Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Development of Cream Formulations Containing Plant Extracts
2.2.2. pH and Conductivity Measurements
2.2.3. Light Microscopy Measurements
2.2.4. Differential Scanning Calorimetry (DSC) Measurements
2.2.5. Rheological Measurements
2.2.6. In Vitro Assessment
Texture Analysis
Spreadability
Skin Occlusivity
Water Washability
Stickiness
Cytotoxicity MTT Assay
Antimicrobial Activity
2.2.7. In Vivo Assessment
Sensory Analysis
Efficacy
Statistical Analysis
3. Results and Discussion
3.1. Formulation Development
3.2. pH and Conductivity
3.3. Light Microscopy
3.4. Differential Scanning Calorimetry (DSC)
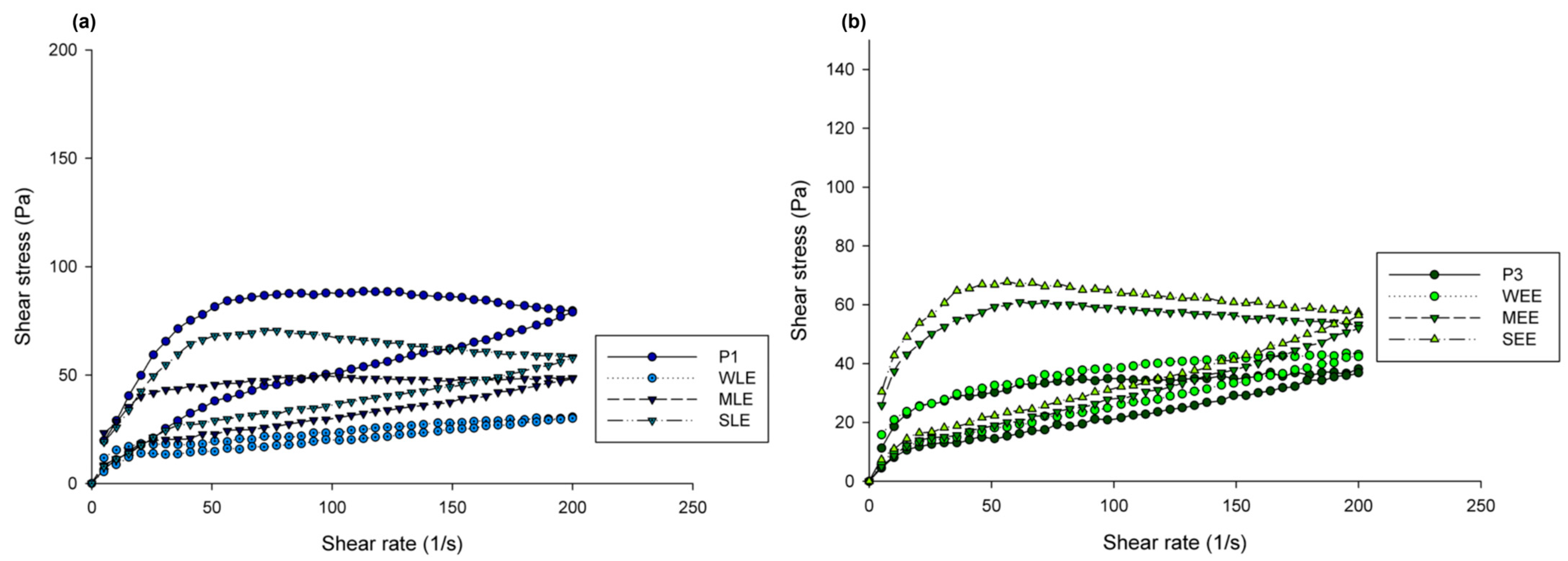
3.5. Rheology
3.6. In Vitro Assessment Analysis
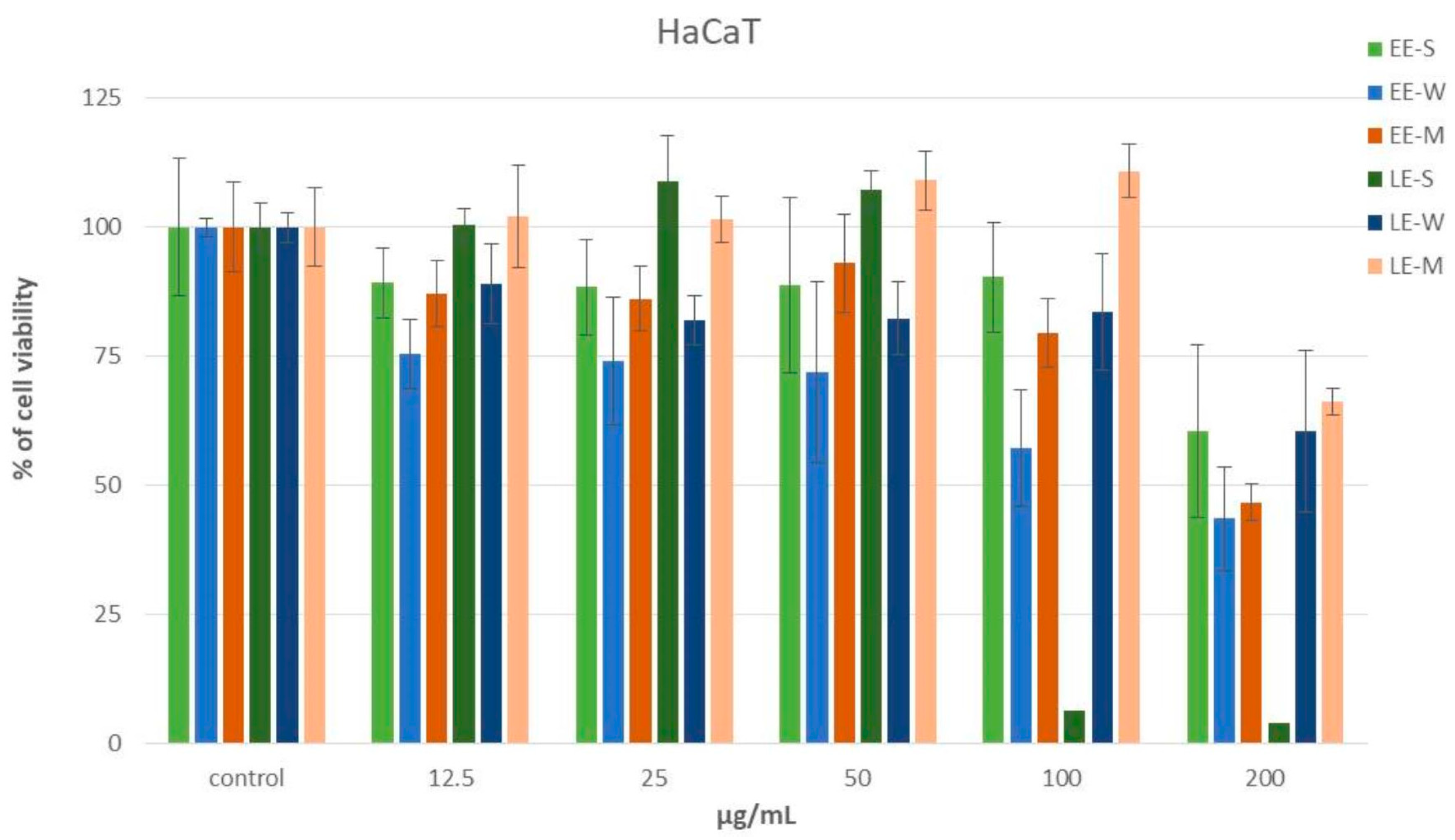
3.6.1. Cytotoxic MTT Assay
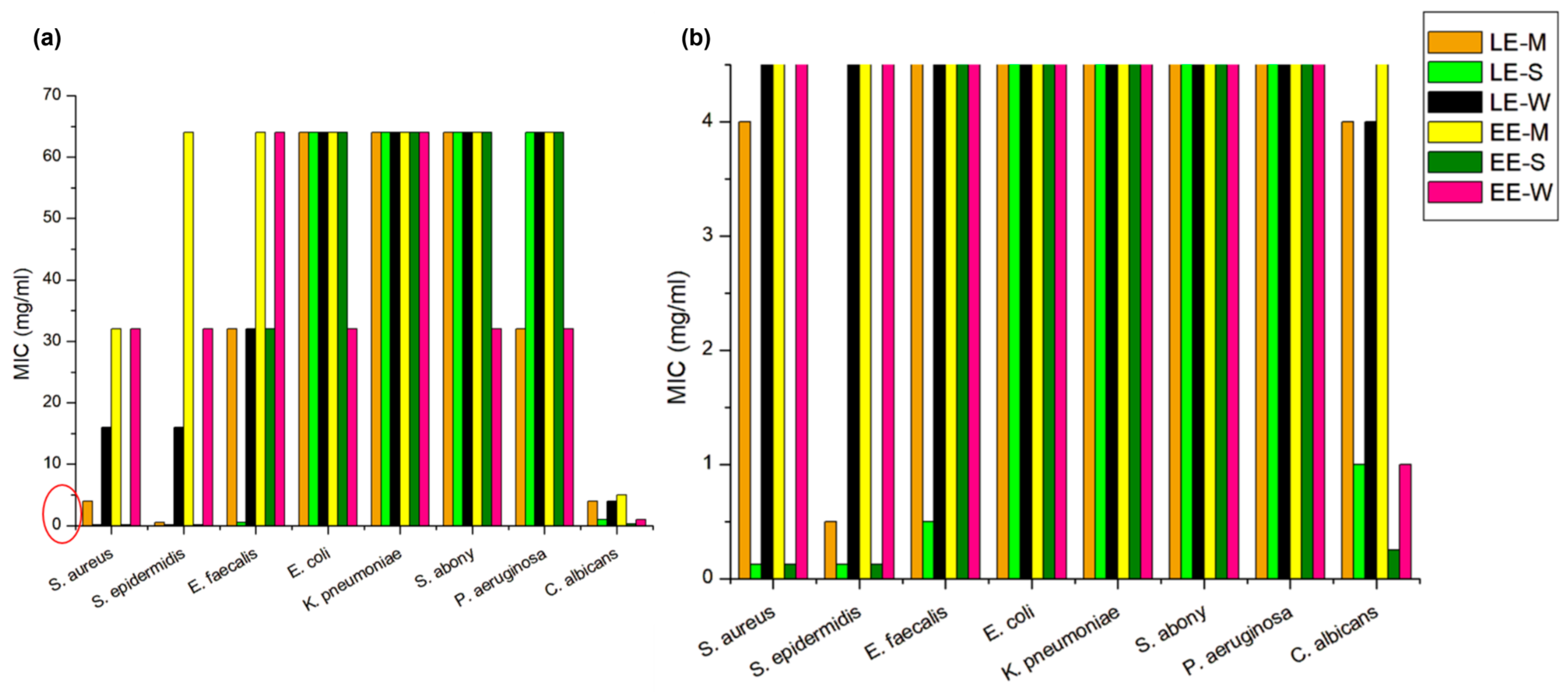
3.6.2. Antimicrobial Activity Analysis
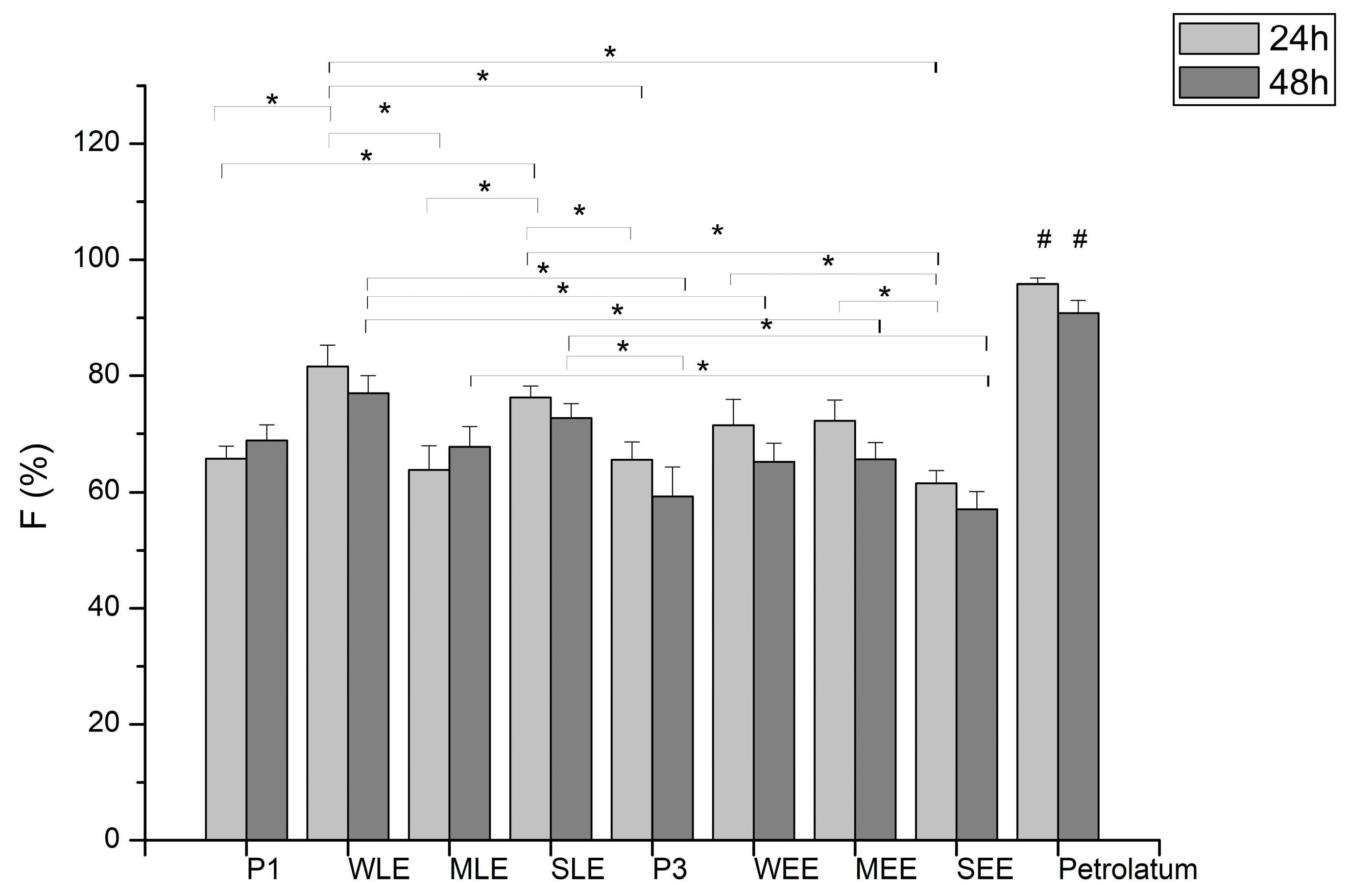
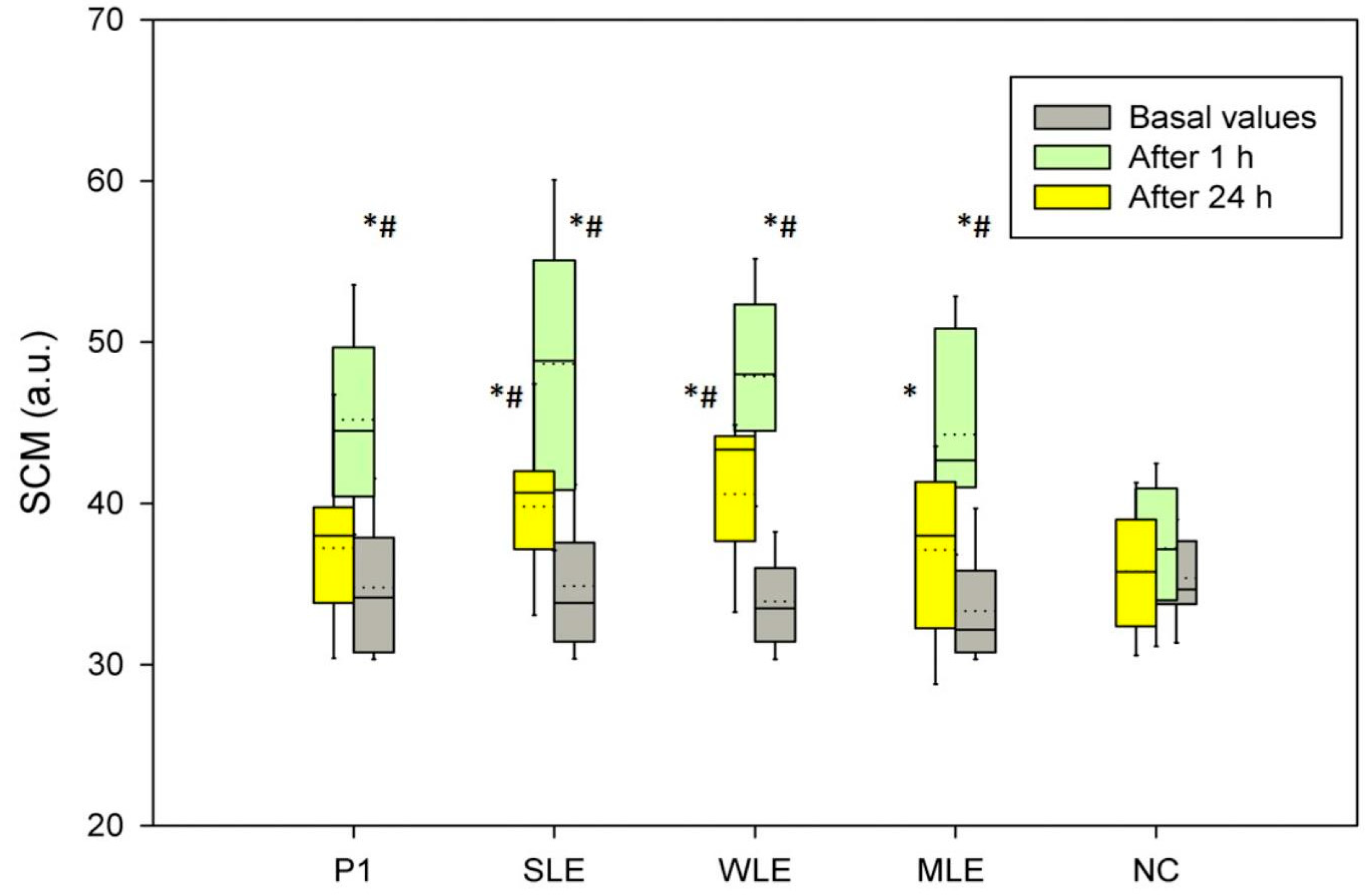
3.7. In Vivo Assessment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Adique, A.; Sarkar, P.; Shenai, V.; Sampath, M.; Lai, R.; Qi, J.; Wang, M.; Farage, M.A. The Impact of Routine Skin Care on the Quality of Life. Cosmetics 2020, 7, 59. [Google Scholar] [CrossRef]
- Draelos, Z. The Use of Cosmetic Products to Improve Self Esteem & Quality of Life. In Essential Psychiatry for the Aesthetic Practitioner; Rieder, E.A., Fried, R.G., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 34–41. [Google Scholar] [CrossRef]
- Beresniak, A.; Auray, J.-P.; Duru, G.; Aractingi, S.; Krueger, G.G.; Talarico, S.; Tsutani, K.; Dupont, D.; de Linares, Y. Quality of life assessment in cosmetics: Specificity and interest of the international BeautyQol instrument. J. Cosmet. Dermatol. 2015, 14, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Marto, J.M. A sustainable life cycle for cosmetics: From design and development to post-use phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European parliament and of the council of 30 november 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- European Consumer Perception Study. 2022. Available online: https://cosmeticseurope.eu/cosmetic-products/european-consumer-perception-study-2022/ (accessed on 9 August 2024).
- Olcer, Z.; Cal, A.; Unal, N.; Oztas, B.; Oge, G. Examining the use of cosmetic products and the awareness of healthy life among university students. Turk. J. Dermatol. 2023, 17, 79–87. [Google Scholar] [CrossRef]
- Amuah, E.; Antwi, K.; Ankamah, N. Elemental burdens of cosmetics and associated health and environmental impacts: A global view. World J. Adv. Res. Reviews. 2023, 20, 76–96. [Google Scholar] [CrossRef]
- Kättström, D.; Beronius, A.; Boije af Gennäs, U.; Rudén, C.; Ågerstrand, M. Out of REACH: Environmental hazards of cosmetic preservatives. Hum. Ecol. Risk Assess. Int. J. 2024, 30, 122–137. [Google Scholar] [CrossRef]
- Ang, K.L.; Saw, E.T.; He, W.; Dong, X.; Ramakrishna, S. Sustainability framework for pharmaceutical manufacturing (PM): A review of research landscape and implementation barriers for circular economy transition. J. Clean. Prod. 2021, 280, 124264. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing Synthetic Ingredients by Sustainable Natural Alternatives: A Case Study Using Topical O/W Emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef]
- Silvério, L.A.L.; Coco, J.C.; de Macedo, L.M.; Mendes dos Santos, E.; Sueiro, A.C.; Ataide, J.A.; Tavares, G.A.; Paiva-Santos, A.C.; Mazzola, P.G. Natural product-based excipients for topical green formulations. Sustain. Chem. Pharm. 2023, 33, 101111. [Google Scholar] [CrossRef]
- Torres, A.; Almeida, I.F.; Oliveira, R. An Overview of Proprietary Vehicles/Bases for Topical Compounding Medicines and Cosmetics. Cosmetics 2024, 11, 16. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) & Organization for Economic Co-Operation and Development (OECD). Background Notes on Sustainable, Productive and Resilient Agro-Food Systems: Value Chains, Human Capital, and the 2030 Agenda. A Report to the G20 Agriculture Deputies July 2019. Available online: https://www.oecd-ilibrary.org/docserver/dca82200-en.pdf?expires=1563959111&id=id&accname=guest&checksum=5BD0A7A51327DB165936B4AE57A0E5CE (accessed on 3 May 2024).
- European Commission. The Post-2020 Common Agricultural Policy: Environmental Benifits and Simplification. 2019. Available online: https://agriculture.ec.europa.eu/system/files/2021-01/cap-post-2020-environ-benefits-simplification_en_0.pdf (accessed on 3 May 2024).
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
- Gupta, S. Agriculture Crop Residue Burning and Its Consequences on Respiration Health of School-Going Children. Glob. Pediatr. Health 2019, 6, 2333794X19874679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Souza, J.R.C.L.; Villanova, J.C.O.; de Souza, T.D.S.; Maximino, R.C.; Menini, L. Vegetable fixed oils obtained from soursop agro-industrial waste: Extraction, characterization and preliminary evaluation of the functionality as pharmaceutical ingredients. Environ. Technol. Innov. 2021, 21, 101379. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Zampini, I.C.; Bravo, K.; Osorio, E.; Isla, M.I. Potential use of native fruits waste from Argentina as nonconventional sources of cosmetic ingredients. J. Cosmet. Dermatol. 2022, 21, 5058–5065. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; RodríguezPadron, D.; Luque, R. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Kwon, K.H. Sustainable Assessment of the Environmental Activities of Major Cosmetics and Personal Care Companies. Sustainability 2023, 15, 13286. [Google Scholar] [CrossRef]
- Postamentel, M.; Coman, C.-N. Analysis of the main technical indicators of corn, wheat and sunflower crops at the level of the European Union. Series Management, Economic Engineering in Agriculture and Rural Development. Sci. Pap. 2022, 22, 591–600. [Google Scholar]
- Grujić Vučkovski, B.; Roljevic, S.; Subic, J. Tendencies of plant production in the Republic of Serbia and at the level NUTS 1. J. Agric. Sci. 2020, 65, 69–84. [Google Scholar] [CrossRef]
- Halkos, G.; Petrou, K.N. Efficient Waste Management Practices: A Review. MPRA Paper No. 71518. 2016. Available online: https://mpra.ub.uni-muenchen.de/71518/ (accessed on 3 May 2024).
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2022, 28, 256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciriminna, R.; Chavarría-Hernández, N.; Demma Carà, P.; Pagliaro, M. Towards Sustainable Production of Cosmetic Ingredients from Plant Wastes: A Review. Cosmetics 2020, 7, 79. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, V.; Rai, M.K.; Singh, N. Exploring Potential of Bio-Based Ingredients in Cosmeceuticals: A Review. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]
- ISO 16128-2:2017; Amd 1: 2022—Cosmetics—Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients—Part 2: Criteria for Ingredients and Products. ISO: Geneva, Switzerland, 2017.
- Glišić, M.; Bošković Cabrol, M.; Cobanović, N.; Baltić, M.Ž.; Vranešević, J.; Samardžić, S.; Maksimović, Z. Antimicrobial activity of ethanolic extracts from wheat, sunflower and maize crop residues. Arch. Vet. Sci. 2023, 16, 53–67. [Google Scholar] [CrossRef]
- Glišić, M.; Bošković Cabrol, M.; Cobanović, N.; Starčević, M.; Samardžić, S.; Veličković, I.; Maksimović, Z. The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés. Foods 2024, 13, 788. [Google Scholar] [CrossRef]
- Romanić, R.; Lužaić, T.; Kravić, S.; Samardžić, S.; Maksimović, Z. Composition of Lipid Extract of Wheat, Corn and Sunflower Harvest Residues. Uljarstvo 2022, 53, 67–74. [Google Scholar]
- Romanić, R.; Lužaić, T.; Kravić, S.; Samardžić, S.; Maksimović, Z. Lipid Extracts of Harvest Residues of Wheat, Corn and Sunflower from Serbia: Investigation of Fatty Acid Composition. In Proceedings of the 19th EuroFed Lipid Congress and Expo: Fats, Oils and Lipids—From Raw Materials to Consumer Expectations, Poznan, Poland, 17–20 September 2023; p. 241. [Google Scholar]
- Djekic, L.; Čalija, B.; Medarević, Đ. Gelation behavior, drug solubilization capacity and release kinetics of poloxamer 407 aqueous solutions: The combined effect of copolymer, cosolvent and hydrophobic drug. J. Mol. Liq. 2020, 303, 112639. [Google Scholar] [CrossRef]
- De Vringer, T.; De Ronde, H.A.G. Preparation and structure of a water-in-oil cream containing lipid nanoparticles. J. Pharm. Sci. 1995, 84, 466–472. [Google Scholar] [CrossRef]
- Umar, A.K.; Butarbutar, M.; Sriwidodo, S.; Wathoni, N. Film-Forming Sprays for Topical Drug Delivery. Drug Des. Devel. Ther. 2020, 14, 2909–2925. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Media Preparation for EUCAST Disk Diffusion Testing and for Determination of MIC Values by the Broth Microdilution Method; V.6.0. January 2020. Available online: https://www.eucast.org/ast_of_bacteria/media_preparation (accessed on 23 March 2024).
- EUCAST Reading Guide for Broth Microdilution; V.5.0. January 2024. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=362&cHash=f2196d399c8618b7313ccb7f048f7af5 (accessed on 23 March 2024).
- Baki, G.; Szoboszlai, M.; Liberatore, M.W.; Chandler, M. Application of Check-All-That-Apply (CATA) Questions for Sensory Characterization of Cosmetic Emulsions by Untrained Consumers. J. Cosmet. Sci. 2018, 69, 83–100. [Google Scholar] [PubMed]
- Berardesca, E. EEMCO guidance for the assessment of stratum corneum hydration: Electrical methods. Ski. Res. Tech. 1997, 3, 126–132. [Google Scholar] [CrossRef]
- Pinagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the standardization group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef]
- ISO/TR 18811:2018; Cosmetics—Guidelines on the Stability Testing of Cosmetic Products. ISO: Geneva, Switzerland, 2018.
- Draft Guideline on Quality and Equivalence of Topical Products. European Medicines Agency (EMA). 2018. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products (accessed on 14 August 2024).
- Qayum, A.; Li, M.; Shi, R.; Bilawal, A.; Gantumur, M.; Hussain, M.; Ishfaq, M.; Waqas Ali Shah, S.; Jiang, Z.; Hou, J. Laccase cross-linking of sonicated α-Lactalbumin improves physical and oxidative stability of CLA oil in water emulsion. Ultrason. Sonochem. 2021, 71, 105365. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Daniels, R.; Müller-Goyman, C.C.; Savic, M.; Savic, S. Moisturizing emulsion systems based on the novel long-chain alkyl polyglucoside emulsifier: The contribution of thermoanalytical methods to the formulation development. J. Therm. Anal. Calorim. 2013, 111, 2045–2057. [Google Scholar] [CrossRef]
- Kaizu, K.; Alexandridis, P. Effect of surfactant phase behavior on emulsification. J. Colloid Interface Sci. 2016, 466, 138–149. [Google Scholar] [CrossRef]
- Naso, J.N.; Bellesi, F.A.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M.R. A new methodology to assess the solubility of fatty acids: Impact of food emulsifiers. Food Res. Int. 2021, 139, 109829. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Li, F.; Chen, M.; Zhang, W. Effect of Binary/Ternary Fatty Acids Ratio and Glycerin on the Phase Behaviors of Soap Solutions. J. Surfact. Deterg. 2017, 20, 425–434. [Google Scholar] [CrossRef]
- Djekic, L.j.; Krajišnik, D.; Mićic, Z.; Čalija, B. Formulation and physicochemical characterization of hydrogels with 18β-glycyrrhetinic acid/phospholipid complex phytosomes. J. Drug Deliv. Sci. Technol. 2016, 35, 81–90. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Lukic, M.; Filipovic, M.; Pajic, N.; Lunter, D.; Bozic, D.; Savic, S. Formulation of topical acidic products and acidification of the skin—Contribution of glycolic acid. Int. J. Cosmet. Sci. 2021, 43, 419–431. [Google Scholar] [CrossRef]
- Lefèbvre, X.; Koch, M.H.J.; Reynaers, H.; David, C. Thermal behavior of poly(hexamethylene terephthalate) oligomers. I. Melting behavior and morphology of the crystalline phase. J. Polym. Sci. B Polym. Phys 1999, 37, 1–18. [Google Scholar] [CrossRef]
- Dahl, V.; Friedrich, A.; Meyer, J.; Venzmer, J.; Belkoura, L.; Strey, R.; Mayer, C.; Michel, R.; Gradzielski, M. Structural Analysis of a Modern o/w-Emulsion Stabilized by a Polyglycerol Ester Emulsifier and Consistency Enhancers. Colloids Interfaces 2018, 2, 3. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil Content and Fatty Acids Composition in Brassica Species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Tamburic, S.; Stevic, C.M.; Sisson, H. Rheological and texture analysis methods for the assessment of viscoelasticity. SOFW J. 2018, 144, 20–25. [Google Scholar]
- Lavuri, R.; Chiappetta Jabbour, C.J.; Grebinevych, O.; Roubaud, D. Green factors stimulating the purchase intention of innovative luxury organic beauty products: Implications for sustainable development. J. Environ. Manag. 2022, 301, 113899. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The Slippery Road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Silva, A.; Costa, P.; Delerue-Matos, C.; Latocha, P.; Rodrigues, F. Extraordinary composition of Actinidia arguta by-products as skin ingredients: A new challenge for cosmetic and medical skincare industries. Trends. Food Sci. Technol. 2021, 116, 842–853. [Google Scholar] [CrossRef]
- Almeida, A.; Sarmento, B.; Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int. J. Pharm. 2017, 519, 178–185. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 26, 22–12827. [Google Scholar] [CrossRef]
- ISO 29621:2017; Cosmetics—Microbiology—Guidelines for the Risk Assessment and Identification of Microbiologically Low-Risk Products. ISO: Geneva, Switzerland, 2017.
- Lukic, M. Preservatives, colorants and UV filters in cosmetic products: Safety aspects. Arh. Farm 2018, 68, 934–948. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements; Recent Trends in Preservation and Legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Thakur, K.; Sharma, V.; Saini, M.; Sharma, D.; Vishwas, S.; Kakoty, V.; Pal, R.S.; Chaitanya, M.V.N.L.; Babu, M.R.; et al. Exploring the multifaceted potential of chlorogenic acid: Journey from nutraceutical to nanomedicine. S. Afr. J. Bot. 2023, 159, 658–677. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świsłocka, R.; Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Kozłowski, M.; Koronkiewicz, K.; Piekut, J.; Lewandowski, W. Structural characterization and evaluation of antimicrobial and cytotoxic activity of six plant phenolic acids. PLoS ONE 2024, 19, e0299372. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.; Smith, V. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Arellano, H.; Nardello-Rataj, V.; Szunerits, S.; Boukherroub, R.; Fameau, A.L. Saturated long chain fatty acids as possible natural alternative antibacterial agents: Opportunities and challenges. Adv. Colloid. Interface Sci. 2023, 318, 102952. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, Y.; Miura, M.; Konno, H.; Yano, S.; Nonomura, Y. Systematic analysis of selective bactericidal activity of fatty acids against Staphylococcus aureus with minimum inhibitory concentration and minimum bactericidal concentration. J. Oleo. Sci. 2019, 68, 291–296. [Google Scholar] [CrossRef]
- Churchward, C.P.; Alany, R.G.; Snyder, L.A.S. Alternative antimicrobials: The properties of fatty acids and monoglycerides. Crit. Rev. Microbiol. 2018, 44, 561–570. [Google Scholar] [CrossRef]
- Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial properties and effects of fruit chilling and extract storage on antioxidant activity, total phenolic and anthocyanin content of four date palm (Phoenix dactylifera) cultivars. Molecules 2016, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Herz, R.S.; Larsson, M.; Trujillo, R.; Casola, M.C.; Ahmed, F.K.; Lipe, S.; Brashear, M.E. A three-factor benefits framework for understanding consumer preference for scented household products: Psychological interactions and implications for future development. Cogn. Res. Princ. Implic. 2022, 7, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, M.; Kashetsky, N.; Feschuk, A.; Maibach, H.I. Transepidermal water loss (TEWL): Environment and pollution-A systematic review. Skin Health Dis. 2022, 2, e104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Ethanol Maize Extract | Ethanol Wheat Extract | Ethanol Sunflower Extract | |
|---|---|---|---|
| Total phenolic content (mg of gallic acid equivalents (GAE) per g) | 20.44 mg GAE/g | 23.19 mg GAE/g | 15.83 mg GAE/g |
| Total flavonoid content (mg of quercetin equivalents (QE) per g) | 9.37 mg QE/g | 15.22 mg QE/g | 8.98 mg QE/g |
| Isolated compounds | p-Coumaric acid = 1.64 mg/g Ferulic acid = 1.21 mg/g Tricin = 1.99 mg/g | p-Coumaric acid = 2.14 mg/g Tricin = 3.92 mg/g | Chlorogenic acid = 2.40 mg/g |
| Fatty Acids | Lipid Wheat Extract (%) | Lipid Maize Extract (%) | Lipid Sunflower Extract (%) |
|---|---|---|---|
| C14:0 | 1.44 ± 0.31 | 0.49 ± 0.02 | nd |
| C16:0 | 23.74 ± 8.23 | 11.84 ± 0.46 | 6.13 ± 0.28 |
| C16:1 | nd | 4.61 ± 0.10 | nd |
| C18:0 | 3.10 ± 0.79 | 2.58 ± 0.08 | 2.56 ± 0.08 |
| C18:1 | 12.93 ± 3.99 | 29.32 ± 0.30 | 26.54 ± 1.02 |
| C18:2 | 26.76 ± 7.61 | 24.01 ± 0.21 | 34.34 ± 0.97 |
| C18:3n3 | 1.59 ± 0.48 | 2.93 ± 0.02 | 0.88 ± 0.12 |
| C20:0 | 6.97 ± 2.94 | 4.37 ± 0.08 | 5.53 ± 0.53 |
| C22:0 | 6.92 ± 3.30 | 4.74 ± 0.36 | 4.84 ± 0.20 |
| C24:0 | 3.16 ± 0.15 | 2.10 ± 0.25 | 2.33 ± 0.18 |
| Other components * | 21.68 ± 6.96 | 13.02 ± 0.10 | 16.85 ± 1.97 |
| Ingredients (INCI) [% wt] | P1 | P3 | WLE | MLE | SLE | WEE | MEE | SEE |
|---|---|---|---|---|---|---|---|---|
| Polyglyceryl-6 distearate, jojoba esters, polyglyceryl-3 beeswax, and cetyl alcohol | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Brassica glycerides | 3.0 | 1.0 | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 1.0 |
| Caprylic/capric triglyceride | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Triticum aestivum lipid extract (wheat lipid extract—LE-W) | / | / | 2.0 | / | / | / | / | / |
| Triticum aestivum ethanol extract (wheat ethanol extract—EE-W) | / | / | / | / | / | 0.1 | / | / |
| Zea mays lipid extract (maize lipid extract—LE-M) | / | / | / | 2.0 | / | / | / | / |
| Zea mays ethanol extract (maize ethanol extract—EE-M | / | / | / | / | / | / | 0.1 | / |
| Helianthus annuus lipid extract (sunflower lipid extract—LE-S) | / | / | / | / | 2.0 | / | / | / |
| Helianthus annuus ethanol extract (sunflower ethanol extract—EE-S | / | / | / | / | / | / | / | 0.1 |
| Phenoxyethanol and ethylhexylglycerol | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Aqua/Water: up to | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Sample | pH ± SD | 90% Confidence Interval # | Conductivity ± SD (μS/cm) | ||
|---|---|---|---|---|---|
| After 7 Days | After 6 Months | After 7 Days | After 6 Months | ||
| P1 | 7.35 ± 0.05 | 7.09 ± 0.03 * | 8.09–6.62 | 13.87 ± 0.28 | 14.63 ± 0.42 |
| WLE | 7.26 ± 0.06 | 6.65 ± 0.05 | 7.98–6.53 | 46.00 ± 0.88 | 20.09 ± 3.40 * |
| MLE | 7.06 ± 0.07 | 6.72 ± 0.06 * | 7.77–6.35 | 56.00 ± 1.22 | 45.63 ± 0.45 * |
| SLE | 6.74 ± 0.05 | 6.67 ± 0.04 | 7.42–6.07 | 64.00 ± 1.57 | 56.03 ± 0.55 |
| P3 | 7.06 ± 0.01 | 7.35 ± 0.03 * | 7.77–6.35 | 31.01 ± 1.94 | 23.87 ± 0.12 |
| WEE | 6.76 ± 0.02 | 7.26 ± 0.02 * | 7.44–6.11 | 83.30 ± 1.73 | 76.11 ± 0.87 * |
| MEE | 6.79 ± 0.01 | 7.06 ± 0.03 * | 7.47–6.11 | 135.68 ± 4.81 | 126.24 ± 2.32 * |
| SEE | 6.49 ± 0.02 | 6.74 ± 0.01 * | 7.14–5.84 | 115.10 ± 5.45 | 104.15 ± 1.32 * |
| Sample | Dimensions (μm) | ||
|---|---|---|---|
| Mean ± SD | Minimum | Maximum | |
| P1 | 39.31 ± 27.76 | 9.49 | 99.77 |
| WLE | 12.90 ± 2.88 | 3.07 | 26.84 |
| MLE | 11.94 ± 8.37 | 3.43 | 40.38 |
| SLE | 11.11 ± 8.14 | 2.75 | 42.86 |
| P3 | 12.71 ± 10.44 | 1.44 | 78.74 |
| WEE | 9.68 ± 8.17 | 1.14 | 56.24 |
| MEE | 7.38 ± 4.07 | 0.51 | 33.62 |
| SEE | 10.96 ± 9.22 | 0.72 | 110.14 |
| Sample | ηmax Initially (Pa·s) | ηmax after 6 Months (Pa·s) | ηmin Initially (Pa·s) | ηmin after 6 Months (Pa·s) |
|---|---|---|---|---|
| P1 | 3.84 | 6.24 | 0.395 | 0.657 |
| WLE | 2.28 | 3.78 | 0.150 | 0.285 |
| MLE | 4.52 | 3.79 | 0.242 | 0.275 |
| SLE | 3.74 | 3.96 | 0.289 | 0.319 |
| P3 | 2.19 | 2.71 | 0.191 | 0.179 |
| WEE | 3.09 | 5.50 | 0.216 | 0.275 |
| MEE | 5.07 | 6.98 | 0.267 | 0.303 |
| SEE | 5.92 | 8.4 | 0.287 | 0.306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, M.; Ćirić, A.; Božić, D.D.; Stanković, J.A.; Medarević, Đ.; Maksimović, Z. Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals. Pharmaceutics 2024, 16, 1182. https://doi.org/10.3390/pharmaceutics16091182
Lukić M, Ćirić A, Božić DD, Stanković JA, Medarević Đ, Maksimović Z. Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals. Pharmaceutics. 2024; 16(9):1182. https://doi.org/10.3390/pharmaceutics16091182
Chicago/Turabian StyleLukić, Milica, Ana Ćirić, Dragana D. Božić, Jelena Antić Stanković, Đorđe Medarević, and Zoran Maksimović. 2024. "Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals" Pharmaceutics 16, no. 9: 1182. https://doi.org/10.3390/pharmaceutics16091182
APA StyleLukić, M., Ćirić, A., Božić, D. D., Stanković, J. A., Medarević, Đ., & Maksimović, Z. (2024). Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals. Pharmaceutics, 16(9), 1182. https://doi.org/10.3390/pharmaceutics16091182







