Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis
Abstract
1. Introduction
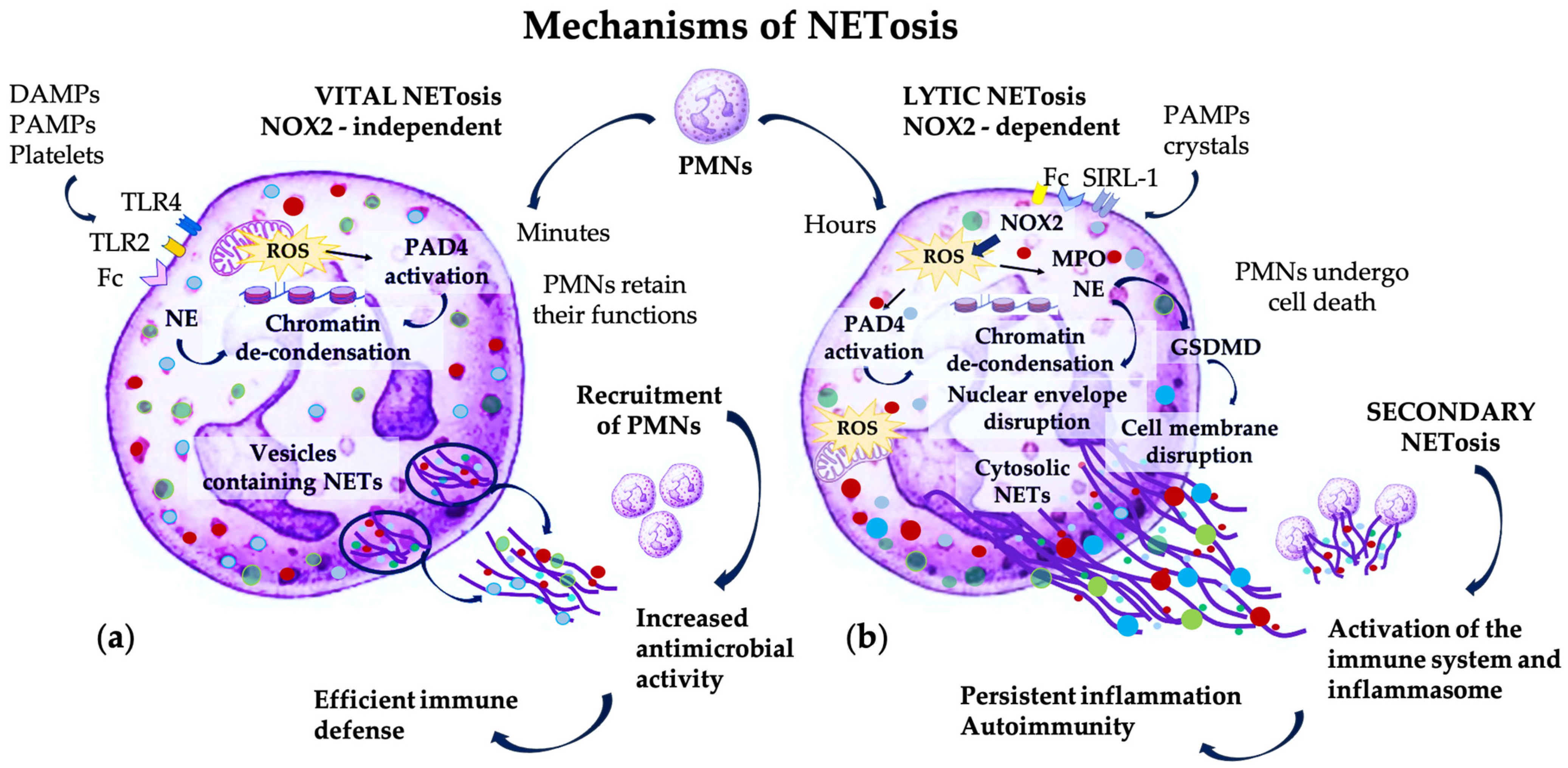
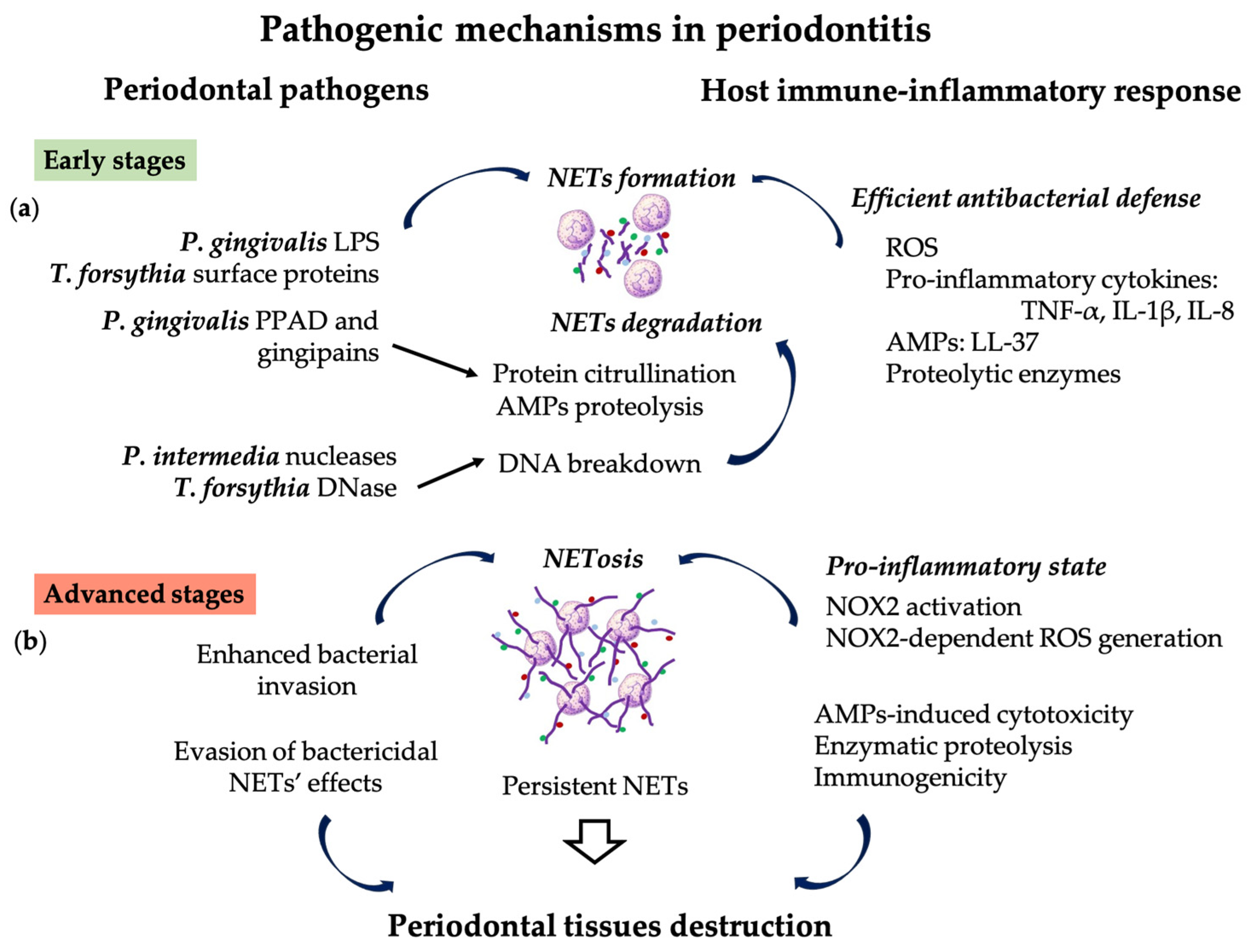
2. Pathogenic Mechanisms Involving PMNs and NET Formation
3. Role of NETs in Periodontitis Initiation and Progression
4. NETosis Biomarkers, and Diagnostic Tools in Periodontitis
4.1. Biomarkers Related to NET Formation
4.2. Biomarkers Related to NET Accumulation
4.3. Associated Non-Specific Biomarkers
4.4. Systemic versus Local Biomarkers
4.5. Novel Tools for Assessing Biomarkers in Oral Biofluids
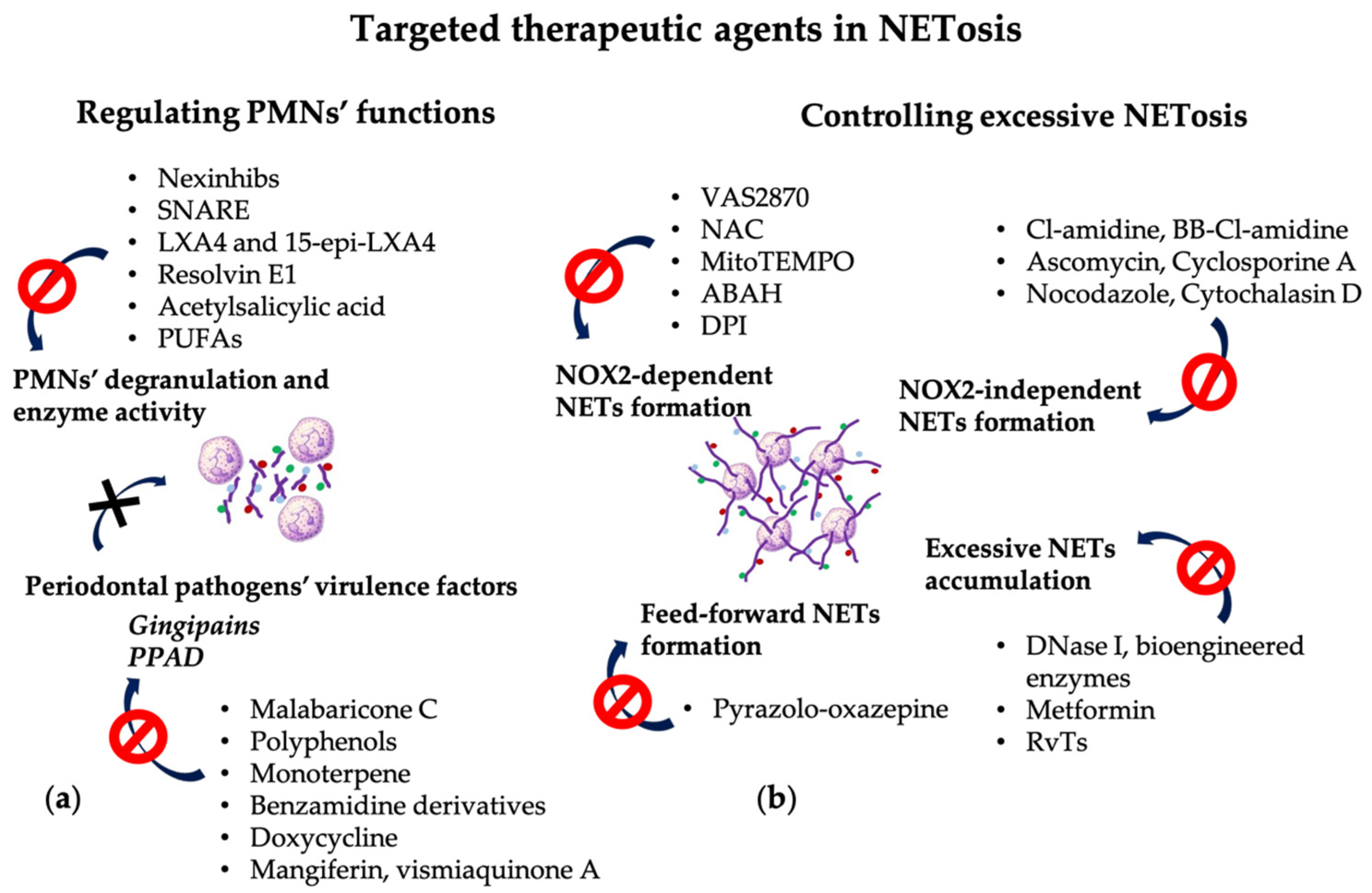
5. NETs as Potential Therapeutic Targets in Periodontitis
5.1. Targeting PMNs’ Degranulation and Enzyme Activity
5.2. Targeting the NOX2-Dependent NET Formation
5.3. Targeting the NOX2-Independent NET Formation
5.4. Targeting Excessive NET Accumulation
5.5. Targeting the Feed-Forward Mechanism of NET Formation
5.6. Targeting Periodontal Pathogens’ Virulence Factors
5.7. Targeting Other Related Pathogenic Mechanisms
6. Local Drug Delivery Systems as Novel Periodontal Therapeutic Approaches
6.1. Required Properties of LDD Systems
6.2. LDD System Constituents and Formulations
6.3. Therapeutic Agents Used in LDD Systems
6.4. Particular Features of LDD Systems for Periodontal Therapy
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A. actinomycetemcomitans | Aggregatibacter actinomycetemcomitans |
| ABAH-4 | Aminobenzoic acid hydrazide |
| A-AMPK | Cyclic adenosine monophosphate-protein kinase |
| AGEs | Advanced glycation end-products |
| AMPs | Antimicrobial peptides |
| APIs | Active pharmaceutical ingredients |
| ARDS | Acute respiratory distress syndrome |
| cAMP | Cyclic adenosine monophosphate |
| cfDNA | Cell-free DNA |
| Co-Q10 | Coenzyme Q10 |
| DAMPs | Damage-associated molecular patterns |
| DNA | Deoxyribonucleic acid |
| DNase I | Deoxyribonuclease I |
| DPI | Diphenyleneiodonium |
| FLCs | Free light chains |
| GCF | Gingival crevicular fluid |
| GSDMD | Gasdermin D |
| H3Cit | Citrullinated histone 3 |
| HMGB1 | High mobility group box 1 |
| IL | Interleukine |
| LDD | Local drug delivery |
| LXA4 | Lipoxin A4 |
| LPS | Lipopolysaccharide |
| MMP | Matrix metalloproteinase |
| MPO | Myeloperoxidase |
| NAC | N-acetyl cysteine |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NCF2 | Neutrophil cytosolic factor 2 |
| NE | Neutrophil elastase |
| NETs | Neutrophil extracellular traps |
| Nexinhibs | Neutrophil-specific exocytosis inhibitors |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| NOX | Nicotinamide adenine dinucleotide phosphate-oxidase |
| PAD4 | Peptidyl arginine deiminase-4 |
| PAMPs | Pathogen-associated molecular patterns |
| PD | Periodontitis |
| P. gingivalis | Porphyromonas gingivalis |
| P. intermedia | Prevotella intermedia |
| PKC | Protein kinase C |
| PMNs | Polymorphonuclear neutrophils |
| PPAD | Peptidyl arginine deiminase |
| PR3 | Proteinase-3 |
| PRR | Pattern recognition receptors |
| PUFAs | Ω-3 polyunsaturated acids |
| RA | Rheumatoid arthrosis |
| RAGEs | Receptors for advanced glycation end-products |
| Rgp | Gingipain R |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RvTs | T-series resolvins |
| SIRL-1 | Signal inhibitory receptor on leukocytes-1 |
| SLE | Systemic lupus erythematosus |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| T. forsythia | Tannerella forsythia |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
References
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, R.A.; Chiesa, S.T.; Hajishengallis, G.; Papayannopoulos, V.; Deanfield, J.E.; D’Aiuto, F. The Roles of Neutrophils Linking Periodontitis and Atherosclerotic Cardiovascular Diseases. Front. Immunol. 2022, 13, 915081. [Google Scholar] [CrossRef] [PubMed]
- Laugisch, O.; Wong, A.; Sroka, A.; Kantyka, T.; Koziel, J.; Neuhaus, K.; Sculean, A.; Venables, P.J.; Potempa, J.; Möller, B.; et al. Citrullination in the periodontium--a possible link between periodontitis and rheumatoid arthritis. Clin. Oral. Investig. 2016, 20, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Pirih, F.Q.; Monajemzadeh, S.; Singh, N.; Sinacola, R.S.; Shin, J.M.; Chen, T.; Fenno, J.C.; Kamarajan, P.; Rickard, A.H.; Travan, S.; et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontology 2000 2021, 87, 50–75. [Google Scholar] [CrossRef]
- Bassani, B.; Cucchiara, M.; Butera, A.; Kayali, O.; Chiesa, A.; Palano, M.T.; Olmeo, F.; Gallazzi, M.; Dellavia, C.P.B.; Mortara, L.; et al. Neutrophils’ Contribution to Periodontitis and Periodontitis-Associated Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 15370. [Google Scholar] [CrossRef]
- Vitkov, L.; Muñoz, L.E.; Knopf, J.; Schauer, C.; Oberthaler, H.; Minnich, B.; Hannig, M.; Herrmann, M. Connection between Periodontitis-Induced Low-Grade Endotoxemia and Systemic Diseases: Neutrophils as Protagonists and Targets. Int. J. Mol. Sci. 2021, 22, 4647. [Google Scholar] [CrossRef]
- White, P.; Sakellari, D.; Roberts, H.; Risafi, I.; Ling, M.; Cooper, P.; Milward, M.; Chapple, I. Peripheral blood neutrophil extracellular trap production and degradation in chronic periodontitis. J. Clin. Periodontol. 2016, 43, 1041–1049. [Google Scholar] [CrossRef]
- Bascones-Martínez, A.; Muñoz-Corcuera, M.; Noronha, S.; Mota, P.; Bascones-Ilundain, C.; Campo-Trapero, J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Med. Oral. Patol. Oral. Cir. Bucal. 2009, 14, e680–e685. [Google Scholar] [CrossRef][Green Version]
- White, P.C.; Chicca, I.J.; Cooper, P.R.; Milward, M.R.; Chapple, I.L. Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue. J. Dent. Res. 2016, 95, 26–34. [Google Scholar] [CrossRef]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontology 2000 2013, 63, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Giaglis, S.; Hahn, S.; Hasler, P. “The NET Outcome”: Are Neutrophil Extracellular Traps of Any Relevance to the Pathophysiology of Autoimmune Disorders in Childhood? Front. Pediatr. 2016, 4, 97. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.Z.; Yu, S.Y.; Pelekos, G.; Yiu, K.H.; Jin, L. Periodontitis links to concurrent systemic comorbidities among ‘self-perceived health’ individuals. J. Periodontal Res. 2022, 57, 632–643. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Filep, J.G. Targeting Neutrophils for Promoting the Resolution of Inflammation. Front. Immunol. 2022, 13, 866747. [Google Scholar] [CrossRef] [PubMed]
- Blanter, M.; Gouwy, M.; Struyf, S. Studying Neutrophil Function in vitro: Cell Models and Environmental Factors. J. Inflamm. Res. 2021, 14, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Li, X.; Dong, G.; Zhang, M.; Xu, Z.; Yang, J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediat. Inflamm. 2020, 2020, 9254087. [Google Scholar] [CrossRef]
- Dömer, D.; Walther, T.; Möller, S.; Behnen, M.; Laskay, T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.J.; Cholok, D.; Li, J.; Bian, G.; Yalavarthi, S.; Li, S.; Carson, W.F.; Hwang, C.; Marini, S.; et al. Disruption of Neutrophil Extracellular Traps (NETs) Links Mechanical Strain to Post-traumatic Inflammation. Front. Immunol. 2019, 10, 2148. [Google Scholar] [CrossRef] [PubMed]
- Tillack, K.; Breiden, P.; Martin, R.; Sospedra, M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J. Immunol. 2012, 188, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ribon, M.; Seninet, S.; Mussard, J.; Sebbag, M.; Clavel, C.; Serre, G.; Boissier, M.C.; Semerano, L.; Decker, P. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 2019, 98, 122–131. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Magán-Fernández, A.; O’Valle, F.; Abadía-Molina, F.; Muñoz, R.; Puga-Guil, P.; Mesa, F. Characterization and comparison of neutrophil extracellular traps in gingival samples of periodontitis and gingivitis: A pilot study. J. Periodontal Res. 2019, 54, 218–224. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Dwivedi, N.; Khan, S.; Radic, M. Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 2009, 1, 194–201. [Google Scholar] [CrossRef]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef]
- Gupta, A.K.; Giaglis, S.; Hasler, P.; Hahn, S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE 2014, 9, e97088. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Ren, B.; Zou, L.; He, B.; Li, M. The Role of Neutrophil Extracellular Traps in Periodontitis. Front. Cell. Infect. Microbiol. 2021, 11, 639144. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Neeli, I.; Khan, S.N.; Radic, M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008, 180, 1895–1902. [Google Scholar] [CrossRef]
- Khan, U.; Chowdhury, S.; Billah, M.M.; Islam, K.M.D.; Thorlacius, H.; Rahman, M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int. J. Mol. Sci. 2021, 22, 7260. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Magán-Fernández, A.; Rasheed Al-Bakri, S.M.; O’Valle, F.; Benavides-Reyes, C.; Abadía-Molina, F.; Mesa, F. Neutrophil Extracellular Traps in Periodontitis. Cells 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef]
- Grégoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52, 1702590. [Google Scholar] [CrossRef]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, with Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Cooper, P.R.; Palmer, L.J.; Chapple, I.L. Neutrophil extracellular traps as a new paradigm in innate immunity: Friend or foe? Periodontology 2000 2013, 63, 165–197. [Google Scholar] [CrossRef]
- Patel, S.; Kumar, S.; Jyoti, A.; Srinag, B.S.; Keshari, R.S.; Saluja, R.; Verma, A.; Mitra, K.; Barthwal, M.K.; Krishnamurthy, H.; et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 2010, 22, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev. Cell 2017, 43, 449–462.e5. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Hilbrands, L.B.; van der Vlag, J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 484. [Google Scholar] [CrossRef]
- Al-Rasheed, A. Elevation of white blood cells and platelet counts in patients having chronic periodontitis. Saudi Dent. J. 2012, 24, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Linhares-Lacerda, L.; Temerozo, J.R.; Ribeiro-Alves, M.; Azevedo, E.P.; Mojoli, A.; Nascimento, M.T.C.; Silva-Oliveira, G.; Savino, W.; Foguel, D.; Bou-Habib, D.C.; et al. Neutrophil extracellular trap-enriched supernatants carry microRNAs able to modulate TNF-α production by macrophages. Sci. Rep. 2020, 10, 2715. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Lazzaretto, B.; Fadeel, B. Intra- and Extracellular Degradation of Neutrophil Extracellular Traps by Macrophages and Dendritic Cells. J. Immunol. 2019, 203, 2276–2290. [Google Scholar] [CrossRef]
- Chiang, N.; Sakuma, M.; Rodriguez, A.R.; Spur, B.W.; Irimia, D.; Serhan, C.N. Resolvin T-series reduce neutrophil extracellular traps. Blood 2022, 139, 1222–1233. [Google Scholar] [CrossRef]
- Schiött, C.R.; Löe, H. The origin and variation in number of leukocytes in the human saliva. J. Periodontal Res. 1970, 5, 36–41. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Imboden, M.A.; Lang, N.P. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J. Periodontol. 1998, 69, 1139–1147. [Google Scholar] [CrossRef]
- Kim, D.; Haynes, C.L. Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal. Chem. 2012, 84, 6070–6078. [Google Scholar] [CrossRef]
- Kim, T.S.; Moutsopoulos, N.M. Neutrophils and neutrophil extracellular traps in oral health and disease. Exp. Mol. Med. 2024, 56, 1055–1065. [Google Scholar] [CrossRef]
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Extracellular neutrophil traps in periodontitis. J. Periodontal Res. 2009, 44, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Muñoz, L.E.; Schoen, J.; Knopf, J.; Schauer, C.; Minnich, B.; Herrmann, M.; Hannig, M. Neutrophils Orchestrate the Periodontal Pocket. Front. Immunol. 2021, 12, 788766. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J. Neutrophil Subsets in Periodontal Health and Disease: A Mini Review. Front. Immunol. 2020, 10, 3001. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.M.; Ling, M.R.; Insall, R.; Kalna, G.; Spengler, J.; Grant, M.M.; Chapple, I.L. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J. Clin. Periodontol. 2015, 42, 1–11. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Konkel, J.; Sarmadi, M.; Eskan, M.A.; Wild, T.; Dutzan, N.; Abusleme, L.; Zenobia, C.; Hosur, K.B.; Abe, T.; et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014, 6, 229ra40. [Google Scholar] [CrossRef]
- Dias, I.H.; Matthews, J.B.; Chapple, I.L.; Wright, H.J.; Dunston, C.R.; Griffiths, H.R. Activation of the neutrophil respiratory burst by plasma from periodontitis patients is mediated by pro-inflammatory cytokines. J. Clin. Periodontol. 2011, 38, 1–7. [Google Scholar] [CrossRef]
- Ling, M.R.; Chapple, I.L.; Matthews, J.B. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015, 21, 714–725. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Ye, S.; Ye, Z.; Yang, L.; Xu, X. Oral microbiota-host interaction: The chief culprit of alveolar bone resorption. Front. Immunol. 2024, 15, 1254516. [Google Scholar] [CrossRef] [PubMed]
- Tervahartiala, T.; Pirilä, E.; Ceponis, A.; Maisi, P.; Salo, T.; Tuter, G.; Kallio, P.; Törnwall, J.; Srinivas, R.; Konttinen, Y.T.; et al. The In Vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 2000, 79, 1969–1977. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Z.; Lee, H.M.; Wolff, M.S.; Golub, L.M.; Sorsa, T.; Kuula, H. Characteristics of collagenase-2 from gingival crevicular fluid and peri-implant sulcular fluid in periodontitis and peri-implantitis patients: Pilot study. Acta Odontol. Scand. 2008, 66, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Muñoz, V.C.; Collados, L.; Oyarzún, A.D. In situ detection of matrix metalloproteinase-9 (MMP-9) in gingival epithelium in human periodontal disease. J. Periodontal Res. 2004, 39, 87–92. [Google Scholar] [CrossRef]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.; White, P.C.; Milward, M.R.; Cooper, P.R.; Chapple, I.L.C. Modulation of Neutrophil Extracellular Trap and Reactive Oxygen Species Release by Periodontal Bacteria. Infect. Immun. 2017, 85, e00297-17. [Google Scholar] [CrossRef]
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Neutrophil fate in gingival crevicular fluid. Ultrastruct. Pathol. 2010, 34, 25–30. [Google Scholar] [CrossRef]
- Domínguez-Díaz, C.; Varela-Trinidad, G.U.; Muñoz-Sánchez, G.; Solórzano-Castanedo, K.; Avila-Arrezola, K.E.; Iñiguez-Gutiérrez, L.; Delgado-Rizo, V.; Fafutis-Morris, M. To Trap a Pathogen: Neutrophil Extracellular Traps and Their Role in Mucosal Epithelial and Skin Diseases. Cells 2021, 10, 1469. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Dommisch, H.; Skora, P.; Horvath, G.; Latz, E.; Hoerauf, A.; Waller, T.; Kawai, T.; Jepsen, S.; Deschner, J.; et al. Neutrophil extracellular trap formation in supragingival biofilms. Int. J. Med. Microbiol. IJMM 2015, 305, 453–463. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Tawee, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral. Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Palmer, L.J.; Chapple, I.L.; Wright, H.J.; Roberts, A.; Cooper, P.R. Extracellular deoxyribonuclease production by periodontal bacteria. J. Periodontal Res. 2012, 47, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.C.; Yam, H.C.; Gunasekaran, B.; Lai, W.Y.; Wo, W.Y.; Agarwal, T.; Ong, Y.Y.; Cheong, S.L.; Tan, S.A. Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases. Front. Cell. Infect. Microbiol. 2022, 12, 987683. [Google Scholar] [CrossRef] [PubMed]
- Prucsi, Z.; Zimny, A.; Płonczyńska, A.; Zubrzycka, N.; Potempa, J.; Sochalska, M. Porphyromonas gingivalis Peptidyl Arginine Deiminase (PPAD) in the Context of the Feed-Forward Loop of Inflammation in Periodontitis. Int. J. Mol. Sci. 2023, 24, 12922. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Collyer, C.A. Gingipains from Porphyromonas gingivalis—Complex domain structures confer diverse functions. Eur. J. Microbiol. Immunol. 2011, 1, 41–58. [Google Scholar] [CrossRef]
- Bryzek, D.; Ciaston, I.; Dobosz, E.; Gasiorek, A.; Makarska, A.; Sarna, M.; Eick, S.; Puklo, M.; Lech, M.; Potempa, B.; et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019, 15, e1007773. [Google Scholar] [CrossRef]
- Chen, J.L.; Tong, Y.; Zhu, Q.; Gao, L.Q.; Sun, Y. Neutrophil extracellular traps induced by Porphyromonas gingivalis lipopolysaccharide modulate inflammatory responses via a Ca2+-dependent pathway. Arch. Oral. Biol. 2022, 141, 105467. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- White, P.; Cooper, P.; Milward, M.; Chapple, I. Differential activation of neutrophil extracellular traps by specific periodontal bacteria. Free Radic. Biol. Med. 2014, 75, S53. [Google Scholar] [CrossRef]
- Doke, M.; Fukamachi, H.; Morisaki, H.; Arimoto, T.; Kataoka, H.; Kuwata, H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol. Oral. Microbiol. 2017, 32, 288–300. [Google Scholar] [CrossRef]
- Schäffer, C.; Andrukhov, O. The intriguing strategies of Tannerella forsythia’s host interaction. Front. Oral. Health 2024, 5, 1434217. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The structure of the antimicrobial human cathelicidin LL-37 shows oligomerization and channel formation in the presence of membrane mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; Orr, D.F.; Yu, Y.; Coulter, W.A.; Manning, G.; Irwin, C.R.; Lundy, F.T. LL-37 in periodontal health and disease and its susceptibility to degradation by proteinases present in gingival crevicular fluid. J. Clin. Periodontol. 2013, 40, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Daleskog, M.; Göransson, S.P.; Schatzberg, D.; Lasselin, J.; Laska, A.C.; Kallner, A.; Helleday, T.; Wallén, H.; Demers, M. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma. Immunol. Res. 2017, 65, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.M.J.; de Smit, M.J.; Withaar, C.; Brouwer, E.; van Winkelhoff, A.J.; Vissink, A.; Westra, J. Autoantibodies against citrullinated histone H3 in rheumatoid arthritis and periodontitis patients. J. Clin. Periodontol. 2017, 44, 577–584. [Google Scholar] [CrossRef]
- Cifcibasi, E.; Ciblak, M.; Kiran, B.; Badur, S.; Firatli, E.; Issever, H.; Cintan, S. The role of activated cytotoxic T cells in etiopathogenesis of periodontal disease: Does it harm or does it heal? Sci. Rep. 2015, 5, 9262. [Google Scholar] [CrossRef]
- Meng, W.; Paunel-Görgülü, A.; Flohé, S.; Hoffmann, A.; Witte, I.; MacKenzie, C.; Baldus, S.E.; Windolf, J.; Lögters, T.T. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care 2012, 16, R137. [Google Scholar] [CrossRef]
- Brebner, J.A.; Stockley, R.A. Polyclonal free light chains: A biomarker of inflammatory disease or treatment target? F1000 Med. Rep. 2013, 5, 4. [Google Scholar] [CrossRef]
- de Pablo, P.; Dietrich, T.; Chapple, I.L.; Milward, M.; Chowdhury, M.; Charles, P.J.; Buckley, C.D.; Venables, P.J. The autoantibody repertoire in periodontitis: A role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Ann. Rheum. Dis. 2014, 73, 580–586. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Shui, Y.; Zhou, X.; Cheng, L.; Ren, B.; Chen, Z.; Li, M. Interactions Between Neutrophils and Periodontal Pathogens in Late-Onset Periodontitis. Front. Cell. Infect. Microbiol. 2021, 11, 627328. [Google Scholar] [CrossRef]
- Garley, M.; Dziemiańczyk-Pakieła, D.; Ratajczak-Wrona, W.; Pryczynicz, A.; Nowak, K.; Łazarczyk, B.; Jabłońska, E. NETs biomarkers in saliva and serum OSCC patients: One hypothesis, two conclusions. Adv. Med. Sci. 2022, 67, 45–54. [Google Scholar] [CrossRef]
- Gavillet, M.; Martinod, K.; Renella, R.; Wagner, D.D.; Williams, D.A. A key role for Rac and Pak signaling in neutrophil extracellular traps (NETs) formation defines a new potential therapeutic target. Am. J. Hematol. 2018, 93, 269–276. [Google Scholar] [CrossRef]
- Gavillet, M.; Martinod, K.; Renella, R.; Harris, C.; Shapiro, N.I.; Wagner, D.D.; Williams, D.A. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am. J. Hematol. 2015, 90, 1155–1158. [Google Scholar] [CrossRef]
- Meschiari, C.A.; Marcaccini, A.M.; Santos Moura, B.C.; Zuardi, L.R.; Tanus-Santos, J.E.; Gerlach, R.F. Salivary MMPs, TIMPs, and MPO levels in periodontal disease patients and controls. Clin. Chim. Acta 2013, 421, 140–146. [Google Scholar] [CrossRef]
- Dagar, M.; Deepa, D.K.; Molly, M.; Sharma, A.; Khattak, B.P. Effect of nonsurgical periodontal therapy on salivary myeloperoxidase levels: A biochemical study. J. Indian. Soc. Periodontol. 2015, 19, 531–536. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Xu, S.; Yin, Y.; Ageno, W.; De Stefano, V.; Zhao, Q.; Qi, X. Clinical significance of neutrophil extracellular traps biomarkers in thrombosis. Thromb. J. 2022, 20, 63. [Google Scholar] [CrossRef]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a Magic Biofluid Available for Multilevel Assessment and a Mirror of General Health—A Systematic Review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sur Chowdhury, C.; Giaglis, S.; Walker, U.A.; Buser, A.; Hahn, S.; Hasler, P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: Analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 2014, 16, R122. [Google Scholar] [CrossRef] [PubMed]
- Balan, P.; Chong, Y.S.; Lin, Q.; Lim, T.K.; Suriyanarayanan, T.; Udawatte, N.S.; Wong, M.L.; Lopez, V.; He, H.G.; Seneviratne, C.J. Salivary Proteomic Profiling Identifies Role of Neutrophil Extracellular Traps Formation in Pregnancy Gingivitis. Immunol. Investig. 2022, 51, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Irimeș, M.B.; Tertiș, M.; Oprean, R.; Cristea, C. Unrevealing the connection between real sample analysis and analytical method. The case of cytokines. Med. Res. Rev. 2024, 44, 23–65. [Google Scholar] [CrossRef]
- Macovei, D.G.; Irimes, M.B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033–1063. [Google Scholar] [CrossRef] [PubMed]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Florea, A.; Feier, B.; Cristea, C. In situ analysis based on molecularly imprinted polymer electrochemical sensors. In Comprehensive Analytical Chemistry, 1st ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2019; Volume 86, pp. 193–234. [Google Scholar] [CrossRef]
- Andrei, V.; Andrei, S.; Gal, A.F.; Rus, V.; Gherman, L.M.; Boșca, B.A.; Niculae, M.; Barabas, R.; Cadar, O.; Dinte, E.; et al. Immunomodulatory Effect of Novel Electrospun Nanofibers Loaded with Doxycycline as an Adjuvant Treatment in Periodontitis. Pharmaceutics 2023, 15, 707. [Google Scholar] [CrossRef]
- Catz, S.D.; McLeish, K.R. Therapeutic targeting of neutrophil exocytosis. J. Leuk. Biol. 2020, 107, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Ramadass, M.; He, J.; Brown, S.J.; Zhang, J.; Abgaryan, L.; Biris, N.; Gavathiotis, E.; Rosen, H.; Catz, S.D. Identification of Neutrophil Exocytosis Inhibitors (Nexinhibs), Small Molecule Inhibitors of Neutrophil Exocytosis and Inflammation: Druggability of the Small Gtpase Rab27a. J. Biol. Chem. 2016, 291, 25965–25982. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Rane, M.J.; Merchant, M.L.; Jin, S.; Lentsch, A.B.; Ward, R.A.; McLeish, K.R. Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock 2013, 39, 286–292. [Google Scholar] [CrossRef]
- Zheng, W.; Warner, R.; Ruggeri, R.; Su, C.; Cortes, C.; Skoura, A.; Ward, J.; Ahn, K.; Kalgutkar, A.; Sun, D.; et al. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J. Pharmacol. Exp. Ther. 2015, 353, 288–298. [Google Scholar] [CrossRef]
- Tremblay, G.M.; Janelle, M.F.; Bourbonnais, Y. Anti-inflammatory activity of neutrophil elastase inhibitors. Curr. Opin. Investig. Drugs 2003, 4, 556–565. [Google Scholar]
- El Kebir, D.; József, L.; Pan, W.; Wang, L.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 2009, 180, 311–319. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Serhan, C.N. Resolution of inflammation: A new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 2003, 82, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Hisada, T.; Aoki, H.; Mori, M. Resolvin E1: A novel lipid mediator in the resolution of allergic airway inflammation. Expert. Rev. Clin. Immunol. 2008, 4, 669–672. [Google Scholar] [CrossRef]
- Fredman, G.; Oh, S.F.; Ayilavarapu, S.; Hasturk, H.; Serhan, C.N.; Van Dyke, T.E. Impaired phagocytosis in localized aggressive periodontitis: Rescue by Resolvin E1. PLoS ONE 2011, 6, e24422. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef]
- Li, M.; Gao, W.; Ma, J.; Zhu, Y.; Li, X. Early-stage lupus nephritis treated with N-acetylcysteine: A report of two cases. Exp. Ther. Med. 2015, 10, 689–692. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Willis, V.C.; Gizinski, A.M.; Banda, N.K.; Causey, C.P.; Knuckley, B.; Cordova, K.N.; Luo, Y.; Levitt, B.; Glogowska, M.; Chandra, P.; et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 2011, 186, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Pottecher, J.; Noll, E.; Borel, M.; Audibert, G.; Gette, S.; Meyer, C.; Gaertner, E.; Legros, V.; Carapito, R.; Uring-Lambert, B.; et al. Protocol for TRAUMADORNASE: A prospective, randomized, multicentre, double-blinded, placebo-controlled clinical trial of aerosolized dornase alfa to reduce the incidence of moderate-to-severe hypoxaemia in ventilated trauma patients. Trials 2020, 21, 274. [Google Scholar] [CrossRef]
- Shinohara, C.; Mori, S.; Ando, T.; Tsuji, T. Arg-gingipain inhibition and anti-bacterial activity selective for Porphyromonas gingivalis by malabaricone C. Biosci. Biotechnol. Biochem. 1999, 63, 1475–1477. [Google Scholar] [CrossRef]
- Okamoto, M.; Sugimoto, A.; Leung, K.P.; Nakayama, K.; Kamaguchi, A.; Maeda, N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral. Microbiol. Immunol. 2004, 19, 118–120. [Google Scholar] [CrossRef]
- Tantivitayakul, P.; Kaypetch, R.; Muadchiengka, T. Thymoquinone inhibits biofilm formation and virulence properties of periodontal bacteria. Arch. Oral. Biol. 2020, 115, 104744. [Google Scholar] [CrossRef] [PubMed]
- Krauser, J.A.; Potempa, J.; Travis, J.; Powers, J.C. Inhibition of arginine gingipains (RgpB and HRgpA) with benzamidine inhibitors: Zinc increases inhibitory potency. Biol. Chem. 2002, 383, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Potempa, J. Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J. Oral. Microbiol. 2014, 6, 24800. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Matsushita, K.; Travis, J.; Potempa, J. Inhibition of trypsin-like cysteine proteinases (gingipains) from Porphyromonas gingivalis by tetracycline and its analogues. Antimicrob. Agents Chemother. 2001, 45, 2871–2876. [Google Scholar] [CrossRef]
- Tan, S.A.; Yam, H.C.; Cheong, S.L.; Chow, Y.C.; Bok, C.Y.; Ho, J.M.; Lee, P.Y.; Gunasekaran, B. Inhibition of Porphyromonas gingivalis peptidyl arginine deiminase, a virulence factor, by antioxidant-rich Cratoxylum cochinchinense: In vitro and in silico evaluation. Saudi J. Biol. Sci. 2022, 29, 2573–2581. [Google Scholar] [CrossRef]
- Campbell, A.M.; Kashgarian, M.; Shlomchik, M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012, 4, 157ra141. [Google Scholar] [CrossRef]
- Winkelstein, J.A.; Marino, M.C.; Johnston, R.B., Jr.; Boyle, J.; Curnutte, J.; Gallin, J.I.; Malech, H.L.; Holland, S.M.; Ochs, H.; Quie, P.; et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 2000, 79, 155–169. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Cedervall, J.; Dragomir, A.; Saupe, F.; Zhang, Y.; Ärnlöv, J.; Larsson, E.; Dimberg, A.; Larsson, A.; Olsson, A.K. Pharmacological targeting of peptidylarginine deiminase 4 prevents cancer-associated kidney injury in mice. Oncoimmunology 2017, 6, e1320009. [Google Scholar] [CrossRef]
- Davis, J.C., Jr.; Manzi, S.; Yarboro, C.; Rairie, J.; Mcinnes, I.; Averthelyi, D.; Sinicropi, D.; Hale, V.G.; Balow, J.; Austin, H.; et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Ko, G.R.; Lee, Y.Y.; Park, J.; Park, W.; Park, T.E.; Jin, Y.; Kim, S.N.; Lee, J.S.; Park, C.G. Polymeric DNase-I nanozymes targeting neutrophil extracellular traps for the treatment of bowel inflammation. Nano Converge 2024, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.B.; Zmijewski, J.W.; Deshane, J.S.; Tadie, J.M.; Chaplin, D.D.; Takashima, S.; Abraham, E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011, 25, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Boșca, A.B.; Dinte, E.; Colosi, H.; Ilea, A.; Câmpian, R.S.; Uifălean, A.; Pârvu, A.E. Curcumin effect on nitro-oxidative stress in ligature-induced rat periodontitis. Rom. Biotechnol. Lett. 2015, 21, 10709–10717. [Google Scholar]
- Greenstein, G.; Tonetti, M. The role of controlled drug delivery for periodontitis. The Research, Science and Therapy Committee of the American Academy of Periodontology. J. Periodontol. 2000, 71, 125–140. [Google Scholar] [CrossRef]
- Dinte, E.; Muntean, D.M.; Andrei, V.; Boșca, B.A.; Dudescu, C.M.; Barbu-Tudoran, L.; Borodi, G.; Andrei, S.; Gal, A.F.; Rus, V.; et al. In Vitro and In Vivo Characterisation of a Mucoadhesive Buccal Film Loaded with Doxycycline Hyclate for Topical Application in Periodontitis. Pharmaceutics 2023, 15, 580. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29–41. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Zakeri-Milani, P.; Khonsari, F. Comparative study of in vitro release and mucoadhesivity of gastric compacts composed of multiple unit system/bilayered discs using direct compression of metformin hydrochloride. BioImpacts BI 2014, 4, 29–38. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Hägerström, H.; Edsman, K. Limitations of the rheological mucoadhesion method: The effect of the choice of conditions and the rheological synergism parameter. Eur. J. Pharm. Sci. 2003, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, e2002153. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Cifuentes, A.; Rodríguez, G.; Aguilar, M.R.; González-Gómez, Á.; Solis, R.; García-Honduvilla, N.; Buján, J.; García-Sanmartin, J.; Martínez, A.; et al. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents. Acta Biomater. 2015, 23, 103–115. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Guo, W.; Dong, H.; Wang, X. Emerging roles of hydrogel in promoting periodontal tissue regeneration and repairing bone defect. Front. Bioeng. Biotechnol. 2024, 12, 1380528. [Google Scholar] [CrossRef]
- Santos, M.S.; dos Santos, A.B.; Carvalho, M.S. New Insights in Hydrogels for Periodontal Regeneration. J. Funct. Biomater. 2023, 14, 545. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Yang, X.; Wu, J.; Kirk, T.B.; Xu, J.; Xu, A.; Xue, W. Injectable and Self-Healing Hydrogels with Double-Dynamic Bond Tunable Mechanical, Gel-Sol Transition and Drug Delivery Properties for Promoting Periodontium Regeneration in Periodontitis. Appl. Mater. Interfaces 2021, 13, 61638–61652. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral. Biol. Craniofac. Res. 2019, 9, 190–200. [Google Scholar] [CrossRef]
- Yan, N.; Xu, J.; Liu, G.; Ma, C.; Bao, L.; Cong, Y.; Wang, Z.; Zhao, Y.; Xu, W.; Chen, C. Penetrating Macrophage-Based Nanoformulation for Periodontitis Treatment. ACS Nano 2022, 16, 18253–18265. [Google Scholar] [CrossRef]
- Chang, K.C.; Chiu, K.C.; Chen, W.C.; Lan, W.C.; Chen, C.Y.; Hsia, S.M.; Wang, T.H.; Tu, H.F.; Shih, Y.H.; Shieh, T.M. Effects of Temoporfin-Based Photodynamic Therapy on the In Vitro Antibacterial Activity and Biocompatibility of Gelatin-Hyaluronic Acid Cross-Linked Hydrogel Membranes. Pharmaceutics 2022, 14, 2314. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Xiao, Z.; Zhang, Z.; Li, Y.; Liu, C.; Chen, X.; Liu, Y.; Wu, D.; Liu, C.; Shuai, X.; et al. Stiffness-tuned and ROS-sensitive hydrogel incorporating complement C5a receptor antagonist modulates antibacterial activity of macrophages for periodontitis treatment. Bioact. Mater. 2023, 25, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, H.; Lei, W.; Tang, Y.; Hong, S.; Yang, H.; Tay, F.R.; Huang, C. MMP-8-Responsive Polyethylene Glycol Hydrogel for Intraoral Drug Delivery. J. Dent. Res. 2019, 98, 564–571. [Google Scholar] [CrossRef]
- Almeida, M.; Magalhães, M.; Veiga, F.; Figueiras, A. Poloxamers, poloxamines and polymeric micelles: Definition, structure and therapeutic applications in cancer. J. Polym. Res. 2018, 25, 31. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, J.-H.; Meng, M.; Cui, N.; Dai, C.-Y.; Jia, Q.; Lee, E.-S.; Jiang, H.-B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials 2021, 14, 4522. [Google Scholar] [CrossRef]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Gupta, P.; Mishra, B.; Nath, G. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J. Oral. Biol. Craniofac. Res. 2018, 8, 126–133. [Google Scholar] [CrossRef]
- Gopalakrishna, P.K.; Jayaramu, R.A.; Boregowda, S.S.; Eshwar, S.; Suresh, N.V.; Abu Lila, A.S.; Moin, A.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels 2023, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored Doxycycline Hyclate Loaded In Situ Gel for the Treatment of Periodontitis: Optimization, In Vitro Characterization, and Antimicrobial Studies. AAPS PharmSciTech 2021, 22, 77. [Google Scholar] [CrossRef]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Dinte, E.; Tomuta, I.; Iovanov, R.I.; Leucuta, S.E. Design and formulation of buccal mucoadhesive preparation based on sorbitan monostearate oleogel. Farmacia 2013, 61, 284–297. [Google Scholar]
- Barabás, R.; Farkas, N.; Cadar, O.; Bizo, L.; Resz, M.A.; Becze, A.; Marincas, L.; Veszi, A.; Bosca, A.B.; Dinte, E.; et al. Release of amoxicillin and doxycycline from PLA nanofibers optimized using factorial experimental design. Appl. Phys. A 2023, 129, 854. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Lashari, D.M.; Aljunaid, M.; Lashari, Y.; Qaid, H.R.; Ridwan, R.D.; Diyatri, I.; Kaid, N.; Alkadasi, B.A. The use of mucoadhesive oral patches containing epigallocatechin-3-gallate to treat periodontitis: An in vivo study. J. Taibah Univ. Med. Sci. 2022, 17, 1014–1020. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Li, X.; Xiao, S.; Filipczak, N.; Yalamarty, S.S.K.; Shang, H.; Zhang, J.; Zheng, Q. Role and Therapeutic Targeting Strategies of Neutrophil Extracellular Traps in Inflammation. Int. J. Nanomed. 2023, 18, 5265–5287. [Google Scholar] [CrossRef]
- Yang, S.C.; Chen, P.J.; Chang, S.H.; Weng, Y.T.; Chang, F.R.; Chang, K.Y.; Chen, C.Y.; Kao, T.I.; Hwang, T.L. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biocheml Pharmacol. 2018, 154, 384–396. [Google Scholar] [CrossRef]
- Zha, Y.F.; Xie, J.; Ding, P.; Zhu, C.L.; Li, P.; Zhao, Z.Z.; Li, Y.H.; Wang, J.F. Senkyunolide I protect against lung injury via inhibiting formation of neutrophil extracellular trap in a murine model of cecal ligation and puncture. Int. Immunopharmacol. 2021, 99, 107922. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xu, M.; Liu, Y. The total terpenoids of Celastrus orbiculatus (TTC) inhibit NOX-dependent formation of PMA-induced neutrophil extracellular traps (NETs). Eur. J. Inflamm. 2018, 16, 2058739218805667. [Google Scholar] [CrossRef]
- Yang, C.; Song, C.; Liu, Y.; Qu, J.; Li, H.; Xiao, W.; Kong, L.; Ge, H.; Sun, Y.; Lv, W. Re-Du-Ning injection ameliorates LPS-induced lung injury through inhibiting neutrophil extracellular traps formation. Phytomedicine 2021, 90, 153635. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xu, M.; Dai, X.; Ni, T.; Li, D.; Jin, F.; Wang, H.; Tao, L.; Pan, B.; Woodgett, J.R.; et al. Polypharmacological Profiles Underlying the Antitumor Property of Salvia miltiorrhiza Root (Danshen) Interfering with NOX-Dependent Neutrophil Extracellular Traps. Oxidative Med. Cell. Longev. 2018, 2018, 4908328. [Google Scholar] [CrossRef] [PubMed]
| Pathogenic Pathway | Therapeutic Agent | Demonstrated Effect | Type of Study |
|---|---|---|---|
| PMNs’ degranulation and enzyme activity | Nexinhibs | Prevented enzymes’ exocytosis | In vitro study on human PMNs [128] |
| SNARE | Controlled granule transport and secretion | Animal models of acute lung injury [129] | |
| LXA4, 15-epi-LXA4 formed after administration of acetylsalicylic acid and statins | Suppressed MPO-induced Mac-1 expression and controlled PMNs tissue infiltration | In vitro study on human PMNs [133] Animal models of PD [133,134] | |
| LXA4 and resolvin E1 | Reduced ROS formation | In vitro study on human PMNs [135] | |
| Acetylsalicylic acid and PUFAs | Reduced salivary levels of pro-inflammatory cytokines | Clinical study on PD patients [136] | |
| NOX2-dependent NET formation | VAS2870 | Inhibited NOX2 activity | In vitro study on human PMNs [22] |
| NAC | Antioxidant activity | Clinical study on patients with SLE [137] | |
| MitoTEMPO | Neutralized mitochondrial ROS | Animal model of SLE [138] | |
| ABAH | Inhibited MPO activity and free radicals’ formation | In vitro study on human PMNs [58] | |
| DPI | Inhibited NOX activity and free radicals’ formation | ||
| NOX2-independent NET formation | Cl-amidine and BB-Cl-amidine | Inhibited PAD4 and histone citrullination | Animal models for autoimmune diseases [56,139] |
| Ascomycin and Cyclosporine A | Inhibited calcineurin pathway | In vitro study on human PMNs [39] | |
| Nocodazole and Cytochalasin D | Inhibited tubulin polymerization and actin filamentation | In vitro study on human PMNs [37] | |
| Excessive NET accumulation | DNase I and other bioengineered enzymes | Degraded the extracellular DNA | Clinical study on ventilated trauma patients [140] |
| Metformin | Activated AMPK and PMNs efferocytosis | Clinical study on patients with ARDS [48] | |
| RvTs | Increased NET phagocytosis by macrophages | In vitro study on human blood [68] | |
| Feed-forward NET formation | Pyrazolo-oxazepine | Controlled GSDMD cleavage, pyroptosis and inflammasome activation | In vitro study on human PMNs [60] |
| Periodontal pathogens’ virulence factors | Malabaricone C derived from nutmeg | Controlled gene expression of gingipains or inhibited the proteolytic activity of gingipains | In vitro study on P. gingivalis culture [141] |
| Polyphenols—catechin derivates | In vitro study on P. gingivalis culture [142] | ||
| Monoterpene from Nigella sativa | In vitro study on P. gingivalis culture [143] | ||
| Benzamidine derivatives | In vitro study on P. gingivalis culture [144] | ||
| Doxycycline | In vitro study on P. gingivalis culture [145,146] | ||
| Mangiferin and vismiaquinone A from Cratoxylum cochinchinense extract | Inhibited PPAD | In vitro study on P. gingivalis culture [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boșca, A.B.; Dinte, E.; Mihu, C.M.; Pârvu, A.E.; Melincovici, C.S.; Șovrea, A.S.; Mărginean, M.; Constantin, A.-M.; Băbțan, A.-M.; Muntean, A.; et al. Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis. Pharmaceutics 2024, 16, 1175. https://doi.org/10.3390/pharmaceutics16091175
Boșca AB, Dinte E, Mihu CM, Pârvu AE, Melincovici CS, Șovrea AS, Mărginean M, Constantin A-M, Băbțan A-M, Muntean A, et al. Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis. Pharmaceutics. 2024; 16(9):1175. https://doi.org/10.3390/pharmaceutics16091175
Chicago/Turabian StyleBoșca, Adina Bianca, Elena Dinte, Carmen Mihaela Mihu, Alina Elena Pârvu, Carmen Stanca Melincovici, Alina Simona Șovrea, Mariana Mărginean, Anne-Marie Constantin, Anida-Maria Băbțan, Alexandrina Muntean, and et al. 2024. "Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis" Pharmaceutics 16, no. 9: 1175. https://doi.org/10.3390/pharmaceutics16091175
APA StyleBoșca, A. B., Dinte, E., Mihu, C. M., Pârvu, A. E., Melincovici, C. S., Șovrea, A. S., Mărginean, M., Constantin, A.-M., Băbțan, A.-M., Muntean, A., & Ilea, A. (2024). Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis. Pharmaceutics, 16(9), 1175. https://doi.org/10.3390/pharmaceutics16091175













