Pharmacokinetics of Piperacillin–Tazobactam in Critically Ill Patients with Open Abdomen and Vacuum-Assisted Wound Closure: Dosing Considerations Using Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Protocol, and Population
2.2. Data Collection and Study Outcome
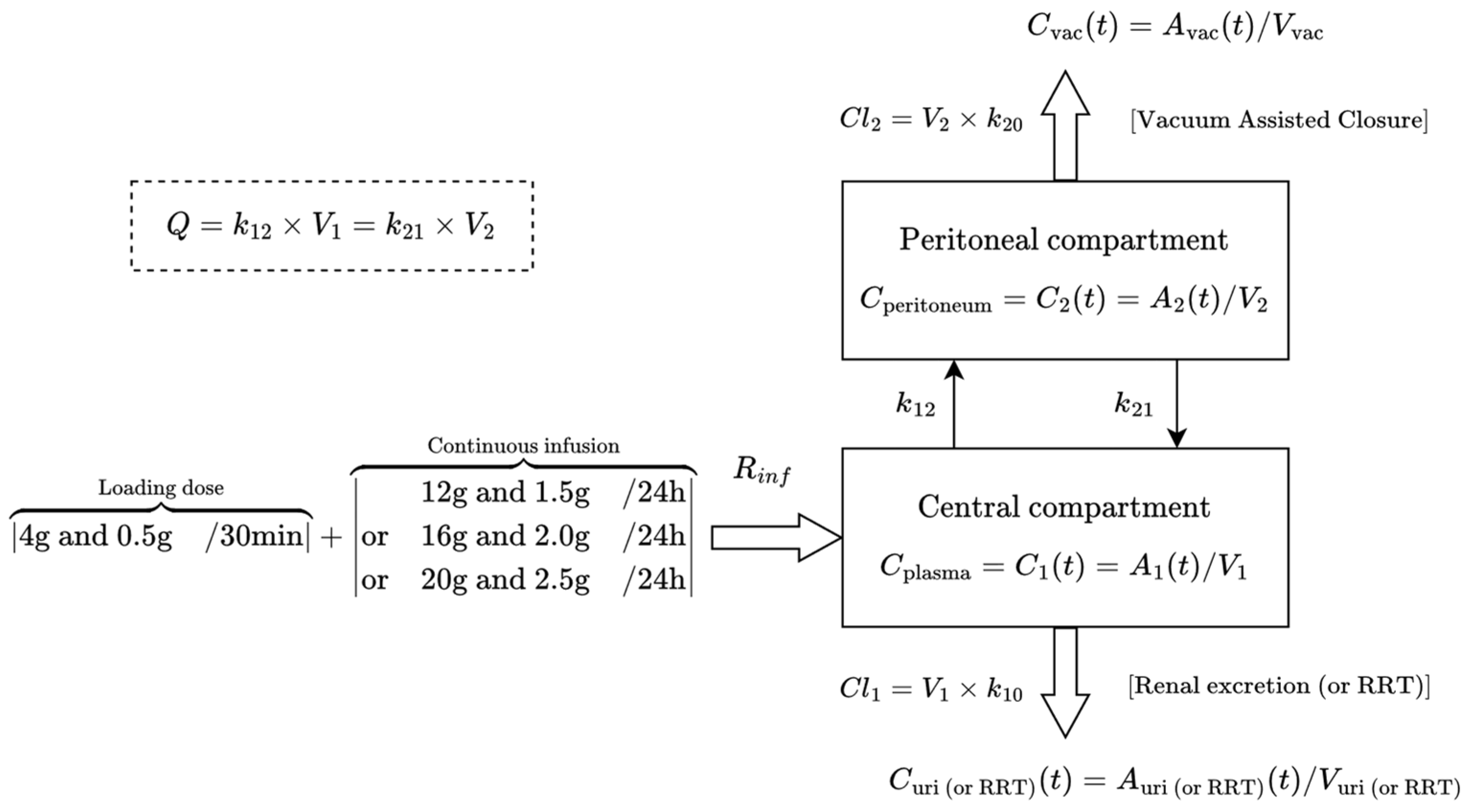
2.3. Population Pharmacokinetic Modeling and Monte Carlo Simulations
3. Results
3.1. Clinical and Pharmacological Data
3.2. Pharmacokinetic Model Building and Monte Carlo Simulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARC | augmented renal clearance |
| BMI | body mass index |
| CL | clearance |
| CLCR | creatinine clearance |
| CRRT | continuous renal replacement therapy |
| Css | concentration at steady state |
| ECOFF | epidemiological cut off |
| IAIs | intra-abdominal infections |
| ICU | Intensive Care Unit |
| MIC | minimum inhibitory concentration |
| NPDE | normalized prediction distribution error |
| OR | operative room |
| PD | Pharmacodynamics |
| PIP | Piperacillin |
| PK | pharmacokinetics |
| PTA | probability of therapeutic target attainment |
| PTZ | piperacillin–tazobactam |
| OA | open abdomen |
| SAPS 2 | simplified acute physiology score 2 |
| SOFA | sepsis-related organ failure assessment |
| TAZ | Tazobactam |
| TBW | total body weight |
| TDM | therapeutic drug monitoring |
| VAC | vacuum-assisted wound closure therapy |
| V1 | central volume of distribution |
| VPC | visual predictive check |
References
- Montravers, P.; Blot, S.; Dimopoulos, G.; Eckmann, C.; Eggimann, P.; Guirao, X.; Paiva, J.A.; Sganga, G.; De Waele, J. Therapeutic management of peritonitis: A comprehensive guide for intensivists. Intensive Care Med. 2016, 42, 1234–1247. [Google Scholar] [CrossRef]
- Montravers, P.; Dupont, H.; Leone, M.; Constantin, J.M.; Mertes, P.M.; Laterre, P.F.; Tuech, J.J. Guidelines for management of intra-abdominal infections. Anaesth. Crit. Care Pain Med. 2015, 34, 117–130. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Garnier, M. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics and the French Society of Anaesthesia and Intensive Care Medicine. Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Carrié, C.; Legeron, R.; Petit, L.; Ollivier, J.; Cottenceau, V.; d’Houdain, N.; Boyer, P.; Lafitte, M.; Xuereb, F.; Sztark, F.; et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J. Crit. Care 2018, 48, 66–71. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Soldatou, O. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- De Waele, J.J.; Kaplan, M.; Sugrue, M.; Sibaja, P.; Björck, M. How to deal with an open abdomen? Anaesthesiol. Intensive Ther. 2015, 47, 372–378. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Roberts, D.J.; Faris, P.D.; Ball, C.G.; Kubes, P.; Tiruta, C.; Jenne, C.N. Active Negative Pressure Peritoneal Therapy After Abbreviated Laparotomy: The Intraperitoneal Vacuum Randomized Controlled Trial. Ann. Surg. 2015, 262, 38–46. [Google Scholar] [CrossRef]
- Bruhin, A.; Ferreira, F.; Chariker, M.; Smith, J.; Runkel, N. Systematic review and evidence based recommendations for the use of negative pressure wound therapy in the open abdomen. Int. J. Surg. 2014, 12, 1105–1114. [Google Scholar] [CrossRef]
- Carrié, C.; Delzor, F.; Roure, S.; Dubuisson, V.; Petit, L.; Molimard, M.; Breilh, D.; Biais, M. Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy. Antimicrob. Agents Chemother. 2020, 64, e02098-19. [Google Scholar] [CrossRef]
- Besnard, T.; Carrié, C.; Petit, L.; Biais, M. Increased dosing regimens of piperacillin-tazobactam are needed to avoid subtherapeutic exposure in critically ill patients with augmented renal clearance. Crit. Care 2019, 23, 13. [Google Scholar] [CrossRef]
- Carrié, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased β-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: A before and after study. Crit. Care 2019, 23, 379. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Sztark, F. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Veillette, J.J.; Winans, S.A.; Forland, S.C.; Maskiewicz, V.K. A simple and rapid RP-HPLC method for the simultaneous determination of piperacillin and tazobactam in human plasma. J. Pharm. Biomed Anal. 2016, 131, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Nicasio, A.M.; VanScoy, B.D.; Mendes, R.E.; Castanheira, M.; Bulik, C.C.; Okusanya, O.O.; Bhavnani, S.M.; Forrest, A.; Jones, R.N.; Friedrich, L.V.; et al. Pharmacokinetics-Pharmacodynamics of Tazobactam in combination with Piperacillin in an in vitro infection model. Antimicrob. Agents Chemother. 2016, 60, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, J.; Carrié, C.; d’Houdain, N.; Djabarouti, S.; Petit, L.; Xuereb, F.; Breilh, D. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob. Agents Chemother. 2019, 63, e02134-18. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Udy, A.A.; Jarrett, P.; Wallis, S.C.; Hope, W.W.; Sharma, R.; Kirkpatrick, C.M.J.; Kruger, P.S.; Roberts, M.S.; Lipman, J. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J. Antimicrob. Chemother. 2015, 70, 1495–1502. [Google Scholar] [CrossRef]
- El-Haffaf, I.; Caissy, J.A.; Marsot, A. Piperacillin-Tazobactam in Intensive Care Units: A Review of Population Pharmacokinetic Analyses. Clin. Pharmacokinet. 2021, 60, 855–875. [Google Scholar] [CrossRef]
- Murao, N.; Ohge, H.; Ikawa, K.; Watadani, Y.; Uegami, S.; Shigemoto, N.; Sueda, T. Pharmacokinetics of piperacillin-tazobactam in plasma, peritoneal fluid and peritoneum of surgery patients, and dosing considerations based on site-specific pharmacodynamic target attainment. Int. J. Antimicrob. Agents 2017, 50, 393–398. [Google Scholar] [CrossRef]
- Leon, L.; Guerci, P.; Pape, E.; Thilly, N.; Luc, A.; Germain, A.; Butin-Druoton, A.-L.; Losser, M.-R.; Birckener, J.; Scala-Bertola, J.; et al. Serum and peritoneal exudate concentrations after high doses of β-lactams in critically ill patients with severe intra-abdominal infections: An observational prospective study. J. Antimicrob. Chemother. 2020, 75, 156–161. [Google Scholar] [CrossRef]
- Finazzi, S.; Luci, G.; Olivieri, C.; Langer, M.; Mandelli, G.; Corona, A.; Di Paolo, A. Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review—Part I. Antibiotics 2022, 11, 1164. [Google Scholar] [CrossRef]
- Akers, K.; Niece, K.; Chung, K.; Cannon, J.; Cota, J.; Murray, C. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J. Trauma Acute Care Surg. 2014, 77, S163–S170. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of β-lactam concentration-toxicity relashionships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J. Using PK/PD to Optimize Antibiotic Dosing for Critically Ill Patients. Curr. Pharm. Biotechnol. 2011, 12, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Greppmair, S.; Brinkmann, A.; Roehr, A.; Frey, O.; Hagel, S.; Dorn, C.; Liebchen, U. Towards model-informed precision dosing of piperacillin: Multicenter systematic external evaluation of pharmacokinetic models in critically ill adults with a focus on Bayesian forecasting. Intensive Care Med. 2023, 49, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Harris, P.N.A.; Mathers, A.J.; Wenzler, E.; Humphries, R.M. Breaking Down the Breakpoints: Rationale for the 2022 Clinical and Laboratory Standards Institute Revised Piperacillin-Tazobactam Breakpoints Against Enterobacterales. Clin. Infect. Dis. 2023, 77, 1585–1590. [Google Scholar] [CrossRef] [PubMed]


| Piperacillin | Tazobactam | |
|---|---|---|
| Observed concentration at steady state (mg/L) | ||
| 83 [52–100] 69 [43–61] | 19 [12–25] 18 [7–24] |
| Drug underdosing | 1 (2) | 5 (11) |
| Peritoneal diffusion (%) | 88 [73–98] | 88 [68–104] |
| Calculated drug clearance | ||
| 129 [62–231] 0.6 [0.3–1.0] | 65 [45–150] 0.7 [0.3–1.0] |
| Overall Population n = 45 | |
|---|---|
| Demographic and anthropometric data at T0 | |
| 66 [57–72] 36 (80) 28 [25–30] 83 [71–92] 79 [69–87] |
| Medical history | |
| 52 [39–71] 4 [3–5] |
| Initial surgical management and indication for OA/VAC | |
| 33 (73) vs. 12 (27) 10 (22) vs. 35 (78) 37 (82) vs. 8 (18) |
| Type of intra-abdominal infection | |
| 11 (24) 11 (24) 10 (22) 9 (20) 4 (9) |
| Microbiological documentation | |
| 34 (76) 17 (38) 11 (24) 8 (18) 1 (2) 18 (40) |
| Clinical and biological data at T1 | |
| 48 [48–72] 3 [3–7] 25 [20–28] 22 (49) 12 (27) 8 (18) 3 (7) |
| Patient’s outcome | |
| 2 [1–3] 4 [3–8] 11 (24) 5 (11) 15 [10–21] 8 (18) |
| Piperacillin | Tazobactam | |||
|---|---|---|---|---|
| Parameter | Value | SE (RSE %) | Value | SE (RSE %) |
| Fixed effects | ||||
| (L) | 21.213 | 17.818 (84) | 23.248 | 2.789 (12) |
| −0.016 | 0.004 (29) | / | / | |
| 0.017 | 0.005 (30) | / | / | |
| (L) | 5 | / | 5 | / |
| (L/h) | 23.833 | 5.958 (25) | 20 | / |
| (L/h) | 38.739 | 32,153 (83) | 7.013 | 2.594 (37) |
| 0 | 0 (0) | 0 | 0 (0) | |
| −2.016 | 0.443 (22) | −5.602 | 3.529 (63) | |
| −1.243 | 0.360 (29) | −2.303 | 0.667 (29) | |
| (L/h) | 5.567 | 0.612 (11) | 3.003 | 0.360 (12) |
| Inter-individual variability | ||||
| 0.225 | 0.056 (25) | 0.351 | 0.119 (34) | |
| 0.661 | 0.178 (27) | 0.569 | 0.500 (88) | |
| 0.123 | 0.106 (86) | 0.243 | 0.123 (51) | |
| Intra-individual variability | ||||
| 0.391 | 0.160 (41) | 0.967 | 0.348 (36) | |
| Residual variability | ||||
| 0.207 | 0.028 (14) | 0.371 | 0.063 (17) | |
| 0.239 | 0.033 (14) | 0.372 | 0.048 (13) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrié, C.; Butruille, J.; Maingault, S.; Lannou, A.; Dubuisson, V.; Petit, L.; Biais, M.; Breilh, D. Pharmacokinetics of Piperacillin–Tazobactam in Critically Ill Patients with Open Abdomen and Vacuum-Assisted Wound Closure: Dosing Considerations Using Monte Carlo Simulation. Pharmaceutics 2024, 16, 1191. https://doi.org/10.3390/pharmaceutics16091191
Carrié C, Butruille J, Maingault S, Lannou A, Dubuisson V, Petit L, Biais M, Breilh D. Pharmacokinetics of Piperacillin–Tazobactam in Critically Ill Patients with Open Abdomen and Vacuum-Assisted Wound Closure: Dosing Considerations Using Monte Carlo Simulation. Pharmaceutics. 2024; 16(9):1191. https://doi.org/10.3390/pharmaceutics16091191
Chicago/Turabian StyleCarrié, Cédric, Jesse Butruille, Sophie Maingault, Alexandre Lannou, Vincent Dubuisson, Laurent Petit, Matthieu Biais, and Dominique Breilh. 2024. "Pharmacokinetics of Piperacillin–Tazobactam in Critically Ill Patients with Open Abdomen and Vacuum-Assisted Wound Closure: Dosing Considerations Using Monte Carlo Simulation" Pharmaceutics 16, no. 9: 1191. https://doi.org/10.3390/pharmaceutics16091191
APA StyleCarrié, C., Butruille, J., Maingault, S., Lannou, A., Dubuisson, V., Petit, L., Biais, M., & Breilh, D. (2024). Pharmacokinetics of Piperacillin–Tazobactam in Critically Ill Patients with Open Abdomen and Vacuum-Assisted Wound Closure: Dosing Considerations Using Monte Carlo Simulation. Pharmaceutics, 16(9), 1191. https://doi.org/10.3390/pharmaceutics16091191






