Lipid-Based Gels for Delivery of 3-O-Ethyl L-Ascorbic acid in Topical Applications
Abstract
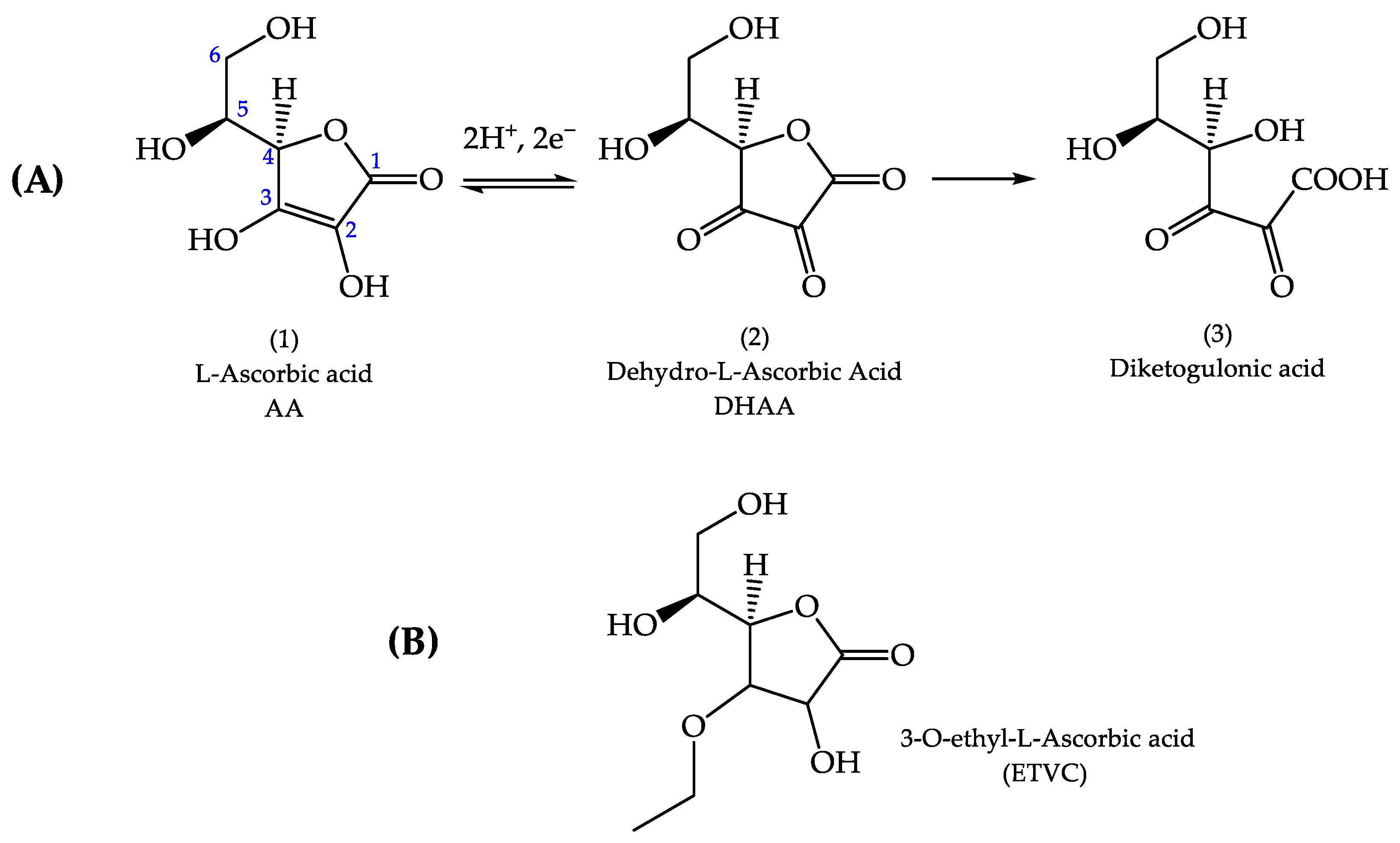
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Preparation
2.2.1. Hydrogel
2.2.2. Bigel
2.3. In Vitro Release Study
2.4. In Vitro Skin Permeation and Kinetics Study
2.5. Skin Surface Lipid Structure
2.6. In Vitro Skin Antioxidant Efficacy
2.7. Statistical Analysis
3. Results and Discussion
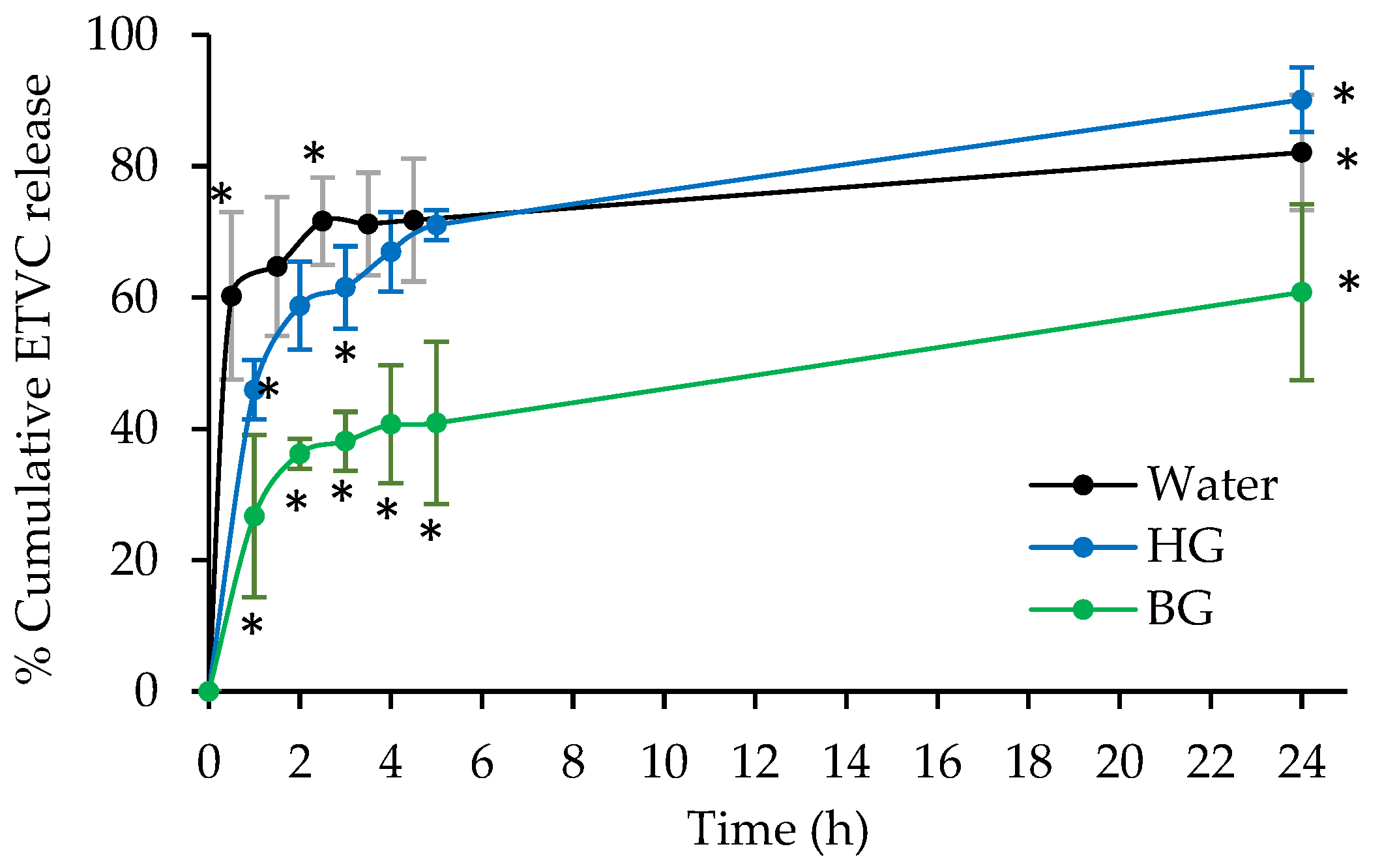
3.1. In Vitro Release Studies
3.2. In Vitro Skin Permeation Studies
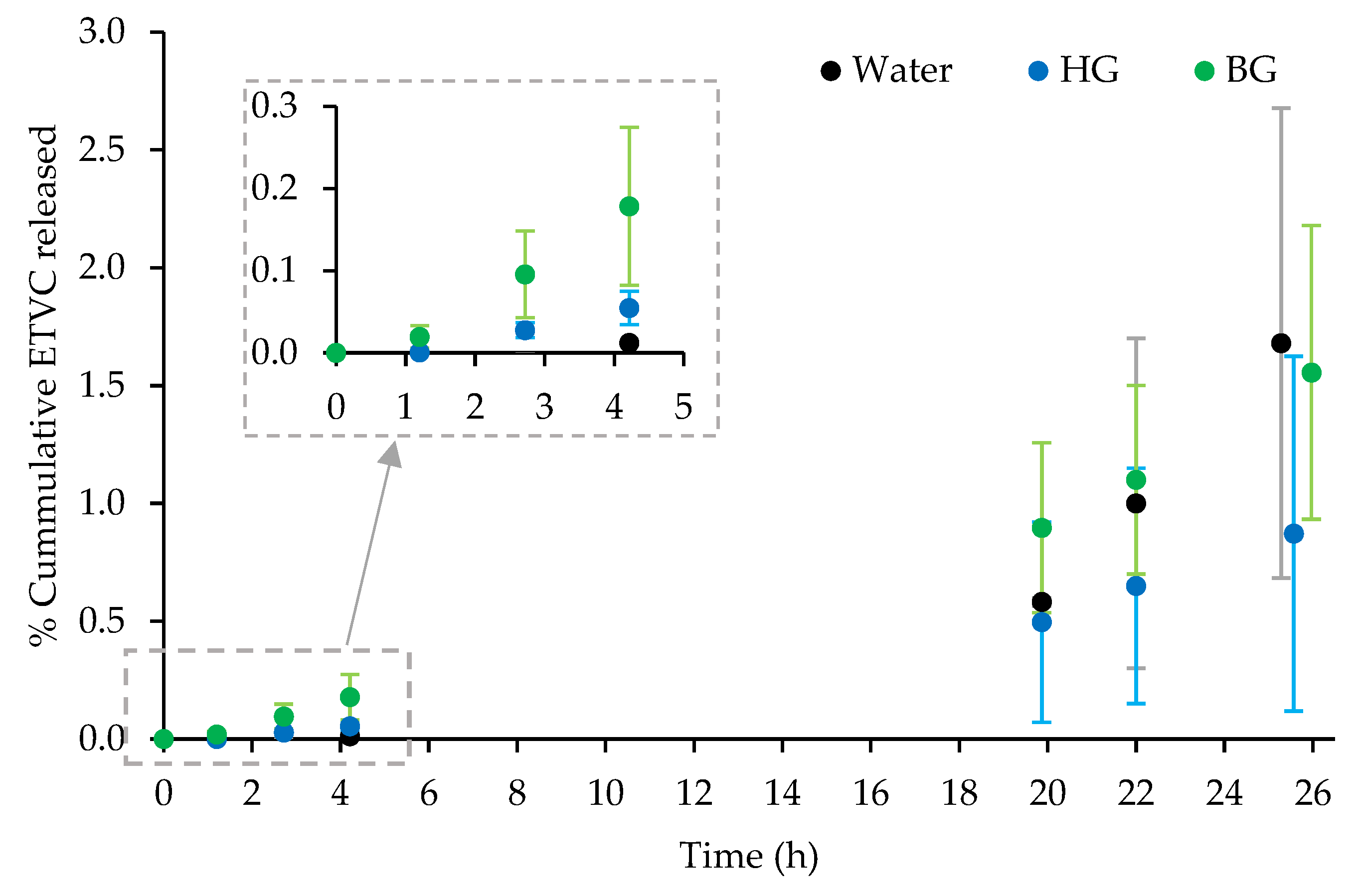
3.2.1. Skin Kinetics
3.2.2. Permeation into and through the Skin
3.2.3. ETVC Distribution into Skin Layers
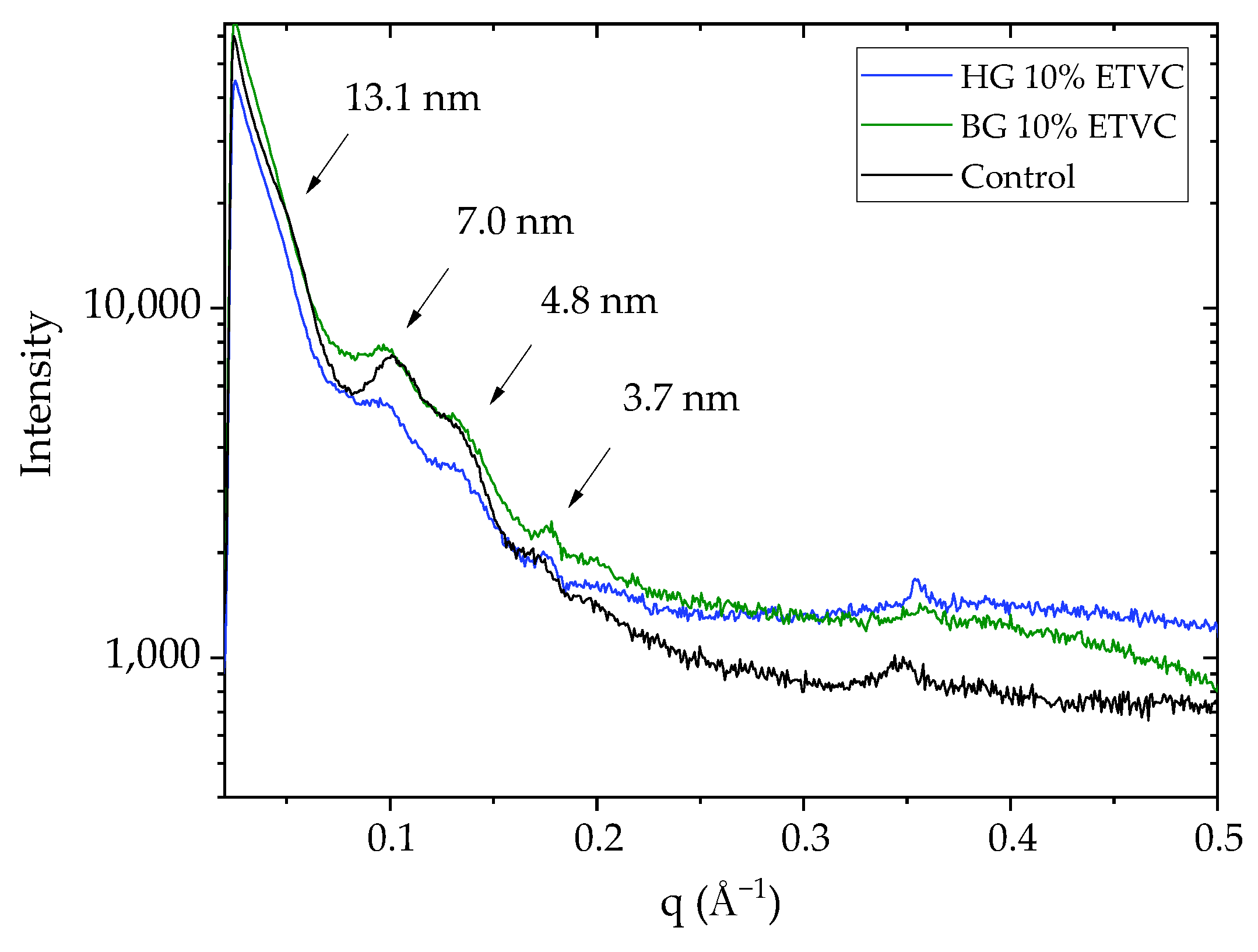
3.3. Skin Surface Lipid Structure
3.4. In Vitro Skin Antioxidant Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. In Vitro Release Studies

| Water Solution 10% ETVC | HG 10% ETVC | BG 10% ETVC | ||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Time (h) | Mean (% ETVC) | SD | Extraction Time (h) | Mean (% ETVC) | SD | Extraction Time (h) | Mean (% ETVC) | SD |
| 0.0 | 0.00 | 0.00 | 0.0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 |
| 0.5 | 60.24 | 12.78 | 1.0 | 45.94 | 4.53 | 1 | 26.72 | 12.37 |
| 1.5 | 64.73 | 10.60 | 2.0 | 58.75 | 6.70 | 2 | 36.20 | 2.27 |
| 2.5 | 71.62 | 6.65 | 3.0 | 61.55 | 6.26 | 3 | 38.10 | 4.48 |
| 3.5 | 71.22 | 7.80 | 4.0 | 66.96 | 6.07 | 4 | 40.70 | 8.98 |
| 4.5 | 71.79 | 9.36 | 5.0 | 71.03 | 2.27 | 5 | 40.89 | 12.36 |
| 24.0 | 82.10 | 8.81 | 24.0 | 90.14 | 4.91 | 24 | 60.80 | 13.42 |
| Time Point | Material Interaction | Q Value | Individual p Value | Significant Differences |
|---|---|---|---|---|
| 1 h | HG–water | 0.0228 | 0.0653 | Yes |
| BG–water | 0.0042 | 0.0040 | Yes | |
| BG–HG | 0.081 | 0.0155 | Yes | |
| 2 h | HG–water | 0.1038 | 0.2966 | No |
| BG–water | 0.0023 | 0.0044 | Yes | |
| BG–HG | 0.0017 | 0.0016 | Yes | |
| 3 h | HG–water | 0.0374 | 0.0356 | Yes |
| BG–water | 0.0007 | 0.0002 | Yes | |
| BG–HG | 0.0051 | 0.0032 | Yes | |
| 4 h | HG–water | 0.1168 | 0.3338 | No |
| BG–water | 0.0012 | 0.0011 | Yes | |
| BG–HG | 0.0012 | 0.0023 | Yes | |
| 5 h | HG–water | 0.3003 | 0.8579 | No |
| BG–water | 0.0007 | 0.0014 | Yes | |
| BG–HG | 0.0007 | 0.0010 | Yes | |
| 24 h | HG–water | 0.0392 | 0.1121 | Yes |
| BG–water | 0.0048 | 0.0091 | Yes | |
| BG–HG | 0.0005 | 0.0005 | Yes |
| Parameter | Material Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| Bmax | HG–water | 3.04 | p > 0.05 | No |
| BG–water | 5.53 | p < 0.01 | Yes | |
| BG–HG | 8.57 | p < 0.001 | Yes | |
| Kd | HG–water | 3 | p > 0.05 | No |
| BG–water | 5 | p < 0.05 | Yes | |
| BG–HG | 2 | p > 0.05 | No |
Appendix B. In Vitro Skin Kinetic Studies
| Water Solution 10% ETVC | HG 10% ETVC | BG 10% ETVC | ||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Time (h) | Mean % ETVC | SD | Extraction Time (h) | Mean % ETVC | SD | Extraction Time (h) | Mean % ETVC | SD |
| 0.0 | 0.00 | 0.00 | 0.0 | 0 | 0 | 0.0 | 0 | 0 |
| 1.2 | 0.00 | 0.00 | 1.2 | 0.00 | 0.00 | 1.2 | 0.02 | 0.01 |
| 2.7 | 0.00 | 0.00 | 2.7 | 0.03 | 0.01 | 2.7 | 0.10 | 0.05 |
| 4.2 | 0.01 | 0.00 | 4.2 | 0.05 | 0.02 | 4.2 | 0.18 | 0.10 |
| 19.9 | 0.58 | 0.02 | 19.9 | 0.50 | 0.43 | 19.9 | 0.90 | 0.36 |
| 22.0 | 1.00 | 0.70 | 22.0 | 0.65 | 0.50 | 22 | 1.10 | 0.40 |
| 25.3 | 1.68 | 1.00 | 25.6 | 0.87 | 0.75 | 26.0 | 1.56 | 0.62 |
| Time Point | Material Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| 1 h | HG–water | 0.1611 | 0.1534 | No |
| BG–water | 0.0264 | 0.0126 | Yes | |
| BG–HG | 0.5708 | 0.8154 | No | |
| 3h | HG–water | 0.0836 | 0.0796 | No |
| BG–water | 0.0092 | 0.0044 | Yes | |
| BG–HG | 0.6687 | 0.9552 | No | |
| 4 h | HG–water | 0.1012 | 0.0964 | No |
| BG–water | 0.0109 | 0.0052 | Yes | |
| BG–HG | 0.6011 | 0.8586 | No | |
| 20 h | HG–water | 0.3470 | 0.2203 | No |
| BG–water | 0.1998 | 0.0634 | No | |
| BG–HG | 0.4612 | 0.4392 | No | |
| 22 h | HG–water | 0.5619 | 0.3568 | No |
| BG–water | 0.7497 | 0.7140 | No | |
| BG–HG | 0.1550 | 0.0492 | No | |
| 24 h | HG–water | 0.7702 | 0.4890 | No |
| BG–water | 0.8388 | 0.7968 | No | |
| BG–HG | 0.7702 | 0.4132 | No |
| Kinetic Parameter | Material Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| J (µg/cm2·h) | HG–water | 0.0808 | 0.0513 | No |
| BG–water | 0.9017 | 0.8588 | No | |
| BG–HG | 0.0653 | 0.0207 | No | |
| Tl (h) | HG–water | 0.6215 | 0.3277 | No |
| BG–water | 0.6215 | 0.5919 | No | |
| BG–HG | 0.6215 | 0.5919 | No | |
| Kp (cm/h) | HG–water | 0.0298 | 0.0142 | Yes |
| BG–water | 0.3316 | 0.4738 | No | |
| BG–HG | 0.0513 | 0.0488 | No | |
| P1 (cm) | HG–water | 0.0300 | 0.0143 | Yes |
| BG–water | 0.3320 | 0.4743 | No | |
| BG–HG | 0.0516 | 0.0491 | No | |
| P2 (h−1) | HG–water | 0.7415 | 0.4589 | No |
| BG–water | 0.7415 | 0.4708 | No | |
| BG–HG | 0.9746 | 0.9282 | No |
Appendix C. In Vitro Permeation Studies
| Material | Total Skin Permeation | Receptor Fluid |
|---|---|---|
| Water solution 10% ETVC | 43.88 ± 14.80 | 56.12 ± 14.80 |
| HG 10% ETVC | 80.99 ± 19.30 | 11.24 ± 3.57 |
| BG 10% ETVC | 77.78 ± 11.42 | 22.22 ± 11.42 |
| One-Way ANOVA | Material Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| RF | HG–water | 0.0019 | 0.0019 | Yes |
| BG–water | 0.0019 | 0.0035 | Yes | |
| BG–HG | 0.0233 | 0.0665 | Yes | |
| Total skin permeation | HG–water | 0.0030 | 0.0057 | Yes |
| BG–water | 0.0030 | 0.0035 | Yes | |
| BG–HG | 0.2572 | 0.7348 | No |
| Material | Stratum Corneum | Epidermis | Dermis | Receptor Fluid |
|---|---|---|---|---|
| Water solution 10% ETVC | 15.75 ± 7.07 | 9.75 ± 5.20 | 18.37 ± 5.96 | 56.12 ± 14.80 |
| HG 10% ETVC | 30.60 ± 10.42 | 27.91 ± 11.26 | 22.48 ± 7.62 | 11.24 ± 3.57 |
| BG 10% ETVC | 15.44 ± 8.47 | 35.59 ± 11.18 | 26.74 ± 7.00 | 22.22 ± 11.42 |
| Material | Skin Layer Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| Water solution 10% ETVC | SC–epidermis | 0.1061 | 0.1684 | No |
| SC–dermis | 0.2859 | 0.5445 | No | |
| SC–RF | 0.0028 | 0.0018 | Yes | |
| Epidermis–dermis | 0.0325 | 0.0413 | Yes | |
| Epidermis–RF | 0.0028 | 0.0012 | Yes | |
| Dermis–RF | 0.0029 | 0.0028 | Yes | |
| HG 10% ETVC | SC–epidermis | 0.3549 | 0.6760 | No |
| SC–dermis | 0.1237 | 0.1571 | No | |
| SC–RF | 0.0145 | 0.0047 | Yes | |
| Epidermis–dermis | 0.2232 | 0.3543 | No | |
| Epidermis–RF | 0.0145 | 0.0135 | Yes | |
| Dermis–RF | 0.0145 | 0.0138 | Yes | |
| BG 10% ETVC | SC–epidermis | 0.0325 | 0.0062 | Yes |
| SC–dermis | 0.0820 | 0.0312 | No | |
| SC–RF | 0.2860 | 0.2724 | No | |
| Epidermis–dermis | 0.1801 | 0.1372 | No | |
| Epidermis–RF | 0.1185 | 0.0677 | No | |
| Dermis–RF | 0.3777 | 0.4317 | No |
| Time Point | Material Interaction | Q Value | Individual p Value | Significant Difference |
|---|---|---|---|---|
| SC | HG–water | 0.0112 | 0.0213 | Yes |
| BG–water | 0.3320 | 0.9486 | No | |
| BG–HG | 0.0112 | 0.0207 | Yes | |
| Epidermis | HG–water | 0.0047 | 0.0090 | Yes |
| BG–water | 0.0014 | 0.0013 | Yes | |
| BG–HG | 0.0921 | 0.2631 | No | |
| Dermis | HG–water | 0.3602 | 0.3431 | No |
| BG–water | 0.1922 | 0.0610 | No | |
| BG–HG | 0.3602 | 0.3368 | No | |
| RF | HG–water | 0.0019 | 0.0018 | Yes |
| BG–water | 0.0019 | 0.0035 | Yes | |
| BG–HG | 0.0233 | 0.0665 | Yes |
Appendix D. GISAXS Profiles with the HG and BG without ETVC

References
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical Ascorbic Acid on Photoaged Skin. Clinical, Topographical and Ultrastructural Evaluation: Double-Blind Study vs. Placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapière, C.M. Topically Applied Vitamin C Enhances the mRNA Level of Collagens I and III, Their Processing Enzymes and Tissue Inhibitor of Matrix Metalloproteinase 1 in the Human Dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Colven, R.M.; Pinnell, S.R. Topical Vitamin C in Aging. Clin. Dermatol. 1996, 14, 227–234. [Google Scholar] [CrossRef]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of Vitamin C and Its Derivatives on Collagen Synthesis and Cross-Linking by Normal Human Fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and Photoaging-Dependent Changes of Enzymic and Nonenzymic Antioxidants in the Epidermis and Dermis of Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. UVR-Induced Oxidative Stress in Human Skin in Vivo: Effects of Oral Vitamin C Supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and Non-Enzymic Antioxidants in Epidermis and Dermis of Human Skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Packer, L. Dose-Response Effects of Acute Ultraviolet Irradiation on Antioxidants and Molecular Markers of Oxidation in Murine Epidermis and Dermis. J. Investig. Dermatol. 1994, 102, 470–475. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant Defense Mechanisms in Murine Epidermis and Dermis and Their Responses to Ultraviolet Light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.U.; Thiele, J.J.; Packer, L.; Cross, C.E. Vitamin C, Uric Acid, and Glutathione Gradients in Murine Stratum Corneum and Their Susceptibility to Ozone Exposure. J. Investig. Dermatol. 1999, 113, 1128–1132. [Google Scholar] [CrossRef]
- Vasques, L.I.; Vendruscolo, C.W.; Leonardi, G.R. Topical Application of Ascorbic Acid and Its Derivatives: A Review Considering Clinical Trials. Curr. Med. Chem. 2022, 30, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- Telang, P. Vitamin C in Dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef]
- Stamford, N.P.J. Stability, Transdermal Penetration, and Cutaneous Effects of Ascorbic Acid and Its Derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-Ethyl-l-Ascorbic Acid: Characterisation and Investigation of Single Solvent Systems for Delivery to the Skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Riviere, N.M.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-Ascorbic Acid: Percutaneous Absorption Studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the Stability of Ascorbic Acid in Emulsified Systems for Topical and Cosmetic Use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Mosca, M.; Ceglie, A.; Ambrosone, L. Biocompatible Water-in-Oil Emulsion as a Model to Study Ascorbic Acid Effect on Lipid Oxidation. J. Phys. Chem. B 2008, 112, 4635–4641. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A.; Ahmad, Z.; Javed, M.S.; Sharif, H.R.; Shah, F.-H.; Imran, M.; Abdelgawad, M.A.; Murtaza, S. Physicochemical Characteristics of Mixed Surfactant-Stabilized l-Ascorbic Acid Nanoemulsions during Storage. Langmuir 2022, 38, 9500–9506. [Google Scholar] [CrossRef]
- Serrano, G.; Almudéver, P.; Serrano, J.-M.; Milara, J.; Torrens, A.; Expósito, I.; Cortijo, J. Phosphatidylcholine Liposomes as Carriers to Improve Topical Ascorbic Acid Treatment of Skin Disorders. Clin. Cosmet. Investig. Dermatol. 2015, 8, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Lee, S.; Han, Y.-S.; Park, K.-C.; Choy, J.-H. Efficient Transdermal Penetration and Improved Stability of L-Ascorbic Acid Encapsulated in an Inorganic Nanocapsule. Bull. Korean Chem. Soc. 2003, 24, 499–503. [Google Scholar] [CrossRef]
- Xu, T.-H.; Chen, J.Z.S.; Li, Y.-H.; Wu, Y.; Luo, Y.-J.; Gao, X.-H.; Chen, H.-D. Split-Face Study of Topical 23.8% L-Ascorbic Acid Serum in Treating Photo-Aged Skin. J. Drugs. Dermatol. 2012, 11, 51–56. [Google Scholar] [PubMed]
- Mottola, M.; Vico, R.V.; Villanueva, M.E.; Fanani, M.L. Alkyl Esters of L-Ascorbic Acid: Stability, Surface Behaviour and Interaction with Phospholipid Monolayers. J. Colloid. Interface Sci. 2015, 457, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hibino, M.; Murata, I.; Kanamoto, I. A Nanocarrier Skin-Targeted Drug Delivery System Using an Ascorbic Acid Derivative. Pharm. Res. 2017, 35, 1. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Hossain, A.S.M.M.A.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of 3-O-Ethyl l-Ascorbic Acid from Complex Solvent Systems. Sci. Pharm. 2020, 88, 19. [Google Scholar] [CrossRef]
- Golonka, I.; Kizior, B.; Szyja, B.M.; Damek, M.P.; Musiał, W. Assessment of the Influence of the Selected Range of Visible Light Radiation on the Durability of the Gel with Ascorbic Acid and Its Derivative. Int. J. Mol. Sci. 2022, 23, 8759. [Google Scholar] [CrossRef]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-Ethyl-l-Ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef]
- Talló, K.; López, O.; Moner, V. Gel Lipídico Nanoestructurado, Procedimiento de Preparación y Uso. WO 2020/079302 A2, 17 April 2020. [Google Scholar]
- Talló, K.; Pons, R.; González, C.; López, O. Monitoring the Formation of a Colloidal Lipid Gel at the Nanoscale: Vesicle Aggregation Driven by a Temperature-Induced Mechanism. J. Mater. Chem. B 2021, 9, 7472–7481. [Google Scholar] [CrossRef]
- Talló, K.; Pons, R.; López, O.; Bosch, M.; Bosch, M.; Cocera, M. Preparation and Characterization of a Supramolecular Hydrogel Made of Phospholipids and Oleic Acid with a High Water Content. J. Mat. Chem. B 2020, 8, 161–167. [Google Scholar] [CrossRef]
- Talló, K.; Vílchez, S.; Pons, R.; López, O. Gels Formed from the Interaction of Lipid Vesicles: Influence of Charge in Their Structural and Rheological Properties. J. Mol. Liq. 2021, 322, 114957. [Google Scholar] [CrossRef]
- Loza-Rodríguez, N.; Millán-Sánchez, A.; López, O. A Biocompatible Lipid-Based Bigel for Topical Applications. Eur. J. Pharm. Biopharm. 2023, 190, 24–34. [Google Scholar] [CrossRef]
- Loza-Rodríguez, N.; Millán-Sánchez, A.; López, O. Characteristics of a Lipid Hydrogel and Bigel as Matrices for Ascorbic Acid Stabilization. Gels 2023, 9, 649. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A Unique Class of Materials for Drug Delivery Applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Starr, N.J.; Abdul Hamid, K.; Wibawa, J.; Marlow, I.; Bell, M.; Pérez-García, L.; Barrett, D.A.; Scurr, D.J. Enhanced Vitamin C Skin Permeation from Supramolecular Hydrogels, Illustrated Using in Situ ToF-SIMS 3D Chemical Profiling. Int. J. Pharm. 2019, 563, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; de Cindio, B. Olive Oil and Hyperthermal Water Bigels for Cosmetic Uses. J. Colloid. Interface Sci. 2015, 459, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as Drug Delivery Systems: From Their Components to Their Applications. Drug Discov. Today 2022, 27, 1008–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and Characterization of Novel Bigels Based on Monoglyceride-Beeswax Oleogel and High Acyl Gellan Gum Hydrogel for Lycopene Delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Singh, V.K.; Qureshi, D.; Nayak, S.K.; Pal, K. Bigels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 265–282. [Google Scholar]
- Singhai, M.; Bhattacharya, S. Bigels; A Charismatic Drug Delivery Formulation. Drug Metab. Bioanal. Lett. 2023, 16, 27–49. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef]
- Vergara, D.; Loza-Rodríguez, N.; Acevedo, F.; Bustamante, M.; López, O. Povidone-Iodine Loaded Bigels: Characterization and Effect as a Hand Antiseptic Agent. J. Drug Deliv. Sci. Technol. 2022, 72, 103427. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Hosseini, E.; Seyed Dorraji, M.S.; Sheikh Mohammadi, S.; Pourmansouri, Z.; Rasoulifard, M.H.; Doosti, M.; Chiti, H. Synthesis of a Green Bigel Using Cottonseed Oil/Cannabis Oil/Alginate/Ferula Gum for Quercetin Release: Synergistic Effects for Treating Infertility in Rats. Int. J. Biol. Macromol. 2021, 177, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zulfakar, M.H.; Chan, L.M.; Rehman, K.; Wai, L.K.; Heard, C.M. Coenzyme Q10-Loaded Fish Oil-Based Bigel System: Probing the Delivery Across Porcine Skin and Possible Interaction with Fish Oil Fatty Acids. AAPS Pharm. Sci. Tech. 2018, 19, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Magalhães, W.V.; da Silva Sufi, B.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; da Silva Lannes, S.C.; Baby, A.R. Vitamin E-Loaded Bigels and Emulsions: Physicochemical Characterization and Potential Biological Application. Colloids Surf. B Biointerfaces 2021, 201, 111651. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Chaparro, M.Y.; Vargas-Riveros, D.; Mora-Huertas, C.E. Hypromellose—Collagen Hydrogels/Sesame Oil Organogel Based Bigels as Controlled Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 75, 103637. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Huang, J.; Xia, N.; Li, T.; Xia, Q. Self-Double-Emulsifying Drug Delivery System Incorporated in Natural Hydrogels: A New Way for Topical Application of Vitamin C. J. Microencapsul. 2018, 35, 90–101. [Google Scholar] [CrossRef]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic Acid Encapsulated into Negatively Charged Liposomes Exhibits Increased Skin Permeation, Retention and Enhances Collagen Synthesis by Fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Formation of Self-Assembled Polyelectrolyte Complex Hydrogel Derived from Salecan and Chitosan for Sustained Release of Vitamin C. Carbohydr. Polym. 2020, 234, 115920. [Google Scholar] [CrossRef]
- Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics 2022, 9, 85. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Chapman, A.; Lane, M.E. A Comparison of the in Vitro Permeation of 3-O-Ethyl-l-Ascorbic Acid in Human Skin and in a Living Skin Equivalent (LabSkinTM). Int. J. Cosmet. Sci. 2021, 43, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Tojo, K. Characterization of Skin Permeation of Vitamin C: Theoretical Analysis of Penetration Profiles and Differential Scanning Calorimetry Study. Chem. Pharm. Bull. 1998, 46, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key Characteristics and Modelling of Bigels Systems: A Review. Mater. Sci. Eng. C 2019, 97, 932–953. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Alonso, C.; Rodríguez, G.; Barbosa-Barros, L.; Coderch, L.; De la Maza, A.; Parra, J.L.; López, O. Bicellar Systems for in Vitro Percutaneous Absorption of Diclofenac. Int. J. Pharm. 2010, 386, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Folle, C.; Marqués, A.M.; Mallandrich, M.; Suñer-Carbó, J.; Halbaut, L.; Sánchez-López, E.; López-Machado, A.L.; Díaz-Garrido, N.; Badia, J.; Baldoma, L.; et al. Colloidal Hydrogel Systems of Thymol-Loaded PLGA Nanoparticles Designed for Acne Treatment. Colloids Surf. B Biointerfaces 2024, 234, 113678. [Google Scholar] [CrossRef]
- Salim, N.; Jose García-Celma, M.; Escribano, E.; Nolla, J.; Llinàs, M.; Basri, M.; Solans, C.; Esquena, J.; Tadros, T.F. Formation of Nanoemulsion Containing Ibuprofen by PIC Method for Topical Delivery. Mater. Today Proc. 2018, 5, S172–S179. [Google Scholar] [CrossRef]
- Escribano, E.; Calpena, A.C.; Queralt, J.; Obach, R.; Doménech, J. Assessment of Diclofenac Permeation with Different Formulations: Anti-Inflammatory Study of a Selected Formula. Eur. J. Pharm. Sci. 2003, 19, 203–210. [Google Scholar] [CrossRef]
- Okamoto, H.; Komatsu, H.; Hashida, M.; Sezaki, H. Effects of β-Cyclodextrin and Di-O-Methyl-β-Cyclodextrin on the Percutaneous Absorption of Butylparaben, Indomethacin and Sulfanilic Acid. Int. J. Pharm. 1986, 30, 35–45. [Google Scholar] [CrossRef]
- Bragg, W.L. The Diffraction of Short Electromagnetic Waves by a Crystal. Proc. Cambridge Philos. Soc. 1929, 17, 43–57. [Google Scholar]
- Fernández-García, E.; Heluani-Gahete, H.; Wellinger, R.E. A New Colorimetric Assay for Antioxidant Capacity and Photostability. Color. Technol. 2016, 132, 195–200. [Google Scholar] [CrossRef]
- Alhelal, H.M.; Mehta, S.; Kadian, V.; Kakkar, V.; Tanwar, H.; Rao, R.; Aldhubiab, B.; Sreeharsha, N.; Shinu, P.; Nair, A.B. Solid Lipid Nanoparticles Embedded Hydrogels as a Promising Carrier for Retarding Irritation of Leflunomide. Gels 2023, 9, 576. [Google Scholar] [CrossRef]
- Khan, N.; Jain, P.; Mohapatra, S.; Hassan, N.; Farooq, U.; Khan, R.; Talegaonkar, S.; Mirza, M.A.; Iqbal, Z. In Vitro, Ex Vivo, and in Vivo Appraisal of Clobetasol Propionate Microparticles Embedded Topical Bigel for Psoriasis Management. J. Disper. Sci. Technol. 2023, 1–9. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Jaglal, Y.; Singh, S.; Devnarain, N.; Mocktar, C.; Govender, T. A Transferosome-Loaded Bigel for Enhanced Transdermal Delivery and Antibacterial Activity of Vancomycin Hydrochloride. Int. J. Pharm. 2021, 607, 120990. [Google Scholar] [CrossRef]
- Ali, S.; Shabbir, M.; Nabeel Shahid, M. The Structure of Skin and Transdermal Drug Delivery System-A Review. Res. J. Pharm. Technol. 2015, 8, 103. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Liu, C.; Liu, W.; Tong, G.; Zheng, H.; Zhou, W. Characterization and Bioavailability of Vitamin C Nanoliposomes Prepared by Film Evaporation-Dynamic High Pressure Microfluidization. J. Disper. Sci. Technol. 2012, 33, 1608–1614. [Google Scholar] [CrossRef]
- Banov, D.; Song, G.; Carvalho, M.; Bassani, A.S.; Valdez, B.C. Evaluation of a Compounding Phospholipid Base for the Percutaneous Absorption of High Molecular Weight Drugs Using the Franz Finite Dose Model. Skin. Res. Technol. 2024, 30, e13610. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, M.; Mönkkönen, J.; Saukkosaari, M.; Valjakka-Koskela, R.; Kiesvaara, J.; Urtti, A. Phospholipids Affect Stratum Corneum Lipid Bilayer Fluidity and Drug Partitioning into the Bilayers. J. Control. Release 1999, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Mohd Amin, M.C.I.; Zulfakar, M.H. Development and Physical Characterization of Polymer-Fish Oil Bigel (Hydrogel/Oleogel) System as a Transdermal Drug Delivery Vehicle. J. Oleo. Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Alaayedi, M.; Ashoor, J.A.; Alghurabi, H. The enhancement effect of olive and almond oils on permeability of nimesulide as transdermal gel. Int. J. Pharm. Res. 2019, 11, 1200–1206. [Google Scholar]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A Minireview of Its Antimicrobial Activity and Its Application in Medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The Structure, Function, and Importance of Ceramides in Skin and Their Use as Therapeutic Agents in Skin-Care Products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Rodríguez, G.; Barbosa-Barros, L.; Rubio, L.; Cocera, M.; Díez, A.; Estelrich, J.; Pons, R.; Caelles, J.; De La Maza, A.; López, O. Conformational Changes in Stratum Corneum Lipids by Effect of Bicellar Systems. Langmuir 2009, 25, 10595–10603. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Cócera, M.C.; Rubio, L.; López-Iglesias, C.; Pons, R.; De La Maza, A.; López, O. A Unique Bicellar Nanosystem Combining Two Effects on Stratum Corneum Lipids. Mol. Pharm. 2012, 9, 482–491. [Google Scholar] [CrossRef] [PubMed]
- De Jager, M.W.; Gooris, G.S.; Ponec, M.; Bouwstra, J.A. Lipid Mixtures Prepared with Well-Defined Synthetic Ceramides Closely Mimic the Unique Stratum Corneum Lipid Phase Behavior. J. Lipid Res. 2005, 46, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Moner del Moral, V. Sistemas Miméticos de los Cuerpos Laminares Epidérmicos para el Tratamiento de la Piel. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2018. [Google Scholar]
- Caussin, J.; Gooris, G.S.; Janssens, M.; Bouwstra, J.A. Lipid Organization in Human and Porcine Stratum Corneum Differs Widely, While Lipid Mixtures with Porcine Ceramides Model Human Stratum Corneum Lipid Organization Very Closely. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1472–1482. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J.; Sánchez-López, E.; Halbaut, L.; Marqués, A.M.; Espina, M.; Badia, J.; Baldoma, L.; et al. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]



| Material | Hydrogel 25% ETVC | Hydrogel 10% ETVC | Bigel 10% ETVC |
|---|---|---|---|
| %w/w | %w/w | %w/w | |
| Water | 70 | 85 | 28 |
| HSPC | 4.8 | 4.8 | 1.9 |
| DOTAP | 0.2 | 0.2 | 0.1 |
| ETVC | 25 | 10 | 10 |
| OO | - | - | 53.4 |
| BW | - | - | 6 |
| α-Tocopherol | - | - | 0.6 |
| Material | R2 | Bmax ± SD (% ETVC) | Kd ± SD (h) |
|---|---|---|---|
| Water solution 10% ETVC | 0.998 | 79.91 ± 4.08 | 0.382 ± 0.15 |
| HG 10% ETVC | 0.997 | 90.35 ± 7.33 | 1.16 ± 0.37 |
| BG 10% ETVC | 0.992 | 60.90 ± 8.44 | 1.636 ± 0.76 |
| Material | R2 | J (µg/cm2·h) | Tl (h) | Kp (cm/h) | P1 (cm) | P2 (h−1) |
|---|---|---|---|---|---|---|
| Water solution 10% ETVC | 0.999 | 10.33 (3.56–17.08) | 14.83 (11.38–18.15) | 1.09 × 10−3 (0.38–1.80)·10−3 | 0.40 × 10−3 (0.20–0.60)·10−3 | 2.47 (1.90–3.03) |
| HG 10% ETVC | 0.999 | 4.61 (1.46–7.77) | 12.65 (12.51–12.72) | 0.46 × 10−3 a (0.14–0.77)·10−3 | 0.22 × 10−3 a (0.07–0.36)·10−3 | 2.11 (2.09–2.12) |
| BG 10% ETVC | 0.999 | 8.45 (3.97–10.43) | 12.73 (11.27–13.56) | 0.78 × 10−3 (0.37–0.96)·10−3 | 0.41 × 10−3 (0.17–0.43)·10−3 | 2.12 (1.88–2.26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loza-Rodríguez, N.; Millán-Sánchez, A.; Mallandrich, M.; Calpena, A.C.; López, O. Lipid-Based Gels for Delivery of 3-O-Ethyl L-Ascorbic acid in Topical Applications. Pharmaceutics 2024, 16, 1187. https://doi.org/10.3390/pharmaceutics16091187
Loza-Rodríguez N, Millán-Sánchez A, Mallandrich M, Calpena AC, López O. Lipid-Based Gels for Delivery of 3-O-Ethyl L-Ascorbic acid in Topical Applications. Pharmaceutics. 2024; 16(9):1187. https://doi.org/10.3390/pharmaceutics16091187
Chicago/Turabian StyleLoza-Rodríguez, Noèlia, Aina Millán-Sánchez, Mireia Mallandrich, Ana Cristina Calpena, and Olga López. 2024. "Lipid-Based Gels for Delivery of 3-O-Ethyl L-Ascorbic acid in Topical Applications" Pharmaceutics 16, no. 9: 1187. https://doi.org/10.3390/pharmaceutics16091187








