Abstract
The space environment can affect the function of all physiological systems, including the properties of cell membranes. Our goal in this study was to explore the effect of simulated microgravity (SMG) on the cellular uptake of small molecules based on reported microgravity-induced changes in membrane properties. SMG was applied to cultured cells using a random-positioning machine for up to three hours. We assessed the cellular accumulation of compounds representing substrates of uptake and efflux transporters, and of compounds not shown to be transported by membrane carriers. Exposure to SMG led to an increase of up to 60% (p < 0.01) in the cellular uptake of efflux transporter substrates, whereas a glucose transporter substrate showed a decrease of 20% (p < 0.05). The uptake of the cathepsin activity-based probe GB123 (MW, 1198 g/mol) was also enhanced (1.3-fold, p < 0.05). Cellular emission of molecules larger than ~3000 g/mol was reduced by up to 50% in SMG (p < 0.05). Our findings suggest that short-term exposure to SMG could differentially affect drug distribution across membranes. Longer exposure to microgravity, e.g., during spaceflight, may have distinct effects on the cellular uptake of small molecules.
1. Introduction
Microgravity, defined as the condition of experiencing very low or negligible gravitational forces, is a crucial aspect of the space environment. The microgravity of space can considerably alter human physiology, affecting almost every system of the body. Examples include fluid shifts toward the head and chest, an approximately 20-fold faster increase in carotid artery stiffness as compared to healthy aging, and accelerated bone and muscle mass loss [1].
The physiological changes that occur during spaceflight can result in medical conditions that require drug treatment and can affect the pharmacokinetics and pharmacodynamics of medications taken in space [1]. One factor that may contribute to altered pharmacokinetics and pharmacodynamics is an increase in membrane fluidity, which was observed during a parabolic flight mission and in simulated microgravity (SMG). In a study conducted onboard a sounding rocket, altered fluidity was shown to affect lidocaine integration into membranes. Microgravity was also associated with reduced transmembranal transport activity of the multidrug resistance–associated transporter (MRP) 2 [1].
Based on the changes in membrane properties, we hypothesized that microgravity could affect the distribution of small molecules into cells, due to changes in passive diffusion, carrier-mediated transport, or both. In this pilot study, we tested our hypothesis using SMG. We focused on the activity of the efflux transporters P-glycoprotein (P-gp), the breast cancer resistance protein (BCRP), and MRP1. We used selective transporter inhibitors and cell lines that overexpress these carriers to isolate the effects of SMG on distinct transporters. We also assessed the distribution into cells of an uptake transporter substrate, a compound which is not a P-gp/BCRP substrate, and several compounds of larger molecular weights. Our preferred probes were molecules trapped within cells (Table 1), which helped combat the reversibility of SMG effects when cells are removed from the microgravity simulator.

Table 1.
Physicochemical properties of the studied compounds.1
2. Materials and Methods
2.1. Materials
GB123 [2] and the FITC-labeled nuclear localization sequence (NLS)-peptide nucleic acid (PNA) [3] were synthesized as previously described. Bovine serum albumin (BSA), Biotechnology Grade, was obtained from Tamar laboratory supplies (Mevaseret Zion, Israel). The other cell culture reagents, phosphate-buffered saline (PBS), trypsin EDTA Solution C (0.05%), and 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) were purchased from Biological Industries Ltd. (Beit Haemek, Israel). Dimethyl sulfoxide (extra dry 99.7% DMSO) was obtained from Acros Organics (Geel, Belgium). Calcein AM and boron-dipyrromethene (BODIPY) prazosin were obtained from Thermo Fisher Scientific (Waltham, MA USA). Hoechst 33342 was obtained from Abcam (Cambridge, UK). 2-Deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose (2-NBDG) was obtained from Cayman Chemical (Ann Arbor, MI, USA). 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Fluoromount G (SouthernBiotech, Birmingham, AL, USA). Valspodar (PSC-833) was obtained from Tocris Bioscience (Bristol, UK). All other reagents were obtained from Sigma-Aldrich (Rehovot, Israel).
2.2. Cell Lines and Cell Culture
The cell lines were selected based on their profile of efflux transporter expression. Several species were chosen in order to enhance extrapolation potential. The RAW 264.7 line is derived from murine macrophages and has been commonly utilized in microgravity research [4]. These cells express functional P-gp [5]. Madin–Darby canine kidney (MDCK) II cells transfected with cDNA coding for the human P-gp (MDCK-MDR1 cells) are a well-characterized in vitro model for assessment of P-gp activity [6,7,8]. U87 human glioblastoma cells were selected based on their low level of P-gp expression [9].
RAW 264.7 cells (from Prof. Boaz Tirosh, The Hebrew University) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, L-glutamine (2 mM), sodium pyruvate (1 mM), non-essential amino acids, penicillin (100 Units/mL), and streptomycin (100 μg/mL). U87-MG cells (from Prof. Ofra Benny, The Hebrew University) were cultured in Eagle’s MEM medium supplemented with 10% fetal calf serum, L-glutamine (2 mM), sodium pyruvate (1 mM), and 1% penicillin/streptomycin. MDCK-MDR1 cells were provided by Prof. Alfred Schinkel (The Netherlands Cancer Institute). Cells were maintained in Dulbecco’s modified Eagle’s phenol-free low-glucose medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 5% CO2 incubator. The cells were harvested after achieving 70–80% confluence. The transmembranal transfer studies were all conducted using suspended cells. Cell viability was analyzed using the XTT assay kit according to the manufacturer’s instructions.
2.3. Tested Compounds
Calcein AM and Hoechst 33342 were used at 0.25 μM [10,11] and 60 μM (determined after several preliminary experiments), respectively. Doxorubicin was used at 5 μM, based on preliminary XTT experiments showing that at this concentration, it does not affect the viability of the studied cells. The concentration of BODIPY prazosin was 1 μM (based on preliminary experiments) and that of 2-NBDG was 100 μM [12]. Valspodar (a P-gp inhibitor [13]) was used at 1.65 μM [14] and fumitremorgin C (FTC; a BCRP inhibitor [13]) at 10 μM [14,15]. FITC-labeled dextrans were used at 2 mg/mL [16,17] (molar values are only an approximation, see Table 1) and NLS-PNA-FITC was used at 5 μM [3].
2.4. Experiments in Simulated Microgravity
Microgravity conditions were simulated by a random positioning machine (RPM) 2.0 (purchased from Yuri GmbH, Meckenbeuren, Germany; originally from DutchSpace Airbus, Leiden, The Netherlands). The RPM continuously and randomly changes the orientation of the accommodated experiments relative to the Earth gravity vector. Objects cannot adjust or react to the gravitational pull due to rapid and continuous changes in orientation. This simulates a microgravity environment by neutralizing the net effect of gravity on the objects, allowing gravity levels that replicate those of the Moon (0.16 g) and of Mars (0.38 g), with a minimum of 10−3 g. The RPM was maintained within a cell culture incubator (3 °C, 5% CO2).
Cells were removed from the culture plates according to the instructions from the American Type Culture Collection (ATCC) website; 0.05% trypsin for MDCK cells, 0.25% trypsin for U87 cells, and scraping for RAW 264.7 cells. Cells in media containing the fluorescent probe were transferred to 0.5 mL Eppendorf tubes placed at the center of the simulator (Appendix A, Figure A1) or on a shelf within the same incubator. Potential effects of the stationary position were excluded based on comparison to samples placed on a shaker (Appendix B, Figure A2). To enable the placement of all tubes on the central part of the RPM, all experiments were conducted using up to six tubes per treatment group. Incubation in the RPM or under control conditions was for the duration of one hour unless otherwise stated (due to slow uptake kinetics). In experiments with transporter inhibitors, the cells were preincubated with the inhibitor for one hour under Earth conditions. The inhibitor was also present throughout the experiment. The RPM was set at Partial G, Loop path, and No Motion mode.
At the end of the experiments, all tubes were immediately placed on ice and the remaining procedures were carried out at 4 °C. The cells were washed with PBS, filtered using a 40 µm nylon mesh filter, and fluorescence was measured by flow cytometry using a Fortessa FACS Analyzer (BD LSRFortessa™ Flow Cytometer, BD Biosciences, San Jose, CA, USA). The results were analyzed using FlowJo™ v10.7 analysis software (https://www.flowjo.com; accessed on 1 August 2024).
For the analyses with GB123, RAW 264.7 cells were divided into four groups. Two groups were incubated with 0.25 μM GB123 for two hours, washed three times, and incubated with PBS for an additional two hours. The other two groups were incubated with the medium over the first two hours, then with 0.25 μM GB123 under Earth conditions for the next two hours. In the cellular uptake experiments, the chambers were washed with phosphate-buffered saline (PBS) three times and stained with DAPI (1:1000) diluted in PBS. The cells were then imaged by a Nikon motorized Ti2E confocal fluorescent microscope with a Yokogawa W1 Spinning Disk (Olympus, Tokyo, Japan). The cathepsin binding analysis was conducted after the cells were lysed in RIPA buffer. Equal amounts of protein (100 μg per sample) were loaded onto the gel and separated on a 12.5% SDS PAGE (Appendix D, Figure A3). Instead of transferring the proteins to a membrane and using a loading control, we scanned the gel for Cy5 using a Typhoon laser scanner and compared the fluorescence intensity of GB123 between the groups. This method allowed direct assessment and comparison of cathepsin expression levels [14].
Statistical Analysis
Two-way ANOVA, the Mann–Whitney test or the Kruskal–Wallis test, as appropriate, were used for comparisons across treatment groups (Prism 10.2.3; GraphPad, La Jolla, CA, USA). Flow cytometry results are expressed as the medians of the fluorescence signal obtained in 10,000 cells. Results are reported as individual values with group medians. A p value ≤ 0.05 was considered significant.
3. Results
3.1. Simulated Microgravity Can Enhance the Cellular Uptake of Efflux Transporter Substrates
Exposure to SMG increased the cellular uptake of calcein AM (Figure 1a–c). The effect was highest in U87 cells (a median 60% increase in emission; Figure 1b). Overall, the magnitude of change in calcein AM emission did not reflect P-gp abundance. However, in MDCK-MDR1 cells, the SMG-inhibition interaction was significant, implying that both efflux transport and diffusion were affected (Figure 1c). In the doxorubicin experiment, only the effect of gravity was statistically significant (Figure 1d). The fold change in doxorubicin (Figure 1d) and BODIPY prazosin uptake (Figure 1e) was similar to that of calcein AM. Hoechst 33342 emission was not significantly affected by SMG (Figure 1f). We did not pursue the experiments with BODIPY prazosin in MDCK-MDR1 and U87 cells any further because these cells have no functional BCRP activity [18] or express low BCRP levels [9].
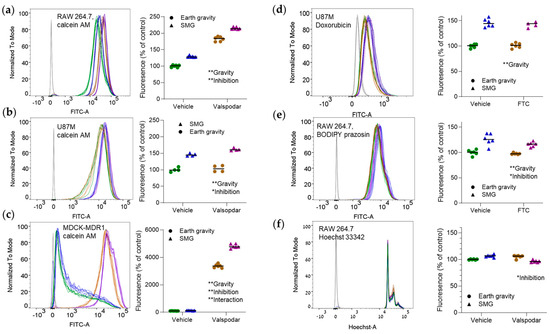
Figure 1.
The uptake of efflux transporter substrates can be increased in simulated microgravity (SMG). Cells were incubated at Earth gravity or SMG (target: 0.001 g; Appendix C) with efflux transporter substrates in the presence or the absence of transporter inhibitors. Emission intensity was measured by flow cytometry. The left part of each panel shows a representative emission distribution curve. The right part is a quantitative analysis of the results. Shifts to the right indicate a higher uptake of the fluorescent probe by the cells. (a–c) Raw 264.7 (n = 6/group) (a), U87 (n = 4/group) (b), or MDCK-MDR1 (n = 6/group) (c) cells, incubated with 0.25 μM calcein AM with or without 1.65 μM valspodar (a P-gp inhibitor) [13]). (d) U877 cells incubated with 5 μM doxorubicin with or without 1.65 μM valspodar (n = 6/group). (e) RAW 264.7 cells incubated with 1 μM BODIPY prazosin with or without 10 μM fumitremorgin C (FTC; a BCRP inhibitor [13]; n = 6/group). (f) RAW 264.7 cells incubated with 60 μM Hoechst 33342 with or without 1.65 μM valspodar (n = 6/group, except for n = 5 in the SMG + valspodar group). Dark gray lines in the distribution curves denote non-stained cells. * p < 0.05; ** p < 0.01; 2-way ANOVA. The test assesses whether the emission is significantly affected by SMG, the inhibitor, and their interaction. The figures are labeled accordingly. Experiments were repeated thrice with similar results. The numbers of replicates per group denote biological replicates (samples cultured separately). Heights are normalized (“to mode”) to highlight shifts to the right or the left. Green, vehicle and Earth gravity; blue, vehicle and SMG; brown, transporter inhibitor and Earth gravity; purple, transporter inhibitor and SMG.
3.2. Glucose Transporter Activity/Metabolism Is Reduced in Simulated Microgravity
Unlike the efflux transporter substrates, the emission intensity of the glucose analog 2-NBDG was 20% lower under SMG conditions targeting 0.001 g (p < 0.01; Appendix E, Figure A4). Similar results were obtained at 0.16 g.
3.3. Simulated Microgravity Can Enhance the Cellular Uptake of a Molecule Larger Than 1000 g/mol
GB123, a cathepsin activity-based probe, is larger than the transporter substrates described above (Table 1). Under simulated Moon gravity (0.16 g), the cellular accumulation of this compound was also enhanced by 30% (Appendix F, Figure A5a–c). The experiments were conducted at higher gravity as compared to the others in order to minimize the potential of cathepsin activation. Indeed, the change in GB123 uptake was not due to increased cathepsin activity (which tended to decrease and not increase under SMG conditions; Appendix F, Figure A5d,e).
3.4. The Cellular Distribution of Molecules that Weigh 3000 g/mol or More Is Reduced in Simulated Microgravity
To assess the effect of size and charge on cellular uptake in SMG, we used positively charged, negatively charged, and neutral FITC-labeled dextrans. The signal from neutral dextran was similar to that of non-treated cells and we did not further explore this compound. Positive dextran yielded a considerably higher signal than negative dextran. The emission of both dextrans was approximately half in SMG (Figure 2a,b).
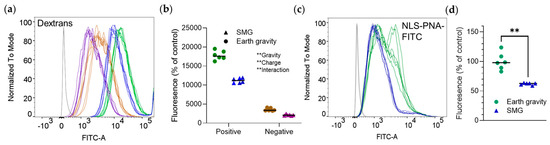
Figure 2.
Simulated Moon gravity reduces the uptake of molecules larger than 3000 g/mol into RAW 264.7 cells. Cells were incubated at Earth gravity or SMG with positively charged, negatively charged, or neutral dextrans (Table 1) for one hour, or with NLS−PNA−FITC for three hours. Emission intensity was measured by flow cytometry. Shown are representative distribution curves of emission intensity obtained by flow cytometry (a,c) and quantitative analyses of the results (b,d). (a,b) Fluorescently labeled dextrans. (b,c) NLS−PNA−FITC. ** p < 0.01, two−way ANOVA (b) or Mann-–Whitney test (d). Experiments were repeated thrice (n = 6 biological replicates per group in each experiment), with similar results. (a,b) Green, positively-charged dextran and Earth gravity; blue, positively-charged dextran and SMG; brown, negatively-charged dextran and Earth gravity; purple, negatively-charged and SMG.
The effect of SMG on large, positively charged molecules was confirmed with NLS-PNA-FITC, [6253.70 g/mol] whose fluorescence intensity at SMG was lower as well (Figure 2c,d).
4. Discussion
Since the beginning of human spaceflight, significant time, resources, and effort have been devoted to the study of space physiology, whereas space pharmacology was far less thoroughly explored. Few pharmacokinetic studies have been conducted in astronauts and cosmonauts, with conflicting findings. The relatively consistent delayed absorption and other pharmacokinetic changes were mostly attributed to fluid shifts and changes in gastrointestinal function [1]. However, the microgravity of space might also affect membrane permeability, as was previously demonstrated in isolated vesicles [19]. This prompted us to explore the transmembranal distribution of smaller and larger molecules. We investigated small molecule (<1000 g/mol) efflux transporter substrates, an uptake transporter substrate, and larger molecules to rule out globally enhanced diffusion into cells. To avoid altered gene expression as a confounder, we limited our analyses to one hour (except for the GB123 and NLS-PNA-FITC studies).
This analysis is a pilot study, yet we could identify several physicochemical properties of the tested compounds that could affect their behavior in SMG. To our knowledge, such an analysis has not yet been reported. One notable observation was the higher cellular uptake of efflux transporter substrates in SMG. The magnitude of change was modest but statistically significant. The SMG effect on calcein AM kinetics was independent of P-gp abundance (Figure 1a–c; low, moderate, and high in U87 [9], RAW 264.7 [5], and MDCK-MDR1 cells, respectively). It was likely related to both enhanced diffusion and reduced P-gp activity (Figure 1c). The altered P-gp function could reflect changes in substrate or ATP binding, altered internalization, or cytoskeletal changes. The reduced 2-NBDG accumulation coupled with the lack of SMG effect on Hoechst 33342 suggest that the higher distribution of small molecules into cells in SMG is not a global phenomenon.
The SMG effect on a larger molecule, GB123, was similar to those observed with the efflux transporter substrates (although GB123 is not a P-gp or BCRP substrate [14]). Our analysis excluded enhanced cathepsin activity as the cause because activity tended to be lower when the cells were exposed to SMG. The enhanced GB123 uptake did not extend to larger molecules, at least not to those with the molecular properties described above.
This pilot study has several limitations. First, lower gravity (0.001 g) is achieved only after prolonged incubation (e.g., 24 h) and the one-hour exposure did not suffice. Yet, gravity was 0.01 g at 10 min and 0.006 g at 30 min after the onset of the experiment (Appendix C). This issue was less pronounced with lunar gravity, because the target value (0.16 g) was obtained within 30 min. Second, the closed Eppendorf tubes minimized exposure to CO2, which could potentially affect the results However, preliminary experiments with Falcon flasks that allow gas exchange yielded similar results, probably because the incubation was relatively short. Third, we took all available steps in order to remove bubbles, but their presence in the vials (and consequent shear forces) cannot be completely ruled out. Therefore, our findings might misinterpret the magnitude of the SMG effect. Additionally, this study assessed only short-time exposure and only transcellular transport. Longer exposure to microgravity has been shown to affect transporter gene expression [1] and could change the magnitude or reverse the direction of the changes observed in the current study. Altered paracellular transport (e.g., tight junction opening) might potentially also affect the transfer of small and large molecules across biological barriers in space. Finally, the cells were cultured in 2D. A study in cardiomyocytes demonstrated similar cellular responses to pharmacological agents in 2D and 3D under SMG conditions [20]. Hence dimensionality is not necessarily expected to affect our results, but further experiments should be performed to ensure that this is the case.
If our findings are applied to humans, membrane permeability to small- and large-molecule drugs could be altered. This could result in enhanced absorption, reduced systemic elimination, and enhanced distribution across the BBB, which may require dosing adjustment. One example is apixaban, a small molecule, P-gp substrate oral anticoagulant [21].
5. Conclusions
SMG selectively affects the uptake of small molecules into cells, but the direction of change varies across molecules. The changes were observed across several cell lines of various origins, suggesting that this phenomenon is not cell-type specific. In space, this could alter drug absorption, distribution, and elimination, and potentially also drug effects. Future research may shed light on the clinical implications of altered transmembranal transport of small molecules during spaceflight.
Author Contributions
Conceptualization, O.T.-S. and S.E.; methodology, O.T.-S., N.T., R.H., N.E., G.D., E.M. and S.E.; validation, R.H. and N.T.; formal analysis, O.T.-S.; investigation, O.T.-S., N.T., R.H., N.E., G.D., E.M., A.H., T.D., N.D. and T.K.; resources, G.B. and E.Y.; data curation, O.T.-S.; writing—original draft preparation, O.T.-S.; writing—review and editing, O.T.-S., N.T., R.H., N.E., G.D., E.M., E.Y., G.B. and S.E.; visualization, G.B., E.Y. and S.E.; project administration, O.T.-S.; funding acquisition, S.E. and E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Israel Space Agency, grant numbers 0004079 and 0006055, and by the Israel Science Foundation, grant number 1366/23.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from S.E. upon request.
Acknowledgments
We are grateful to Boaz Tirosh from the Hebrew University for providing RAW 264.7 cells; Ofra Benny from the Hebrew University for the U87 cell line; and Alfred Schinkel from The Netherlands Cancer Institute for MDCK-MDR1 cells. We thank Omer Eyal for language editing. Sara Eyal is affiliated with the David R. Bloom Centre for Pharmacy and Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem, Israel, and is holding a Dame Susan Garth Chair of Cancer Research.
Conflicts of Interest
S.E. has served as an advisor to SpacePharma, Israel. The other authors have no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Figure A1.
Placement of vials within the RPM.
Figure A1.
Placement of vials within the RPM.

Appendix B
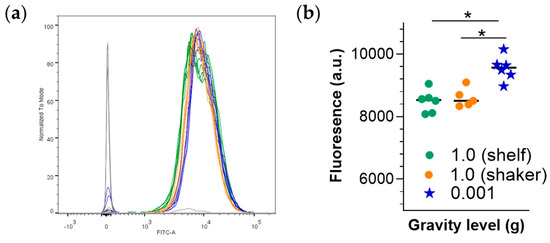
Figure A2.
Comparison of shelf- versus shaker placement of samples. (a) Histograms showing the distribution of cells by emission intensity within each treatment group. (b) quantitative analysis of emission intensity by group. In this experiment, calcein AM uptake into RAW 264.7 cells was assessed as described above, with additional control of “ground” samples placed on a shaker. No difference was observed between the two “ground controls” (Figure). * p < 0.05, Kruskal–Wallis test.
Figure A2.
Comparison of shelf- versus shaker placement of samples. (a) Histograms showing the distribution of cells by emission intensity within each treatment group. (b) quantitative analysis of emission intensity by group. In this experiment, calcein AM uptake into RAW 264.7 cells was assessed as described above, with additional control of “ground” samples placed on a shaker. No difference was observed between the two “ground controls” (Figure). * p < 0.05, Kruskal–Wallis test.

Appendix C

Table A1.
Mean Gravity throughout a One-Hour Experiments That Target 0.16 g or 0.001 g.
Table A1.
Mean Gravity throughout a One-Hour Experiments That Target 0.16 g or 0.001 g.
| Time (min) | Gravity | |
|---|---|---|
| Setting: 0.16 g | Setting: 0.001 g | |
| 10 | 0.17 | 0.011 |
| 20 | 0.163 | 0.008 |
| 30 | 0.155 | 0.006 |
| 40 | 0.155 | 0.005 |
| 50 | 0.156 | 0.005 |
| 60 | 0.157 | 0.003 |
Appendix D

Figure A3.
Protein loading in the cathepsin activity analysis.
Figure A3.
Protein loading in the cathepsin activity analysis.

Appendix E
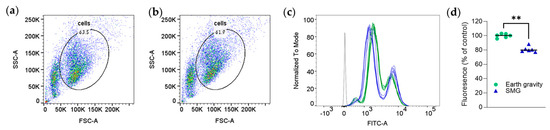
Figure A4.
Simulated microgravity reduces the cellular accumulation of the glucose transporter substrate 2-NBDG. RAW 264.7 cells were incubated in Earth gravity or SMG (target, 0.001 g; Appendix C) with 2-NBDG for one hour. Emission intensity was measured by flow cytometry. (a,b) Gating for the cells exposed to Earth (a) or SMG (b) conditions. (c). A representative distribution curve of emission intensity obtained by flow cytometry. Dark gray lines in the distribution curves denote non-stained cells. (d) Quantitative analyses of the results. n = 6/group (biological replicates); ** p < 0.01, Mann–Whitney test. Experiments were repeated twice at target 0.001 and thrice at 0.16 g with similar results.
Figure A4.
Simulated microgravity reduces the cellular accumulation of the glucose transporter substrate 2-NBDG. RAW 264.7 cells were incubated in Earth gravity or SMG (target, 0.001 g; Appendix C) with 2-NBDG for one hour. Emission intensity was measured by flow cytometry. (a,b) Gating for the cells exposed to Earth (a) or SMG (b) conditions. (c). A representative distribution curve of emission intensity obtained by flow cytometry. Dark gray lines in the distribution curves denote non-stained cells. (d) Quantitative analyses of the results. n = 6/group (biological replicates); ** p < 0.01, Mann–Whitney test. Experiments were repeated twice at target 0.001 and thrice at 0.16 g with similar results.

Appendix F
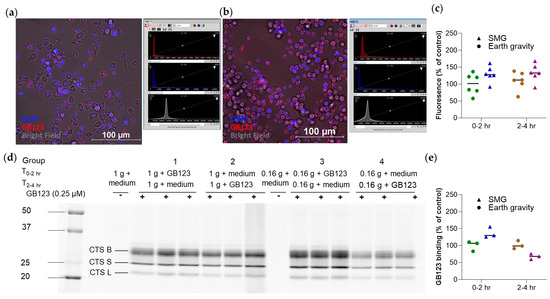
Figure A5.
Simulated Moon gravity modestly increases GB123 uptake into RAW 264.7 cells. Cells were divided into four groups, n = 6/group: two groups (1—Earth and 3—Moon) were incubated with GB123 at 0–2 h under simulated Earth or Moon conditions, washed three times, and incubated with PBS for an additional two hours (under the same respective conditions). The other groups (2—Earth and 4—Moon) were treated similarly, except that the first two hours were with the medium only (without GB123), to assess the effect of SMG on cathepsin activity. Cells were either imaged by confocal microscopy or lysed in RIPA buffer and subjected to Western blot analysis. Experiments were repeated twice, with similar results. (a,b) Confocal microscopy images of RAW 264.7 cells treated with the medium (a) or GB123 (b) for two hours. Red, GB123. Blue, DAPI. Also shown are the imaging settings. For both the Earth and the Moon images, ranges were set as 0–11,000 for the red channel, 0–14,000 for the blue channel, and 12,000–60,000 for the bright field. (c) Quantitative analysis of the GB123 signal. (d) Images of cathepsin-bound GB123 following separation on a gel. (e) Quantitative analysis of the respective GB123 emission, n = 3/group (biological replicates). The bands represent cathepsins (CTS) B, S, and L.
Figure A5.
Simulated Moon gravity modestly increases GB123 uptake into RAW 264.7 cells. Cells were divided into four groups, n = 6/group: two groups (1—Earth and 3—Moon) were incubated with GB123 at 0–2 h under simulated Earth or Moon conditions, washed three times, and incubated with PBS for an additional two hours (under the same respective conditions). The other groups (2—Earth and 4—Moon) were treated similarly, except that the first two hours were with the medium only (without GB123), to assess the effect of SMG on cathepsin activity. Cells were either imaged by confocal microscopy or lysed in RIPA buffer and subjected to Western blot analysis. Experiments were repeated twice, with similar results. (a,b) Confocal microscopy images of RAW 264.7 cells treated with the medium (a) or GB123 (b) for two hours. Red, GB123. Blue, DAPI. Also shown are the imaging settings. For both the Earth and the Moon images, ranges were set as 0–11,000 for the red channel, 0–14,000 for the blue channel, and 12,000–60,000 for the bright field. (c) Quantitative analysis of the GB123 signal. (d) Images of cathepsin-bound GB123 following separation on a gel. (e) Quantitative analysis of the respective GB123 emission, n = 3/group (biological replicates). The bands represent cathepsins (CTS) B, S, and L.

References
- Eyal, S.; Derendorf, H. Medications in Space: In Search of a Pharmacologist’s Guide to the Galaxy. Pharm. Res. 2019, 36, 148. [Google Scholar] [CrossRef] [PubMed]
- Blum, G.; von Degenfeld, G.; Merchant, M.J.; Blau, H.M.; Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 2007, 3, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Soudah, T.; Mogilevsky, M.; Karni, R.; Yavin, E. CLIP6-PNA-Peptide Conjugates: Non-Endosomal Delivery of Splice Switching Oligonucleotides. Bioconjug. Chem. 2017, 28, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Ludtka, C.; Silberman, J.; Moore, E.; Allen, J.B. Macrophages in microgravity: The impact of space on immune cells. NPJ Microgravity 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Wang, G.; Xu, W.; Liu, X.; Wang, Y.; Zha, B.S.; Shi, J.; Zhao, Q.; Gerk, P.M.; Studer, E.; et al. Inhibition of P-glycoprotein by HIV protease inhibitors increases intracellular accumulation of berberine in murine and human macrophages. PLoS ONE 2013, 8, e54349. [Google Scholar] [CrossRef] [PubMed]
- FDA. In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. Guidance for Industry. Available online: https://www.fda.gov/media/134582/download (accessed on 23 June 2023).
- Irvine, J.D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J.W.; Selick, H.E.; Grove, J.R. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 1999, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tohyama, K.; Kosugi, Y. Investigation of MDR1-overexpressing cell lines to derive a quantitative prediction approach for brain disposition using in vitro efflux activities. Eur. J. Pharm. Sci. 2020, 142, 105119. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.A.; Rodgers, L.T.; Kryscio, R.J.; Hartz, A.M.; Bauer, B. Characterization and comparison of human glioblastoma models. BMC Cancer 2022, 22, 844. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, E.; Gurina, M.; Magdassi, S.; Eyal, S. Evaluation of the near infrared compound indocyanine green as a probe substrate of P-glycoprotein. Mol. Pharm. 2012, 9, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Guberović, I.; Marjanović, M.; Mioč, M.; Ester, K.; Martin-Kleiner, I.; Ramljak, T.; Mlinarić-Majerski, K.; Kralj, M. Crown ethers reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Sci. Rep. 2018, 8, 14467. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Sasaki, A.; Ono, K.; Ohshika, S.; Ishibashi, Y.; Yamada, K. Uptake of fluorescent D- and L-glucose analogues, 2-NBDG and 2-NBDLG, into human osteosarcoma U2OS cells in a phloretin-inhibitable manner. Hum. Cell 2021, 34, 634–643. [Google Scholar] [CrossRef] [PubMed]
- FDA. Drug Development and Drug Interactions|Table of Substrates, Inhibitors and Inducers. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 25 July 2024).
- Hamed, R.; Merquiol, E.; Zlotver, I.; Blum, G.; Eyal, S.; Ekstein, D. Challenges in Batch-to-Bed Translation Involving Inflammation-Targeting Compounds in Chronic Epilepsy: The Case of Cathepsin Activity-Based Probes. ACS Omega 2024, 9, 6965–6975. [Google Scholar] [CrossRef] [PubMed]
- Rubinchik-Stern, M.; Shmuel, M.; Eyal, S. Antiepileptic drugs alter the expression of placental carriers: An in vitro study in a human placental cell line. Epilepsia 2015, 56, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Nevo, N.; Chossat, N.; Gosgnach, W.; Logeart, D.; Mercadier, J.-J.; Michel, J.-B. Increasing endothelial cell permeability improves the efficiency of myocyte adenoviral vector infection. J. Gene Med. 2001, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Marino, N.; Curran, C.; McHale, A.P.; Callan, J.F.; Callan, B. Cholesteryl to improve the cellular uptake of polymersomes within HeLa cells. Int. J. Pharm. 2016, 511, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Strab, R.; Kardos, P.; Ferguson, C.; Li, J.; Owen, A.; Hidalgo, I.J. Application and limitation of inhibitors in drug-transporter interactions studies. Int. J. Pharm. 2008, 356, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Vaquer, S.; Cuyàs, E.; Rabadán, A.; González, A.; Fenollosa, F.; de la Torre, R. Active transmembrane drug transport in microgravity: A validation study using an ABC transporter model [version 1; peer review: 2 approved]. F1000Research 2014, 3, 201. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Wu, Q.; Singh, M.; Preininger, M.K.; Han, P.; Ding, G.; Cho, H.C.; Jo, H.; Maher, K.O.; Wagner, M.B.; et al. Simulated Microgravity and 3D Culture Enhance Induction, Viability, Proliferation and Differentiation of Cardiac Progenitors from Human Pluripotent Stem Cells. Sci. Rep. 2016, 6, 30956. [Google Scholar] [CrossRef] [PubMed]
- FDA (USA Food and Drug Administration). Eliquis (Apixaban). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202155s034lbl.pdf (accessed on 22 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


