Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems
Abstract
:1. Introduction
2. Gut Barrier in IBD
3. Traditional Treatments of IBD
3.1. Aminosalicylates
3.2. Corticosteroids
3.3. Immunomodulators
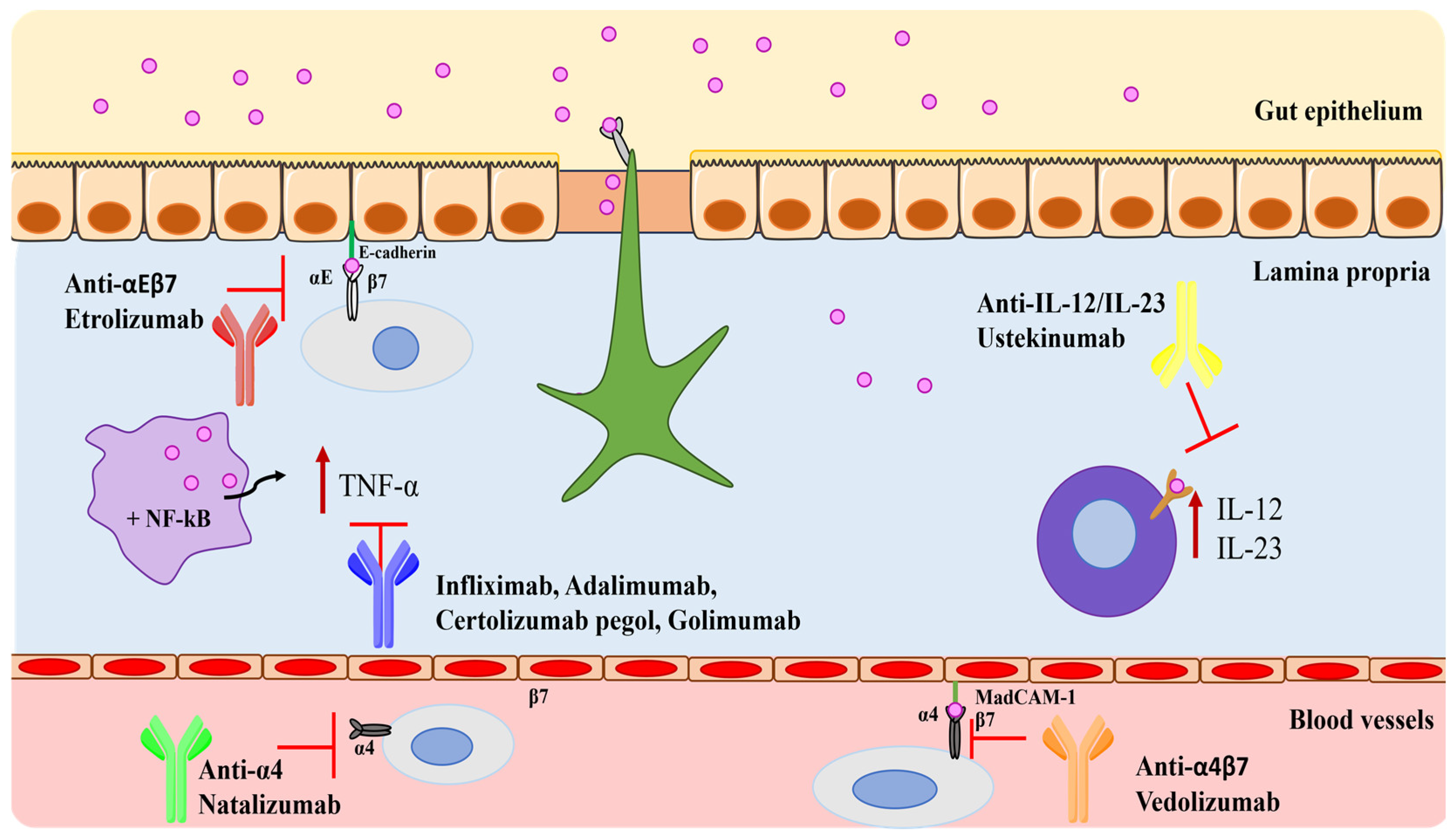
4. Monoclonal Antibodies in IBD
4.1. Tumor Necrosis Factor-Alpha (TNF-α) Blockers
4.2. Integrin Blockers
4.3. Interleukins Blockers
4.4. Monoclonal Antibodies in Development
| Drugs | Mechanism of Action | Administration Route | Doses | Adverse Effects | Ref. |
|---|---|---|---|---|---|
| Infliximab (REMICADE®) | Anti-TNF-α | Intravenous | 5 mg/kg Induction doses: 0, 2, and 6 weeks. Maintenance doses: 8-weekly intervals. | Hypersensitivity reactions. | [44] |
| Infliximab (ZYMFENTRA®) | Anti-TNF-α | Subcutaneous | Maintenance doses only: 120 mg at week 10, 2-weekly intervals. | COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain (for UC). COVID-19, headache, upper respiratory tract infection, injection site reaction, diarrhea, increased blood creatine phosphokinase, arthralgia, increased alanine aminotransferase, hypertension, urinary tract infection, neutropenia, dizziness, and leukopenia (for CD). | [54] |
| Adalimumab (HUMIRA®) | Anti-TNF-α | Intramuscular | Induction doses: 80/160 mg at week 0 and 40/80 mg at week 2 (for CD); 80/160 mg at week 0, 40/80 mg at week 1, and 40/80 mg at week 2 (for UC). Maintenance doses: 20/40 mg every week starting from week 4 (for CD); 40/80 mg every week starting from week 4 or 20/40 mg every week (for UC). | Infections (e.g., upper respiratory, sinusitis), injection site reactions, headache, and rash. | [55] |
| Golimumab (SIMPONI®) | Anti-TNF-α | Subcutaneous | Induction doses: 200 mg week 0 and 100 mg at week 2. Maintenance doses: 50/100 mg 4-weekly intervals. | Paraesthesia, cutaneous infection, pneumonitis, and the recurrence of cervical neoplasia. | [58] |
| Certolizumab pegol (CIMZIA®) | Anti-TNF-α | Subcutaneous | Induction doses: 400 mg at 0, 2, and 4 weeks. Maintenance doses: 400 mg 4-weekly intervals. | Upper respiratory tract infection, rash, and urinary tract infection. | [61] |
| Natalizumab (TYSABRI®) | Integrin blocker | Intravenous | 300 mg 4-weekly intervals. | Headache, the exacerbation of CD, nausea, nasopharyngitis, and progressive multifocal leukoencephalopathy. | [69,70] |
| Vedolizumab (ENTYVIO®) | Integrin blocker | Intravenous and subcutaneous | Induction doses: 300 mg at weeks 0, 2, and 6. Maintenance doses: 300 mg 8-weekly intervals. | Nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, rash, pruritus, sinusitis, oropharyngeal pain, pain in extremities, and injection site reactions with subcutaneous administration. | [72,73] |
| Etrolizumab | Integrin blocker | Subcutaneous | 105 mg every 4 weeks for 14 weeks (phase 3 clinical program). | UC flare, appendicitis, and anemia. | [75] |
| Ustekinumab (STELARA®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: depends on the patient’s body weight. Maintenance doses: 90 mg 8–12-weekly intervals. | Headache, nasopharyngitis, inflammation of the nose and throat, and hypersensitivity (allergic reaction). | [79] |
| Mirikizumab (OMVOH®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 300 mg at weeks 0, 4, and 8; 4-weekly intervals. Maintenance doses: 200 mg 4-weekly intervals. | Infections (active tuberculosis). | [83] |
| Briakinumab | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 200, 400, and 700 mg at weeks 0, 4, and 8. Maintenance doses: 200, 400, and 700 mg after 12 weeks (phase 2 clinical program). | Upper respiratory tract infection, nausea, abdominal pain, and headache. | [84] |
| Ontamalimab | Integrin blocker | Subcutaneous | Induction doses: 25/75 mg once 4-weekly intervals to week 12. Maintenance doses: 25/75 mg once at 4-weekly intervals to week 52 (phase 3 clinical program). | Serious infections, gastroenteritis, pelvic abscess, pneumonia, arthralgia, and nasopharyngitis. | [85] |
| Risankizumab (SKYRIZI®) | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 600 mg at weeks 0, 4, and 8. Maintenance doses: 360 mg at week 12, 8-weekly intervals. | Upper respiratory infection, tinea infection, folliculitis, headache, pruritus, rash, urticaria, fatigue, and injection site reactions. | [86] |
| Brazikumab | Interleukin blocker | Intravenous and subcutaneous | Induction doses: 700 mg at weeks 0 and 4. Maintenance doses: 210 mg from week 12. (Phase 2 clinical program.) | Nasopharyngitis, headache, and abdominal pain. | [86] |
| Guselkumab (TREMFYA®) | Interleukin blocker | Intravenous | Induction doses: 200 mg at weeks 0, 4, and 8 for 12 weeks. | Headache, joint pain, upper respiratory infections, diarrhea, and stomach pain. | [88] |
4.5. Other Treatments
5. Drug Delivery Systems
5.1. General Features of DDSs in the IBD
5.2. Antibody-Loaded DDSs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kontola, K.; Oksanen, P.; Huhtala, H.; Jussila, A. Increasing Incidence of Inflammatory Bowel Disease, with Greatest Change Among the Elderly: A Nationwide Study in Finland, 2000–2020. J. Crohn’s Colitis 2023, 17, 706–711. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Rieder, F.; Kucharzik, T.; Peyrin-Biroulet, L.; Schoepfer, A.M.; Rubin, D.T.; Vavricka, S.R. Emerging treatment options for extraintestinal manifestations in IBD. Gut 2021, 70, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Raj, J.P. Role of biologics and biosimilars in inflammatory bowel disease: Current trends and future perspectives. J. Inflamm. Res. 2018, 11, 215–226. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, W.M.; Guo, Z. Current status of novel biologics and small molecule drugs in the individualized treatment of inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 6888–6899. [Google Scholar] [CrossRef]
- Faris, A.; Cacciatore, I.; Ibrahim, I.M.; Al Mughram, M.H.; Hadni, H.; Tabti, K.; Elhallaoui, M. In silico computational drug discovery: A Monte Carlo approach for developing a novel JAK3 inhibitors. J. Biomol. Struct. Dyn. 2023, 1–23. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.J.; Panaccione, R.; Domènech, E.; Pouillon, L.; Siegmund, B.; Danese, S.; Ghosh, S. Tumour necrosis factor inhibitors in inflammatory bowel disease: The story continues. Therap. Adv. Gastroenterol. 2021, 14, 17562848211059954. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Zhang, D.; Gordon, M.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 9, CD003715. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Kuenzig, M.E.; Hazlewood, G.; Clement, F.; McBrien, K.; Holmes, R.; Panaccione, R.; Ghosh, S.; Seow, C.H.; Rezaie, A.; et al. Comparative Effectiveness of Mesalamine, Sulfasalazine, Corticosteroids, and Budesonide for the Induction of Remission in Crohn’s Disease: A Bayesian Network Meta-analysis. Inflamm. Bowel. Dis. 2017, 23, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Krnjic, A.; Frick, A.; Gmainer, C.; Asboth, M.; Jimenez, K.; Lang, M.; Baumgartner, M.; Evstatiev, R.; Gasche, C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci. Rep. 2019, 9, 2842. [Google Scholar] [CrossRef]
- Mehta, R.S.; Mayers, J.R.; Zhang, Y.; Bhosle, A.; Glasser, N.R.; Nguyen, L.H.; Ma, W.; Bae, S.; Branck, T.; Song, K.; et al. Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease. Nat. Med. 2023, 29, 700–709. [Google Scholar] [CrossRef]
- Gisbert, J.P.; González-Lama, Y.; Maté, J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2007, 13, 629–638. [Google Scholar] [CrossRef]
- Becker, H.E.F.; Demers, K.; Derijks, L.J.J.; Jonkers, D.M.A.E.; Penders, J. Current evidence and clinical relevance of drug-microbiota interactions in inflammatory bowel disease. Front. Microbiol. 2023, 14, 1107976. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.; Saxena, S.; Pollok, R. Using corticosteroids appropriately in inflammatory bowel disease: A guide for primary care. Br. J. Gen. Pract. 2018, 68, 497–498. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef] [PubMed]
- Manguso, F.; Bennato, R.; Lombardi, G.; Riccio, E.; Costantino, G.; Fries, W. Efficacy and Safety of Oral Beclomethasone Dipropionate in Ulcerative Colitis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166455. [Google Scholar] [CrossRef]
- Gabbani, T.; Manetti, N.; Bagnoli, S.; Annese, V. Beclomethasone dipropionate for the treatment of ulcerative colitis. Expert Opin. Orphan Drugs 2015, 3, 87–96. [Google Scholar] [CrossRef]
- Oancea, I.; Movva, R.; Das, I.; Aguirre de Cárcer, D.; Schreiber, V.; Yang, Y.; Purdon, A.; Harrington, B.; Proctor, M.; Wang, R.; et al. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut 2017, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Diall, A.; Dvorsky, R.; Atreya, R.; Henninger, C.; Grün, M.; Hofmann, U.; Schaeffeler, E.; López-Posadas, R.; Daehn, I.; et al. Designer Thiopurine-analogues for Optimised Immunosuppression in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2016, 10, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: A systematic review and meta-analysis of randomized, controlled trials. eClinicalMedicine 2020, 20, 100271. [Google Scholar] [CrossRef]
- Rosh, J.R. The Current Role of Methotrexate in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 43–46. [Google Scholar]
- Weissman, S.; Chris-Olaiya, A.; Mehta, T.I.; Aziz, M.; Alshati, A.; Berry, R.; Fatima, R.; Kolli, S.; Hassan, A.; Sciarra, M.A. A novel player: Cyclosporine therapy in the management of inflammatory bowel disease. Transl. Gastroenterol. Hepatol. 2019, 4, 67. [Google Scholar] [CrossRef]
- Wu, B.; Tong, J.; Ran, Z. Tacrolimus Therapy in Steroid-Refractory Ulcerative Colitis: A Review. Inflamm. Bowel Dis. 2020, 26, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Fujii, T.; Kinoshita, K.; Kawamoto, A.; Hibiya, S.; Takenaka, K.; Saito, E.; Nagahori, M.; Ohtsuka, K.; Watanabe, M.; et al. Intravenous tacrolimus is a superior induction therapy for acute severe ulcerative colitis compared to oral tacrolimus. BMC Gastroenterol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Rehman, M.; Cancarevic, I.; Iskander, B.; Lalani, S.; Malik, B.H. Biologics Targeting in the Treatment of Inflammatory Bowel Disease: A Conundrum. Cureus 2020, 12, e10621. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Billmeier, U.; Dieterich, W.; Neurath, M.F.; Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 9300–9313. [Google Scholar] [CrossRef]
- Avedillo-Salas, A.; Corral-Cativiela, S.; Fanlo-Villacampa, A.; Vicente-Romero, J. The Efficacy and Safety of Biologic Drugs in the Treatment of Moderate-Severe Crohn’s Disease: A Systematic Review. Pharmaceuticals 2023, 16, 1581. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Begue, B.; Wajant, H.; Bambou, J.C.; Dubuquoy, L.; Siegmund, D.; Beaulieu, J.F.; Canioni, D.; Berrebi, D.; Brousse, N.; Desreumaux, P.; et al. Implication of TNF-related apoptosis-inducing ligand in inflammatory intestinal epithelial lesions. Gastroenterology 2006, 130, 1962–1974. [Google Scholar] [CrossRef]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, A. Infliximab approved for use in Crohn’s disease: A report on the FDA GI Advisory Committee conference. Inflamm. Bowel Dis. 1998, 4, 328–329. [Google Scholar] [CrossRef]
- de Vries, H.S.; van Oijen, M.G.; Driessen, R.J.; de Jong, E.M.; Creemers, M.C.; Kievit, W.; de Jong, D.J. Appropriate infliximab infusion dosage and monitoring: Results of a panel meeting of rheumatologists, dermatologists and gastroenterologists. Br. J. Clin. Pharmacol. 2011, 71, 7–19. [Google Scholar] [CrossRef] [PubMed]
- ten Hove, T.; van Montfrans, C.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 2002, 50, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Van den Brande, J.M.; Braat, H.; van den Brink, G.R.; Versteeg, H.H.; Bauer, C.A.; Hoedemaeker, I.; van Montfrans, C.; Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 2003, 124, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic. Dis. 2019, 10, 2040622319838443. [Google Scholar] [CrossRef]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’ Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Rosenstein, S.; Yavzori, M.; Dror, Y.; Fudim, E.; Ungar, B.; Kopylov, U.; Picard, O.; Kigel, A.; Ben-Horin, S.; et al. Molecular Landscape of Anti-Drug Antibodies Reveals the Mechanism of the Immune Response Following Treatment with TNFα Antagonists. Front. Immunol. 2019, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Brembilla, N.C. Immunogenicity of biologic therapies: Causes and consequences. Expert. Rev. Clin. Immunol. 2018, 14, 513–523. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Zymfentra (infliximab-dyyb) Subcutaneous Formulation for the Treatment of People with Inflammatory Bowel Disease. Available online: https://www.drugs.com/newdrugs/fda-approves-zymfentra-infliximab-dyyb-subcutaneous-formulation-inflammatory-bowel-6122.html (accessed on 1 December 2023).
- Humira (Adalimumab): Prescribing Information. 2012, pp. 1–73. Available online: http://www.rxabbvie.com/pdf/humira.pdf (accessed on 3 December 2023).
- European Medicines Agency (EMA). Humira Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (accessed on 5 December 2023).
- Yin, J.; Li, Y.; Chen, Y.; Wang, C.; Song, X. Adalimumab for induction of remission in patients with Crohn’s disease: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 190. [Google Scholar] [CrossRef]
- Cunningham, G.; Samaan, M.A.; Irving, P.M. Golimumab in the treatment of ulcerative colitis. Therap. Adv. Gastroenterol. 2019, 12, 1756284818821266. [Google Scholar] [CrossRef]
- Kay, J.; Matteson, E.L.; Dasgupta, B.; Nash, P.; Durez, P.; Hall, S.; Hsia, E.C.; Han, J.; Wagner, C.; Xu, Z.; et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: A randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008, 58, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Cimzia Prescribing Information. Available online: https://www.cimzia.com/themes/custom/cimzia/docs/CIMZIA_full_prescribing_information.pdf (accessed on 5 December 2023).
- Lang, L. FDA approves Cimzia to treat Crohn’s disease. Gastroenterology 2008, 134, 1819. [Google Scholar] [CrossRef]
- Schreiber, S. Certolizumab pegol for the treatment of Crohn’s disease. Therap. Adv. Gastroenterol. 2011, 4, 375–389. [Google Scholar] [CrossRef]
- Okabayashi, S.; Yamazaki, H.; Yamamoto, R.; Anan, K.; Matsuoka, K.; Kobayashi, T.; Shinzaki, S.; Honzawa, Y.; Kataoka, Y.; Tsujimoto, Y.; et al. Certolizumab pegol for maintenance of medically induced remission in Crohn’s disease. Cochrane Database Syst. Rev. 2022, 6, CD013747. [Google Scholar] [CrossRef]
- Shimaoka, M.; Takagi, J.; Springer, T.A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 485–516. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; O’Byrne, S.; Keir, M.E.; Butcher, E.C. Gut-Selective Integrin-Targeted Therapies for Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, S653–S668. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Allez, M.; Danese, S.; Keir, M.; Tole, S.; McBride, J. The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti-integrin therapies. Med. Res. Rev. 2020, 40, 245–262. [Google Scholar] [CrossRef]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.S.; Wang, J.; Buckman, C.A.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef]
- Nelson, S.M.; Nguyen, T.M.; McDonald, J.W.; MacDonald, J.K. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 8, CD006097. [Google Scholar] [CrossRef]
- Van Assche, G.; Van Ranst, M.; Sciot, R.; Dubois, B.; Vermeire, S.; Noman, M.; Verbeeck, J.; Geboes, K.; Robberecht, W.; Rutgeerts, P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 2005, 353, 362–368. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Drug Safety Communication: New Risk Factor for Progressive Multifocal Leukoencephalopathy (PML) Associated with Tysabri (Natalizumab). Available online: http://www.fda.gov/Drugs/DrugSafety/ucm288186.htm (accessed on 18 May 2024).
- Tamilarasan, A.G.; Cunningham, G.; Irving, P.M.; Samaan, M.A. Recent advances in monoclonal antibody therapy in IBD: Practical issues. Frontline Gastroenterol. 2019, 10, 409–416. [Google Scholar] [CrossRef]
- U.S. FDA Approves Subcutaneous Administration of Takeda’s Entyvio (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Ulcerative Colitis. Entyvio (Vedolizumab) FDA Approval History-Drugs.com. Available online: https://www.drugs.com/newdrugs/u-s-fda-approves-subcutaneous-administration-takeda-s-entyvio-vedolizumab-maintenance-therapy-6099.html (accessed on 6 December 2023).
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Hart, A.; Bossuyt, P.; Long, M.; Allez, M.; Juillerat, P.; Armuzzi, A.; Loftus, E.V., Jr.; Ostad-Saffari, E.; Scalori, A.; et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): A phase 3, randomised, controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 128–140. [Google Scholar] [CrossRef]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor. Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Parigi, T.L.; Iacucci, M.; Ghosh, S. Blockade of IL-23: What is in the Pipeline? J. Crohn’s Colitis 2022, 16, ii64–ii72. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Stelara. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/stelara (accessed on 4 December 2023).
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Gasink, C.; Gao, L.L.; Blank, M.A.; Johanns, J.; Guzzo, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Rutgeerts, P.; et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 2012, 367, 1519–1528. [Google Scholar] [CrossRef]
- Sands, B.E.; D’Haens, G.; Clemow, D.B.; Irving, P.M.; Johns, J.T.; Hunter Gibble, T.; Abreu, M.T.; Lee, S.; Hisamatsu, T.; Kobayashi, T.; et al. Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment for Ulcerative Colitis: Results from the LUCENT-3 Open-Label Extension Study. Inflamm. Bowel Dis. 2024, 30, 1044–1045. [Google Scholar] [CrossRef]
- European Medicines Agency. Omvoh. Available online: https://www.ema.europa.eu/en/documents/product-information/omvoh-epar-product-information_en.pdf (accessed on 24 July 2024).
- Panaccione, R.; Sandborn, W.J.; Gordon, G.L.; Lee, S.D.; Safdi, A.; Sedghi, S.; Feagan, B.G.; Hanauer, S.; Reinisch, W.; Valentine, J.F.; et al. Briakinumab for treatment of Crohn’s disease: Results of a randomized trial. Inflamm. Bowel Dis. 2015, 21, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Danese, S.; Sandborn, W.J.; Schreiber, S.; Hanauer, S.; D’Haens, G.; Nagy, P.; Thakur, M.; Bliss, C.; Cataldi, F.; et al. Efficacy and Safety of the Anti-mucosal Addressin Cell Adhesion Molecule-1 Antibody Ontamalimab in Patients with Moderate-to-Severe Ulcerative Colitis or Crohn’s Disease. J. Crohn’s Colitis 2024, 18, 708–719. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Skyrizi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi#product-info (accessed on 24 July 2024).
- Danese, S.; Beaton, A.; Duncan, E.A.; Mercier, A.K.; Neisen, J.; Seth, H.; Zetterstrand, S.; Sands, B.E. Long-term safety of brazikumab in the open-label period of a randomized phase 2a study of patients with Crohn’s disease. BMC Gastroenterol. 2023, 23, 451. [Google Scholar] [CrossRef]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results from the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef]
- Dimmito, M.P.; Marinelli, L.; Cacciatore, I.; Valeri, A.L.; Rapino, A.; Di Stefano, A. Self-assembling Peptides (SAPs) as Powerful Tools for the Preparation of Antimicrobial and Wound-Healing Nanostructures. Lett. Drug Des. Discov. 2024, 21, 2232–2247. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Peretz, A.; Saliba, W. Role of antibiotics for treatment of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Docherty, M.J.; Jones, R.C.; Wallace, M.S. Managing pain in inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 592–601. [Google Scholar] [PubMed]
- Gao, J.; Li, J.; Luo, Z.; Wang, H.; Ma, Z. Nanoparticle-Based Drug Delivery Systems for Inflammatory Bowel Disease Treatment. Drug Des. Devel. Ther. 2024, 18, 2921–2949. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef] [PubMed]
- Ben Khalifa, R.; Cacciatore, I.; Dimmito, M.P.; Ciulla, M.; Grande, R.; Puca, V.; Robuffo, I.; De Laurenzi, V.; Chekir-Ghedira, L.; Di Stefano, A.; et al. Multiple lipid nanoparticles as antimicrobial drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 10288. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.M. Budesonide loaded nanoparticles with pHsensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control. Release 2014, 183, 167–177. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Niebel, W.; Walkenbach, K.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J. Control. Release 2012, 160, 659–665. [Google Scholar] [CrossRef]
- Jubeh, T.T.; Barenholz, Y.; Rubinstein, A. Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharm. Res. 2004, 21, 447–453. [Google Scholar] [CrossRef]
- Giron, F.; Pastó, A.; Tasciotti, E.; Abraham, B. Nanotechnology in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Moayedi, S.; Mohajeri, S.; Yadegar, A.; Haririan, I. Targeting pathophysiological changes using biomaterials-based drug delivery systems: A key to managing inflammatory bowel disease. Front. Pharmacol. 2022, 13, 1045575. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef]
- Gao, C.; Yu, S.; Zhang, X.; Dang, Y.; Han, D.D.; Liu, X.; Han, J.; Hui, M. Dual Functional Eudragit® S100/L30D-55 and PLGA Colon-Targeted Nanoparticles of Iridoid Glycoside for Improved Treatment of Induced Ulcerative Colitis. Int. J. Nanomed. 2021, 16, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Turanlı, Y.; Acartürk, F. Preparation and characterization of colon-targeted pH/Time-dependent nanoparticles using anionic and cationic polymethacrylate polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, A.; Marinelli, L.; Di Stefano, A.; Vicaretti, G.; Cacciatore, I. Aptamers-based Strategies for the Treatment of Microbial Infections. Lett. Drug Des. Discov. 2024, 21, 858–865. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Wang, L.; Pan, D.; Xu, Y.; Wang, F.; Sheng, J.; Li, X.; Yang, M. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics 2020, 10, 10808–10822. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.H.; Park, H.J.; Ma, H.W.; Park, I.S.; Son, M.; Ro, S.Y.; Hong, S.; Han, H.K.; Lim, S.J.; et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J. Nanobiotechnology 2020, 18, 133. [Google Scholar] [CrossRef]
- Li, X.; Fang, S.; Yu, Y.; Yang, H.; Rao, Y.; Hong, D.; Lu, C.; Yu, M.; Lu, X.; Yu, C.; et al. Oral administration of inflammatory microenvironment-responsive carrier-free infliximab nanocomplex for the targeted treatment of inflammatory bowel disease. Chem. Eng. J. 2022, 445, 136438. [Google Scholar] [CrossRef]
- Ries, M.; Moulari, B.; Shetab Boushehri, M.A.; Ali, M.E.; Molnar, D.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics 2022, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Xu, Y.; Prévost, J.R.C.; McCartney, F.; Brayden, D.; Frédérick, R.; Beloqui, A.; Préat, V. Impact of PEGylation on an antibody-loaded nanoparticle-based drug delivery system for the treatment of inflammatory bowel disease. Acta Biomater. 2022, 140, 561–572. [Google Scholar] [CrossRef] [PubMed]

| Traditional Drugs | Class of Drugs | Administration Route | Mechanism of Action | Refs. |
|---|---|---|---|---|
| Sulfasalazine | Aminosalicylates | Oral | The inhibition of IL-1, TNF-α production, NF-kB, and lipoxygenase pathways | [21,22,23,24,25,26] |
| Prednisone | Corticosteroids first generation | Oral or parenteral | The inhibition of the immune system | [29] |
| Beclomethasone dipropionate | Corticosteroids second generation | Oral or topical | The inhibition of the immune system | [30,31] |
| Azathioprine | Thiopurines | Oral | The inhibition of T lymphocyte proliferation and activation | [32] |
| Methotrexate | Antimetabolites | Parenteral | Decreasing pro-inflammatory cytokine production and leading to the apoptosis of T cells | [33,34] |
| Cyclosporine | Immunosuppressives | Oral or intravenous | Calcineurin inhibition | [35] |
| Tacrolimus | Immunosuppressives | Oral or intravenous | Calcineurin inhibition | [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rienzo, A.; Marinelli, L.; Dimmito, M.P.; Toto, E.C.; Di Stefano, A.; Cacciatore, I. Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics 2024, 16, 1185. https://doi.org/10.3390/pharmaceutics16091185
Di Rienzo A, Marinelli L, Dimmito MP, Toto EC, Di Stefano A, Cacciatore I. Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics. 2024; 16(9):1185. https://doi.org/10.3390/pharmaceutics16091185
Chicago/Turabian StyleDi Rienzo, Annalisa, Lisa Marinelli, Marilisa Pia Dimmito, Eleonora Chiara Toto, Antonio Di Stefano, and Ivana Cacciatore. 2024. "Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems" Pharmaceutics 16, no. 9: 1185. https://doi.org/10.3390/pharmaceutics16091185
APA StyleDi Rienzo, A., Marinelli, L., Dimmito, M. P., Toto, E. C., Di Stefano, A., & Cacciatore, I. (2024). Advancements in Inflammatory Bowel Disease Management: From Traditional Treatments to Monoclonal Antibodies and Future Drug Delivery Systems. Pharmaceutics, 16(9), 1185. https://doi.org/10.3390/pharmaceutics16091185








