Preparation of Glutathione-Responsive Paclitaxel Prodrug Based on Endogenous Molecule of L-Glutathione Oxidized for Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
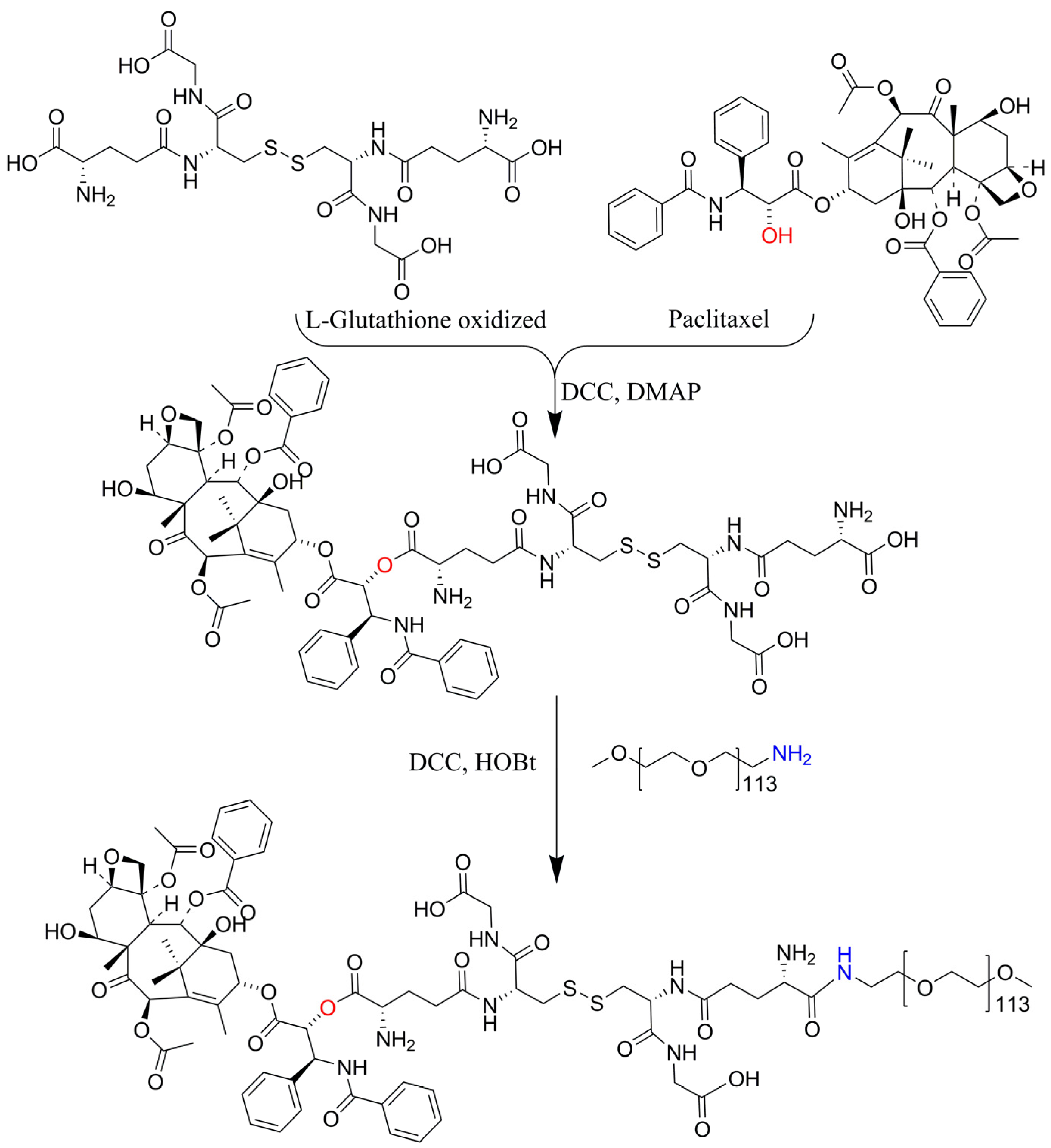
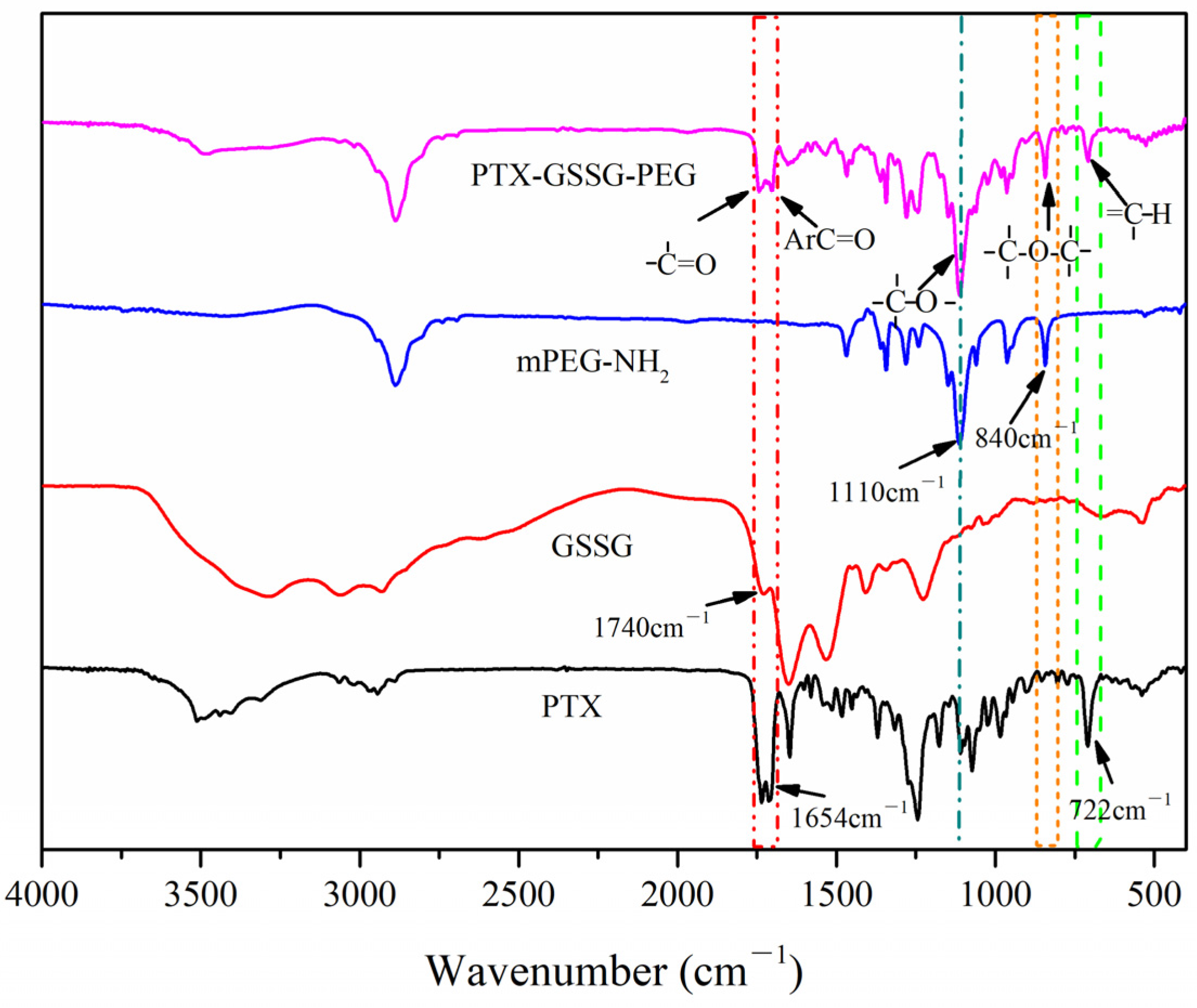
2.3. Synthesis and Characterization of PTX-GSSG-PEG
2.4. Preparation of PTX-GSSG-PEG Micelles
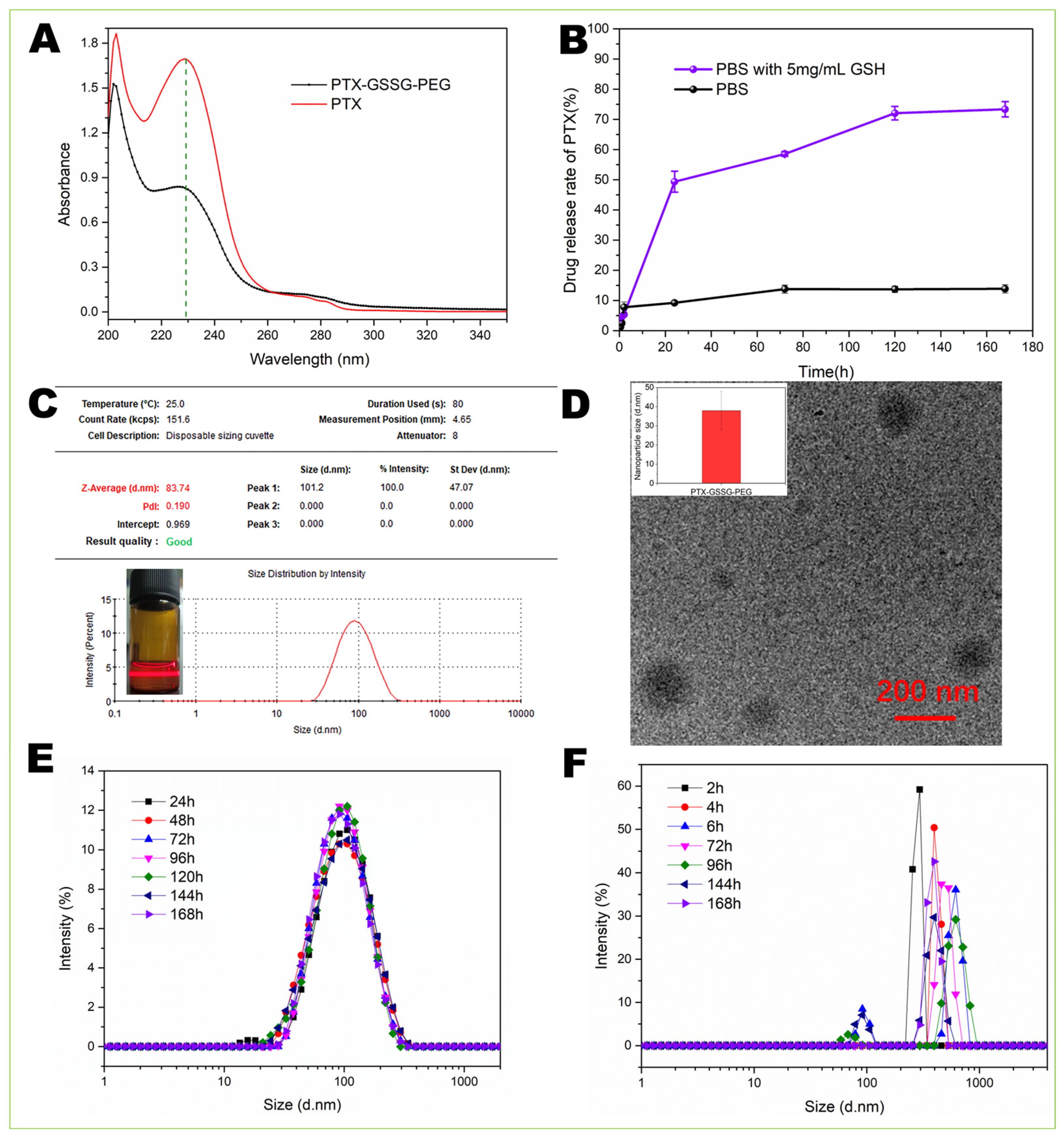
2.5. Drug Loading and Release In Vitro
2.6. Cell Viability and Flow Cytometry Experiments
2.7. Cellular Uptake Experiment
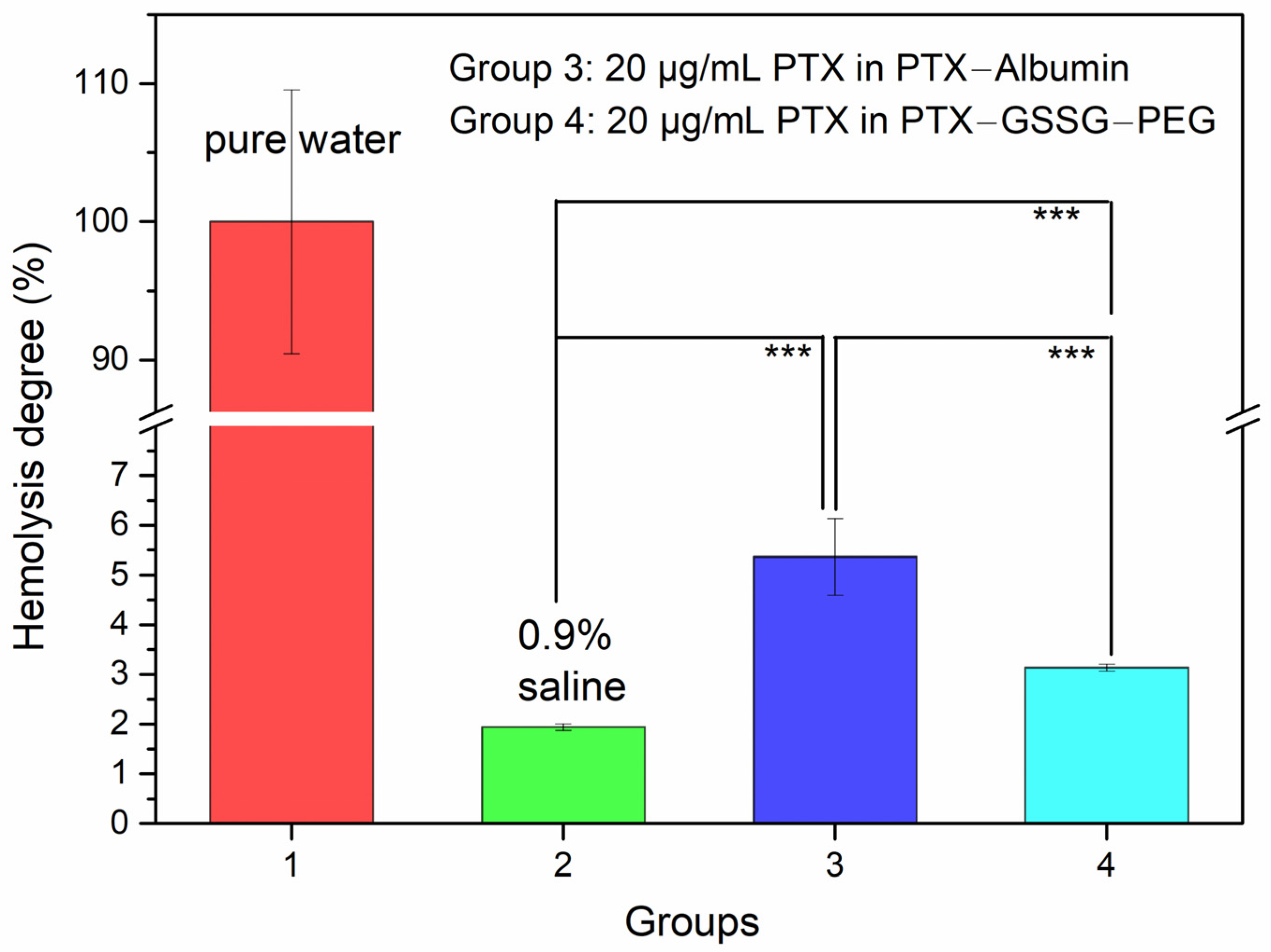
2.8. Hemolysis Test
3. Results and Discussion
3.1. Characterization of PTX-GSSG-PEG
3.2. Drug Loading and Release
3.3. Particle Size and Stability of Micelles
3.4. Cell Viability and Flow Cytometry Experiments
3.5. Cellular Uptake
3.6. Hemolysis Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research Progress on The Source, Production, and Anti-Cancer Mechanisms of Paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Alshhada, N.O.; Alhamdo, H.A.; Thallaj, N. A Review Study of Proline-Derived Paclitaxel as Treatment Anti Cancers. Int. J. Pharm. Sci. Res. 2023, 4, 32–52. [Google Scholar] [CrossRef]
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano Delivery System for Paclitaxel: Recent Advances in Cancer Theranostics. Colloids Surf. B 2023, 228, 113419. [Google Scholar] [CrossRef]
- Hrkach, J.; Hoff, D.V.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; Witt, D.D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Wileński, S.; Koper, A.; Śledzińska, P.; Bebyn, M.; Koper, K. Innovative Strategies for Effective Paclitaxel Delivery: Recent Developments and Prospects. J. Oncol. Pharm. Pract. 2024, 30, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Lepetre-Mouelhi, S.; Garbayo, E.; Blanco-Prieto, M.J. Self-assembled Lipid–Prodrug Nanoparticles. Nat. Rev. Bioeng. 2023, 1, 749–768. [Google Scholar] [CrossRef]
- Sreekanth, V.; Bajaj, A. Recent Advances in Engineering of Lipid Drug Conjugates for Cancer Therapy. ACS Biomater. Sci. Eng. 2019, 5, 4148–4166. [Google Scholar] [CrossRef]
- Pei, Q.; Jiang, B.; Hao, D.; Xie, Z. Self-assembled Nanoformulations of Paclitaxel for Enhanced Cancer Theranostics. Acta Pharm. Sin. B 2023, 13, 3252–3276. [Google Scholar] [CrossRef]
- Sreekanth, V.; Pal, S.; Kumar, S.; Komalla, V.; Yadav, P.; Shyam, R.; Sengupta, S.; Bajaj, A. Self-assembled Supramolecular Nanomicelles from A Bile Acid–Docetaxel Conjugate Are Highly Tolerable with Improved Therapeutic Efficacy. Biomater. Sci. 2021, 9, 5626. [Google Scholar] [CrossRef]
- Hao, D.; Meng, Q.; Jiang, B.; Lu, S.; Xiang, X.; Pei, Q.; Yu, H.; Jing, X.; Xie, Z. Hypoxia-Activated PEGylated Paclitaxel Prodrug Nanoparticles for Potentiated Chemotherapy. ACS Nano 2022, 16, 14693–14702. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Zhao, J.; Cao, S.; Brodie, P.J.; Tamarkin, L.; Huhta, M.; Myer, L.D.; Friedman, J.; Kingston, D.G.I. Synthesis and Evaluation of Paclitaxel-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconjug. Chem. 2016, 27, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, J.; Liu, D.; Sun, B.; Miao, L.; Musetti, S.; Li, J.; Han, X.; Du, Y.; Li, L.; et al. Self-Assembled Redox Dual-Responsive Prodrug-nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy. Nano Lett. 2016, 16, 5401–5408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, H.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Effects of PEGylated Paclitaxel Nanocrystals on Breast Cancer and Its Lung Metastasis. Nanoscale 2015, 7, 10790–10800. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Q.; Xiang, J.; Liu, F.; Zhou, Z.; Shen, Y. Self-assembly of Oxidation-responsive Polyethylene Glycol Paclitaxel Prodrug for Cancer Chemotherapy. J. Control. Release 2020, 321, 529–539. [Google Scholar] [CrossRef]
- Tian, C.; Guo, J.; Miao, Y.; Zheng, S.; Sun, B.; Sun, M.; Ye, Q.; Liu, W.; Zhou, S.; Kamei, K.; et al. Triglyceride-Mimetic Structure-Gated Prodrug Nanoparticles for Smart Cancer Therapy. J. Med. Chem. 2021, 64, 15936–15948. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Q.; Zhu, S.; Wang, X.; Zhang, C.; Ma, D.; Li, N.; Yin, Y. Construction of Glutathione-responsive Paclitaxel Prodrug Nanoparticles for Image-guided Targeted Delivery and Breast Cancer Therapy. RSC Adv. 2024, 14, 12796. [Google Scholar] [CrossRef]
- Caron, J.; Maksimenko, A.; Wack, S.; Lepeltier, E.; Bourgaux, C.; Morvan, E.; Leblanc, K.; Couvreur, P.; Desmaële, D. Improving the Antitumor Activity of Squalenoyl-Paclitaxel Conjugate Nanoassemblies by Manipulating the Linker between Paclitaxel and Squalene. Adv. Healthc. Mater. 2013, 2, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, J.; Bellucci, J.J.; Weitzhandler, I.; McDaniel, J.R.; Spasojevic, I.; Li, X.; Lin, C.; Chi, J.A.; Chilkoti, A. A Paclitaxel-loaded Recombinant Polypeptide Nanoparticle Outperforms Abraxane in Multiple Murine Cancer Models. Nat. Commun. 2015, 6, 7939. [Google Scholar] [CrossRef]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of Glutathione/glutathione Disulphide Ratio and S-glutathionylated Proteins in Human Blood, Solid Tissues, and Cultured Cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef]
- Sofic, E.; Lange, K.W.; Jellinger, K.; Riederer, P. Reduced and Oxidized Glutathione in The Substantia Nigra of Patients with Parkinson’s Disease. Neurosci. Lett. 1992, 142, 128–130. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, Q.; Jin, X.; Lu, H.; Wang, S.; Li, T.; Sheng, Y.; Zhang, F.; Zheng, Y. Drug Release from Disulfide-Linked Prodrugs: Role of Thiol Agents. Mol. Pharm. 2021, 18, 2777–2785. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Duan, X.; Pei, Z.; He, Z.; Guo, W.; Han, L. A Glutathione-Responsive PEGylated Nanogel with Doxorubicin-Conjugation for Cancer Therapy. J. Mater. Chem. B 2023, 11, 11612. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Q.; Che, W.; Li, T.; Zhang, K.; Han, L.; Song, L.; Guo, W. Redox-Responsive Nanomedicine of Doxorubicin-Conjugated Poly-L-glutathione Oxidized for Cancer Therapy. J. Taiwan Inst. Chem. E 2024, 159, 105456. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, B.; Li, Y.; Zhao, Y. Activity Difference between α-COOH and β-COOH in N-phosphorylaspartic Acids. J. Org. Chem. 2003, 68, 4052–4058. [Google Scholar] [CrossRef]
- Tang, X.; Wen, Y.; Zhang, Z.; Zhu, J.; Song, X.; Li, J. Rationally Designed Multifunctional Nanoparticles As GSH-responsive Anticancer Drug Delivery Systems Based on Host-guest Polymers Derived from Dextran and β-cyclodextrin. Carbohydr. Polym. 2023, 320, 121207. [Google Scholar] [CrossRef]
- Lang, W.; Chen, L.Z.; Chen, Y.; Cao, Q.Y. A GSH-activated AIE-based Polymer Photosensitizer for Killing Cancer Cells. Talanta 2023, 258, 124473. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ Entry into the Cell: Relevance to Drug Delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Wang, Q.; Wang, Y.; Liu, X.; Lu, M.; Li, Z.; Jiang, X.; Ji, J. Preparation of Glutathione-Responsive Paclitaxel Prodrug Based on Endogenous Molecule of L-Glutathione Oxidized for Cancer Therapy. Pharmaceutics 2024, 16, 1178. https://doi.org/10.3390/pharmaceutics16091178
Duan X, Wang Q, Wang Y, Liu X, Lu M, Li Z, Jiang X, Ji J. Preparation of Glutathione-Responsive Paclitaxel Prodrug Based on Endogenous Molecule of L-Glutathione Oxidized for Cancer Therapy. Pharmaceutics. 2024; 16(9):1178. https://doi.org/10.3390/pharmaceutics16091178
Chicago/Turabian StyleDuan, Xiao, Qiang Wang, Yue Wang, Xinping Liu, Manman Lu, Zhifang Li, Xuelian Jiang, and Jingquan Ji. 2024. "Preparation of Glutathione-Responsive Paclitaxel Prodrug Based on Endogenous Molecule of L-Glutathione Oxidized for Cancer Therapy" Pharmaceutics 16, no. 9: 1178. https://doi.org/10.3390/pharmaceutics16091178





