. Selective Inhibition of Lysine-Specific Demethylase 5A (KDM5A) Using a Rhodium(III) Complex for Triple-Negative Breast Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 13091–13095. [Google Scholar] [CrossRef]
- Máliková, K.; Masaryk, L.; Štarha, P. Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics 2021, 9, 26. [Google Scholar] [CrossRef]
- Dai, P.; Li, J.; Sun, T.; Chen, C. Rhodium-Based Nanozymes: Recent Advances and Challenges. Chem. Rec. 2023, 23, e202300034. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Nunes, E.; Carneiro, M.L.B.; De Oliveira, R.G.S.; Báo, S.N.; De Souza, A.R. Colloidal Stability, Surface Characterisation and Intracellular Accumulation of Rhodium(II) Citrate Coated Superparamagnetic Iron Oxide Nanoparticles in Breast Tumour: A Promising Platform for Cancer Therapy. J. Nanopartic. Res. 2013, 15, 1683. [Google Scholar] [CrossRef]
- Carneiro, M.L.B.; Lopes, C.A.P.; Miranda-Vilela, A.L.; Joanitti, G.A.; da Silva, I.C.R.; Mortari, M.R.; de Souza, A.R.; Báo, S.N. Acute and Subchronic Toxicity of the Antitumor Agent Rhodium (II) Citrate in Balb/c Mice after Intraperitoneal Administration. Toxicol. Rep. 2015, 2, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B. Metals and Metal Compounds in Cancer Treatment. Anticancer. Res. 2004, 24, 1529–1544. [Google Scholar]
- Lemos Chaves, N.; Pinho Lopes, C.A.; Brettas Carneiro, M.L. Rhodium Citrate Associated with Maghemite Nanoparticles Causes DNA Fragmentation Independently of Caspases 3 and Mediated by Reactive Oxygen Species. J. Nanomed. Nanotechnol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Carneiro, M.L.B.; Peixoto, R.C.A.; Joanitti, G.A.; Oliveira, R.G.S.; Telles, L.A.M.; Miranda-Vilela, A.L.; Bocca, A.L.; Vianna, L.M.S.; da Silva, I.C.R.; de Souza, A.R.; et al. Antitumor Effect and Toxicity of Free Rhodium (II) Citrate and Rhodium (II) Citrate-Loaded Maghemite Nanoparticles in Mice Bearing Breast Cancer. J. Nanobiotechnol. 2013, 11, 4. [Google Scholar] [CrossRef]
- El Sayed, S.M. Baghdadi The Antioxidant Glycolysis Inhibitor (Citric Acid) Induces a Dose-Dependent Caspase-Mediated Apoptosis and Necrosis in Glioma Cells. J. Cancer Res. Treat. 2018, 6, 18–24. [Google Scholar] [CrossRef]
- Korga, A.; Ostrowska, M.; Iwan, M.; Herbet, M.; Dudka, J. Inhibition of Glycolysis Disrupts Cellular Antioxidant Defense and Sensitizes HepG2 Cells to Doxorubicin Treatment. FEBS Open Bio 2019, 9, 959–972. [Google Scholar] [CrossRef]
- Carneiro, M.L.B.; Nunes, E.S.; Peixoto, R.C.A.; Oliveira, R.G.S.; Lourenço, L.H.M.; da Silva, I.C.R.; Simioni, A.R.; Tedesco, A.C.; de Souza, A.R.; Lacava, Z.G.M.; et al. Free Rhodium(II) Citrate and Rhodium(II) Citrate Magnetic Carriers as Potential Strategies for Breast Cancer Therapy. J. Nanobiotechnol. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Shin, W.; Choi, M.-H.; Ahn, M.; Kim, Y.-K.; Kim, S.; Min, D.-H.; Jang, H. Morphology-Controlled Synthesis of Rhodium Nanoparticles for Cancer Phototherapy. ACS Nano 2018, 12, 6997–7008. [Google Scholar] [CrossRef]
- Saladino, G.M.; Kilic, N.I.; Brodin, B.; Hamawandi, B.; Yazgan, I.; Hertz, H.M.; Toprak, M.S. Carbon Quantum Dots Conjugated Rhodium Nanoparticles as Hybrid Multimodal Contrast Agents. Nanomaterials 2021, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Deng, Z.; Gao, J.; Liang, C.; Xia, H.; Zhang, P. An Osmium-Peroxo Complex for Photoactive Therapy of Hypoxic Tumors. Nat. Commun. 2022, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, K.; Lin, W.; Johnstone, T.C.; Bruno, P.M.; Zheng, Y.-R.; Hemann, M.T.; Lippard, S.J. A Breast Cancer Stem Cell-Selective, Mammospheres-Potent Osmium(VI) Nitrido Complex. J. Am. Chem. Soc. 2014, 136, 14413–14416. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive Oxygen Species in Cancer Stem Cells. Antioxid. Redox Signal 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Eskandari, A.; Kundu, A.; Ghosh, S.; Suntharalingam, K. A Triangular Platinum(II) Multinuclear Complex with Cytotoxicity Towards Breast Cancer Stem Cells. Angew. Chem. Int. Ed. 2019, 58, 12059–12064. [Google Scholar] [CrossRef]
- Laws, K.; Bineva-Todd, G.; Eskandari, A.; Lu, C.; O’Reilly, N.; Suntharalingam, K. A Copper(II) Phenanthroline Metallopeptide That Targets and Disrupts Mitochondrial Function in Breast Cancer Stem Cells. Angew. Chem. Int. Ed. 2018, 57, 287–291. [Google Scholar] [CrossRef]
- Huang, C.; Huang, W.; Ji, P.; Song, F.; Liu, T.; Li, M.; Guo, H.; Huang, Y.; Yu, C.; Wang, C.; et al. A Pyrazolate Osmium(VI) Nitride Exhibits Anticancer Activity through Modulating Protein Homeostasis in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 12779. [Google Scholar] [CrossRef]
- Needham, R.J.; Sanchez-Cano, C.; Zhang, X.; Romero-Canelón, I.; Habtemariam, A.; Cooper, M.S.; Meszaros, L.; Clarkson, G.J.; Blower, P.J.; Sadler, P.J. In-Cell Activation of Organo-Osmium(II) Anticancer Complexes. Angew. Chem. Int. Ed. 2017, 56, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Infante-Tadeo, S.; Rodríguez-Fanjul, V.; Habtemariam, A.; Pizarro, A.M. Osmium (II) Tethered Half-Sandwich Complexes: PH-Dependent Aqueous Speciation and Transfer Hydrogenation in Cells. Chem. Sci. 2021, 12, 9287–9297. [Google Scholar] [CrossRef]
- Albu, P.C.; Ferencz (Dinu), A.; Al-Ani, H.N.A.; Tanczos, S.-K.; Oprea, O.; Grosu, V.-A.; Nechifor, G.; Bungău, S.G.; Grosu, A.R.; Goran, A.; et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes 2021, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Spricigo, R.; Richter, C.; Leimkühler, S.; Gorton, L.; Scheller, F.W.; Wollenberger, U. Sulfite Biosensor Based on Osmium Redox Polymer Wired Sulfite Oxidase. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 314–319. [Google Scholar] [CrossRef]
- Kang, S.; Gil, Y.-G.; Yim, G.; Min, D.-H.; Jang, H. Osmium–Tellurium Nanozymes for Pentamodal Combinatorial Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 44124–44135. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J. Osmium and OsOx Nanoparticles: An Overview of Syntheses and Applications. Open Res. Eur. 2023, 2, 39. [Google Scholar] [CrossRef]
- Sovari, S.N.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Yuan, X.; Cen, J.; Chen, X.; Jia, Z.; Zhu, X.; Huang, Y.; Yuan, G.; Liu, J. Iridium Oxide Nanoparticles Mediated Enhanced Photodynamic Therapy Combined with Photothermal Therapy in the Treatment of Breast Cancer. J. Colloid. Interface Sci. 2022, 605, 851–862. [Google Scholar] [CrossRef]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef]
- Hussain, A.; Chakravarty, A.R. Photocytotoxic Lanthanide Complexes. J. Chem. Sci. 2012, 124, 1327–1342. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic Therapy for Prostate Cancer: Recent Advances, Challenges and Opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef] [PubMed]
- Calori, I.R.; Bi, H.; Tedesco, A.C. Expanding the Limits of Photodynamic Therapy: The Design of Organelles and Hypoxia-Targeting Nanomaterials for Enhanced Photokilling of Cancer. ACS Appl. Bio Mater. 2021, 4, 195–228. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Liu, Y.; Lin, L.; Bai, J.; Jia, X.; Tian, H.; Jiang, X. BSA-IrO2: Catalase-like Nanoparticles with High Photothermal Conversion Efficiency and a High X-ray Absorption Coefficient for Anti-inflammation and Antitumor Theranostics. Angew. Chem. 2018, 130, 10466–10470. [Google Scholar] [CrossRef]
- Zhen, W.; Liu, Y.; Wang, W.; Zhang, M.; Hu, W.; Jia, X.; Wang, C.; Jiang, X. Specific “Unlocking” of a Nanozyme-Based Butterfly Effect to Break the Evolutionary Fitness of Chaotic Tumors. Angew. Chem. Int. Ed. 2020, 59, 9491–9497. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, H.; Gao, Y.; Lin, J.; Yang, H.; Yang, S. Photostable Iridium (III)–Cyanine Complex Nanoparticles for Photoacoustic Imaging Guided near-Infrared Photodynamic Therapy In Vivo. ACS Appl. Mater. Interfaces 2019, 11, 15417–15425. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Li, S.; Shen, J.; Mao, Z.W. An Enhanced Photothermal Therapeutic Iridium Hybrid Platform Reversing the Tumor Hypoxic Microenvironment. Molecules 2022, 27, 2629. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, X.; Chen, Y.; Peng, K.; Huang, Y.; Zhao, H.; Chen, Q.; Lv, F.; Liu, L.; Wang, S.; et al. Cyclometalated Iridium(Iii) Complex Nanoparticles for Mitochondria-Targeted Photodynamic Therapy. Nanoscale 2020, 12, 14061–14067. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.N.; Ma, W.; Yang, Y.; Chen, G.; Fu, H.; Cui, C.; Yu, Z.; Wang, X. Multifunctional AIE Iridium (III) Photosensitizer Nanoparticles for Two-Photon-Activated Imaging and Mitochondria Targeting Photodynamic Therapy. J. Nanobiotechnol. 2021, 19, 254. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, Q.; Luo, K.; Zhu, C.; Xie, M.; Wang, S.; Fei, Z.; Zhao, J. Synthesis of Iridium-Based Nanocomposite with Catalase Activity for Cancer Phototherapy. J. Nanobiotechnol. 2021, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhu, M.; Jiang, M.; Yang, F.; Zhang, Z. Current Status of Iridium-Based Complexes against Lung Cancer. Front. Pharmacol. 2022, 13, 1025544. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Winter, H.; Goforth, A.M.; Sahay, G.; Sun, C. Facile Synthesis of Ligand-Free Iridium Nanoparticles and Their In Vitro Biocompatibility. Nanoscale Res. Lett. 2018, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.X.; Zhong, H.; Niu, S.; Ding, C.; Lv, S. Iridium Oxide Nanoparticles-Based Theranostic Probe for In Vivo Tumor Imaging and Synergistic Chem/Photothermal Treatments of Cancer Cells. Chem. Eng. J. 2022, 430, 132675. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zheng, Y.; Zhang, H.; Sun, J.H.; Tan, C.P.; He, L.; Zhang, W.; Ji, L.N.; Mao, Z.W. Delivery of Phosphorescent Anticancer Iridium(III) Complexes by Polydopamine Nanoparticles for Targeted Combined Photothermal-Chemotherapy and Thermal/Photoacoustic/Lifetime Imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, R.; Song, Q.; Zhang, Y.; Zhao, H.; Hu, H.; Zhang, Z.; Liu, W.; Lin, W.; Wang, G. Synthesis of Dual-Stimuli Responsive Metal Organic Framework-Coated Iridium Oxide Nanocomposite Functionalized with Tumor Targeting Albumin-Folate for Synergistic Photodynamic/Photothermal Cancer Therapy. Drug Deliv. 2022, 29, 3142–3154. [Google Scholar] [CrossRef]
- Rogers, N.J.; Jeffery, H.C.; Claire, S.; Lewis, D.J.; Zikeli, G.; Hodges, N.J.; Egginton, S.; Nash, G.B.; Pikramenou, Z. Tailoring Iridium Luminescence and Gold Nanoparticle Size for Imaging of Microvascular Blood Flow. Nanomedicine 2017, 12, 2725–2740. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-González, D.; Zamora-Gálvez, A.; de la Escoura-Muñiz, A.; Merkoçi, A. Label-Free Impedimetric Aptasensor for Ochratoxin—A Detection Using Iridium Oxide Nanoparticles. Anal. Chem. 2015, 85, 5167–5172. [Google Scholar] [CrossRef]
- Quesada-González, D.; Sena-Torralba, A.; Wicaksono, W.P.; de la Escosura-Muñiz, A.; Ivandini, T.; Merkoçi, A. Iridium Oxide (IV) Nanoparticle-Based Lateral Flow Immunoassay. Biosens. Bioelectron. 2019, 132, 132–135. [Google Scholar] [CrossRef]
- Ma, D.-L.; Wu, C.; Tang, W.; Gupta, A.-R.; Lee, F.-W.; Li, G.; Leung, C.-H. Recent Advances in Iridium(III) Complex-Assisted Nanomaterials for Biological Applications. J. Mater. Chem. B 2018, 6, 537–544. [Google Scholar] [CrossRef]
- Alrushaid, N.; Khan, F.A.; Al-Suhaimi, E.A.; Elaissari, A. Nanotechnology in Cancer Diagnosis and Treatment. Pharmaceutics 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]





| Name of the Drug | Active Ingredient | Clinical Trial Number | No of Patients | Details for Which Subtypes of Breast Cancer | Stages the Drug Is Being Tested | Results | Patient Completed Status | Refs. |
|---|---|---|---|---|---|---|---|---|
| Genexol®-PM | Paclitaxel | 0412-CR01-0704-000 | 43 | Metastatic breast cancer (MBC) | Phase II clinical trials | Better maximum tolerated decreased myelosuppression, excellent inhibition of P-glycoprotein, and higher cellular internalisation than Taxol®. | Nonpregnant women having histologically confirmed breast cancer with evidence of metastasis. They also possessed an Eastern Cooperative Oncology Group performance status of 0 or 1, expected survival of >3 months, and measurable disease using the Response Criteria in Solid Tumors. | [34,35] |
| Xyotax® | Poly-L-glutamate and paclitaxel | - | 18 | HER2-negative MBC | Phase II clinical trials | Good anticancer efficacy, but the study was terminated due to unexpected neurotoxicity. | Patients were required to be at least 18 years of age and have histologic confirmation of invasive breast carcinoma, with metastatic disease. | [36] |
| Myocet® | Nonpegylated liposome and doxorubicin | NCT00294996 | 183 | HER2-positive MBC | Phase III clinical studies | Median progression-free survival (PFS) ranging between 14.5 and 16.1 months. | Women with documented HER2-positive MBC who had received no prior chemotherapy for metastatic disease. | [37] |
| Thermodox® | Lyso-thermosensitive liposome and doxorubicin | - | 17 | Breast carcinoma on the chest wall | Partial phase I/II clinical studies | Provision optimistic and patient-friendly treatment choices for patients at a dose of 40 mg/m2. | Patients with breast carcinoma on the chest wall with progression following radiation were eligible; prior chemotherapy and hormone therapy were allowed. | [38] |
| MAG-Tn3 | Carbohydrate peptide lysine and Vaccine | NCT02364492 | 7 | Localised breast cancer | Phase I | All vaccinated women produced high levels of Tn-specific antibodies. | The patients were aged between 44 and 66 with no expressed HER2 receptor. | [39] |
| PGMN | Nanoparticle System | Cell Line(s) Used | Experimental Assessment Methods | Therapeutic Outcomes | Nanoparticle Size | References |
|---|---|---|---|---|---|---|
| Platinum nanoparticles | Pt NPs | MCF-7 | MTT assay | Superior anticancer activity with less than 40% cell viability | 20.12 nm | [52] |
| Pt NPs | MCF-7 | MTT assay | High inhibition against the proliferation of breast cancer cells | 1.3–2.6 nm | [51] | |
| Pt NPs | MCF-7 and MDA-MB-231 | MTT assay | High cytotoxic effects against breast cancer cells | _ | [53] | |
| Pt NPs | MCF-7 | MTT assay | Stimulated apoptosis pathway in the MCF-7 cells with good anticancer activity | 33.49–67.58 nm and 75.56–88.90 nm | [54] | |
| Pt NPs | MCF-7 | MTT assay | Good anticancer efficacy | 20–50 nm | [55] | |
| Pt NPs | MCF-7 | MTT assay | Good anticancer activity of almost 50% | 10 nm | [56] | |
| Pt NPs | MCF-7 | MTT assay | Good anticancer activity with IC50 of 167.2 µg/mL | 113.2 nm | [57] | |
| Pt NPs | MCF-7 | MTT assay | Superior anticancer activity with an IC50 of 96.36 μg·mL−1 | 2–10 nm | [58] | |
| Pt NPs | MBA-MB-231 | MTT assay | Excellent anticancer activity with cell viability of 2% | 50 nm | [59] | |
| Pt NPs | MCF-7/ADR | MTT assay | Controlled release profile of doxorubicin from Pt nanoparticles with good anticancer activity | 67 nm | [60] | |
| Pt NPs | MCF-7 and MCF-10A | MTT assay | Superior cytotoxic effects | 30–60 nm | [61] | |
| Pt NPs | 4 T1 | In vivo, studies combined with X-ray radiation using female BALB/c mice | Superior growth inhibition of breast tumours, which was due to synergistic anticancer effect | 99.09 nm | [62] | |
| Platinum nanoparticles | Pt NPs | MDA-MB-231 | MTT assay | Good anticancer efficacy with IC50 value of 12 ± 2.14 μg/mL | 75–150 nm | [63] |
| Pt NPs | MCF-7 | MTT assay | High cytotoxic effects against breast cancer cells with an IC50 value of 6.8294 mg/mL | 34 nm | [64] | |
| Pt NPs | 4 T1 | In vivo studies using nude mice | Induced lung metastasis at high doses (10 mg/kg) | 5 nm and 70 nm | [65] | |
| Pt NPs | MCF-7 | MTT assay | Excellent anticancer properties | 3 nm | [69] | |
| Pt NPs | MCF-7 and MDA-MB-231 | MTT assay | High cytotoxic effects against breast cancer cells with an IC50 values of less than 4.6 μg/mL | 9.2–13.5 nm | [67] | |
| Palladium nanoparticles | Pd NPs | MDA-MB-231 MDA-MB-468 MCF-7 and MCF-10A | MTT assay | More than 80% cancer cell viability | 6–30 nm | [14]. |
| ACPs-Pd NPs | MCF-7 | MTT assay | Inhibit against breast cancer cell lines with an IC50 value of 66.37 µg/mL | 5–30 nm | [73] | |
| Pd NPs | MDA-MB-231 | WST-8 assay | Induces apoptosis, inhibiting HDAC activity and increases caspase-3 activity | 25 nm | [78] | |
| MnO2@Pd@PPy/GO | MCF-7 | MTT assay and chemodynamic therapy | PTT = An increase of cell viability up to 90% (irradiation) CDT = viability cancer cell is 74.7% (nanocomposites) | 1–3 nm | [81], | |
| Palladium nanoparticles | Pd@COS NPs and Pd@COS-RGD | MDA-MB-231 | In vitro photothermal cytotoxicity and in vivo studies. PTT, PAT imaging and MTT assay | Cell death rate was observed to be 73.32%. | Pd@COS NPs = 23.54 ± 1.25 nm Pd@COS-RGD = 24.28 ± 1.29 nm | [77] |
| Fe3O4@CS-Agarose/Pd | MCF7 Hs 578Bs Hs 319.T and MDA-MB-453 | MTT assay | Excellent cytotoxicity to inhibit cancer line growth | 15.69–35.73 nm | [79] | |
| Pd NPs | MCF-7 | MTT assay | Excellent viability reduction of cancer cells | 5–7 nm | [84] | |
| Ag@Pd | MCF-7 | MTT assay | Inhibit cancer cell line growth | 8 ± 0.26 and 6 ± 0.23 nm | [85] | |
| Ruthenium nanoparticles | IrRu-GOx@PEG | 4T1 | CCK-8 assay, intracellular ROS detection assay, live/dead cell staining assay and flow cytometry | Efficient glucose degradation and ROS generation induced apoptosis in cancer cells | 2, 3, and 4 nm | [107] |
| 4T1 | In vivo studies on 4T1 tumour-bearing BALB/c nude mice | Tumour growth inhibited through synergistic starvation and oxidative therapies | ||||
| Ru-SeNPs | MCF-7 | MTT assay | Lower IC50 value (20.2 ± 2.3 µg/mL) compared to cisplatin (24.4 ± 1.9 µg/mL) | 100 nm | [111] | |
| RuONPs | MCF-7 | MTT assay | Low anticancer efficacy | 7.31 nm | [112] | |
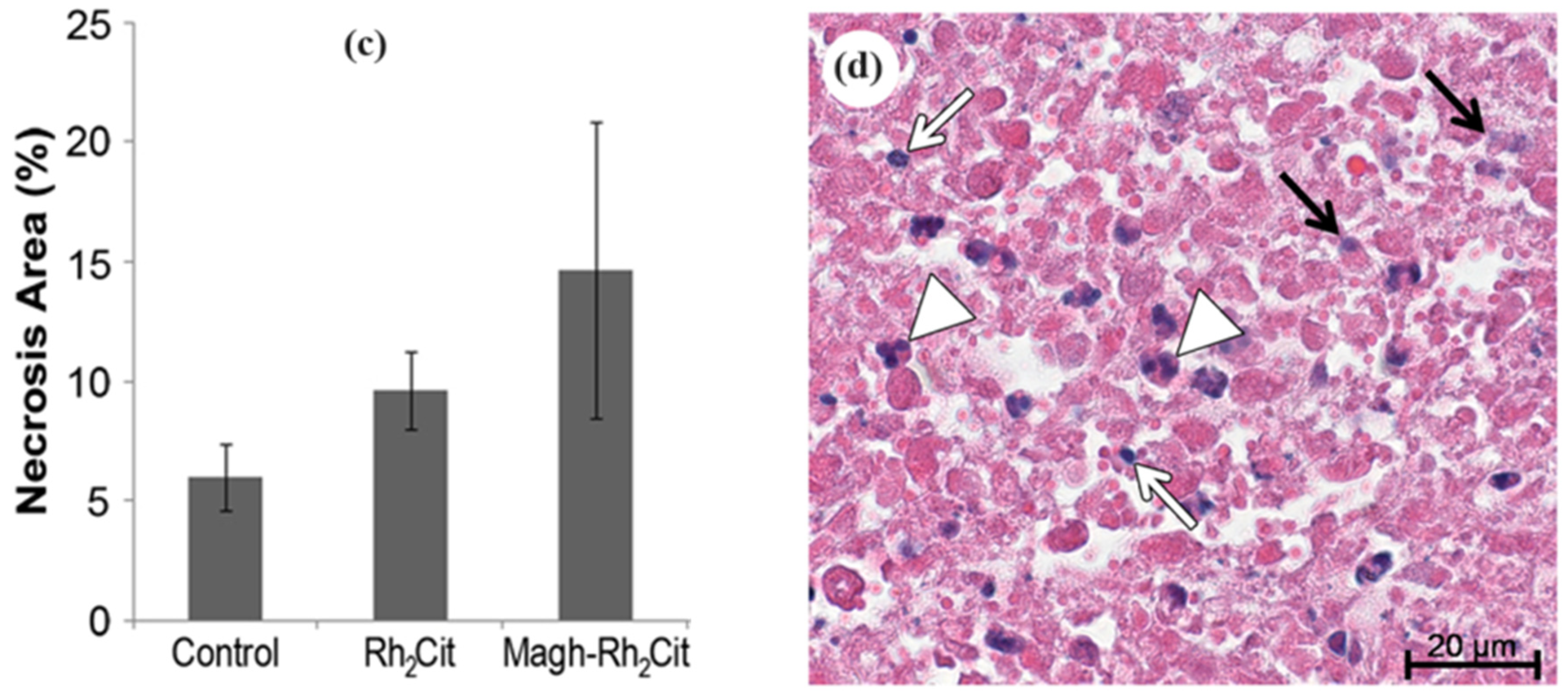
| Rhodium nanoparticles | Magh-Rh2(H2cit)4-250 | 4T1 | Ultrastructural analysis using TEM | NPs distributed in the cytoplasm and nucleus of the tumour cells. Necrosis was induced in tumour tissue | 5 and 60 nm | [138] |
| Magh-Rh2(H2cit)4 | MCF-7 | MTT assay, flow cytometry analysis, confocal microscopy | Apoptosis mediated by ROS and associated with cytochrome C release, caspases 6 and 7 activation, and DNA fragmentation. Minimal toxicity on MCF-10A (non-tumour cells) | |||
| Magh-Rh2Cit | 4T1 | MTT assay | Higher cytotoxic effects on 4T1 and MCF-7 cells compared to MCF-10A cells | 7–8 nm | [141] | |
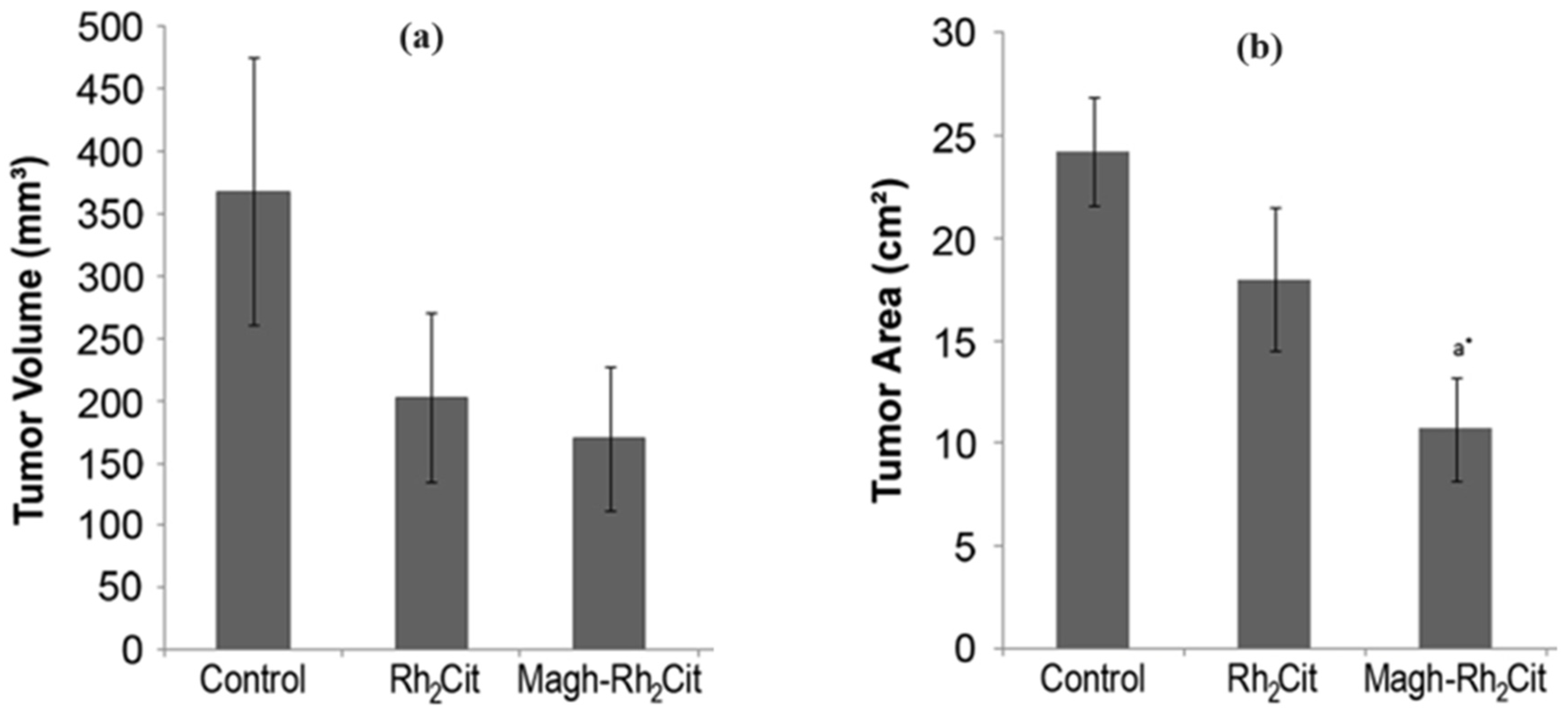
| 4T1 | In vivo studies on Balb/c mice bearing orthotopic 4T1 breast carcinoma | Tumour area reduction without inducing haematotoxic effects. No toxicity to the liver, kidney, and lung tissues. Induction of tumour necrosis | 60 nm | [130,142] | ||
| Magh-Rh2(H2cit)4 | MDA-MB-231 and MCF-7 | MTT assay, cell migration assay, cell cycle analysis, P-glycoprotein level measurement | Reduced migratory capacity of metastatic MDA-MB-231 cells and induced S phase arrest in the cell cycle. MCF-7 cell viability recovered after a 48-h exposure | _ | [131] | |
| Lip-Magh-Rh2(H2cit)4 | MCF-7 and 4T1 | MTT assay | Greater cytotoxic effects on 4T1 and MCF-7 cells compared to normal breast cells (MCF-10A) | 28.19 nm | [145] | |
| Iridium nanoparticles | IrO2-GOx@HA | 4T1 | Cell uptake analysis using ICP-MS, intracellular ROS detection using a fluorescent probe, live/dead cell staining (confocal microscope), flow cytometry | Enhanced type II PDT resulting in induced apoptosis in breast cancer cells | 3.2 nm | [161] |
| In vivo studies on tumour-bearing Balb/c mice | Hypoxia effectively alleviated in tumour tissue. Enhanced type II PDT and improved overall therapeutic effect of PTT | |||||
| IrCy NPs | 4T1 | MTT assay | Cytotoxic effects upon 808 nm laser irradiation (generation of 1O2), with notable cell viability reduction. | 200 nm | [169] | |
| 4T1 | In vivo tests using a 4T1 xenograft mouse model (PAT imaging-guided, PDT) | Significant tumour ablation following PDT, with minimal side effects | ||||
| DPP-Ir | MDA-MB-231 | MTT assay and Photothermal activity | Increase in photothermal conversion efficiency from 42.1% to 67% with a reverse of hypoxia microenvironment | 70 nm | [170] | |
| Iridium nanoparticles | IrRu-Gox@PEG | 4T1 | 3D cell culture model experiments and in vivo tests using BALB/c mice with 4T1 xenograft tumours | Prevention of tumour growth via oxidative and starvation therapy with induced apoptosis | 2–4 nm | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Gandidzanwa, S.; Ngalo, B.; Poswayo, O.; Madanhire, T.; Aderibigbe, B.A.; Tshentu, Z. Platinum Group Metals Nanoparticles in Breast Cancer Therapy. Pharmaceutics 2024, 16, 1162. https://doi.org/10.3390/pharmaceutics16091162
Alven S, Gandidzanwa S, Ngalo B, Poswayo O, Madanhire T, Aderibigbe BA, Tshentu Z. Platinum Group Metals Nanoparticles in Breast Cancer Therapy. Pharmaceutics. 2024; 16(9):1162. https://doi.org/10.3390/pharmaceutics16091162
Chicago/Turabian StyleAlven, Sibusiso, Sendibitiyosi Gandidzanwa, Basabele Ngalo, Olwethu Poswayo, Tatenda Madanhire, Blessing A. Aderibigbe, and Zenixole Tshentu. 2024. "Platinum Group Metals Nanoparticles in Breast Cancer Therapy" Pharmaceutics 16, no. 9: 1162. https://doi.org/10.3390/pharmaceutics16091162
APA StyleAlven, S., Gandidzanwa, S., Ngalo, B., Poswayo, O., Madanhire, T., Aderibigbe, B. A., & Tshentu, Z. (2024). Platinum Group Metals Nanoparticles in Breast Cancer Therapy. Pharmaceutics, 16(9), 1162. https://doi.org/10.3390/pharmaceutics16091162









