A Patent-Pending Ointment Containing Extracts of Five Different Plants Showed Antinociceptive and Anti-Inflammatory Mechanisms in Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Gas Chromatography–Mass Spectrometry Analysis
2.2. Animals
2.3. Drugs and Reagents
2.4. Plant Material
2.5. Plant Extract Preparation
2.6. Ointment Preparation
2.7. Ointment Physicochemical and Organoleptic Characterization
2.8. Skin Irritation Test
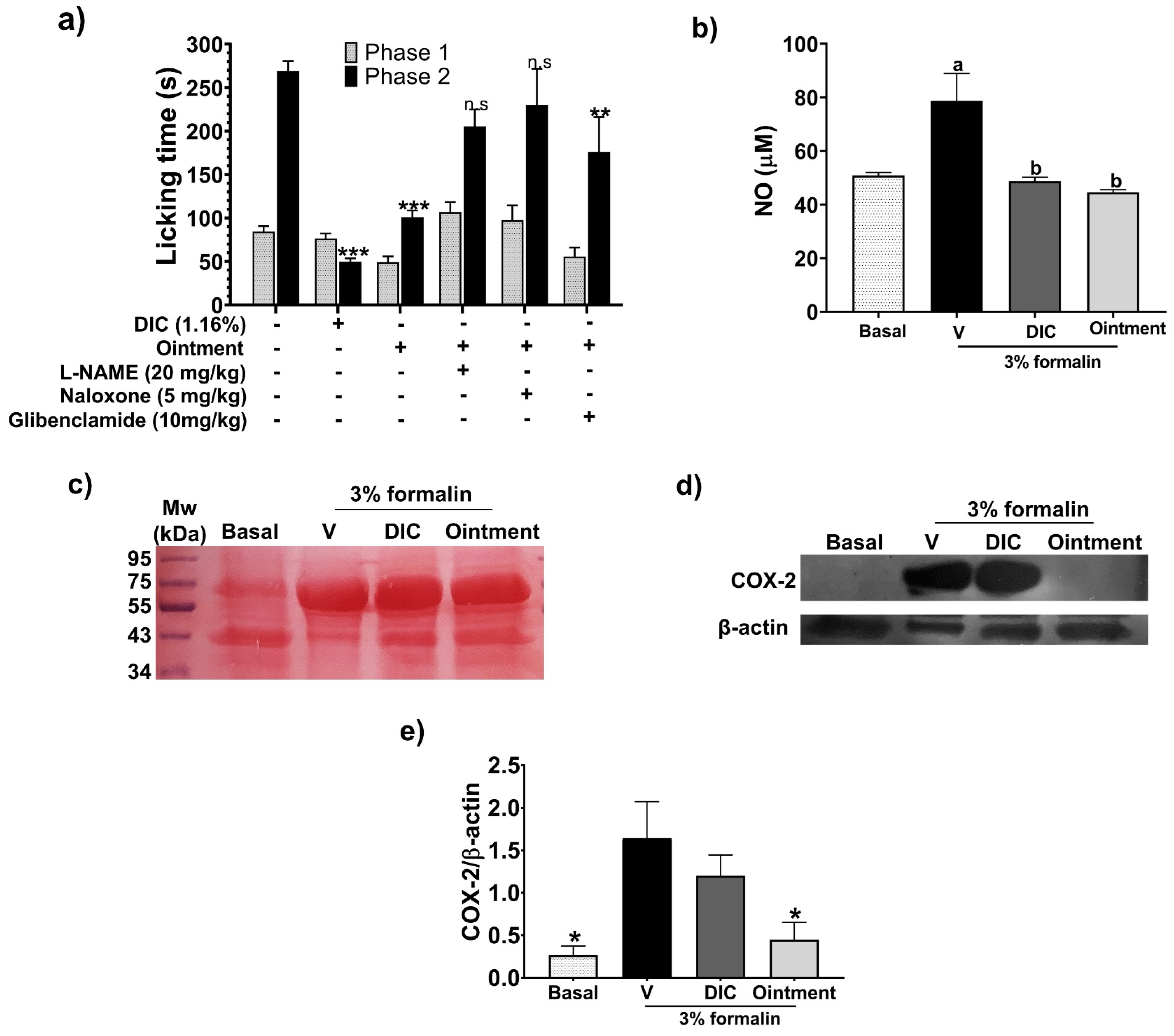
2.9. Formalin Test (FT)
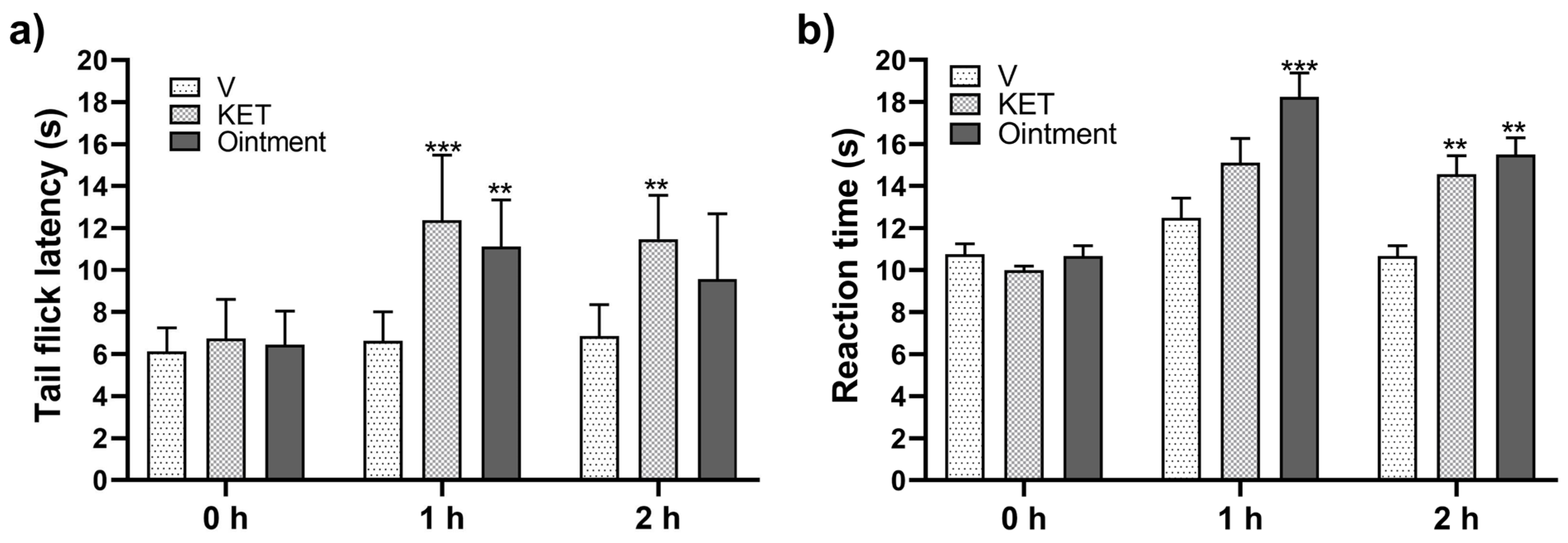
2.10. Tail Flick Test (TFT)
2.11. Hot Plate Test (HPT)
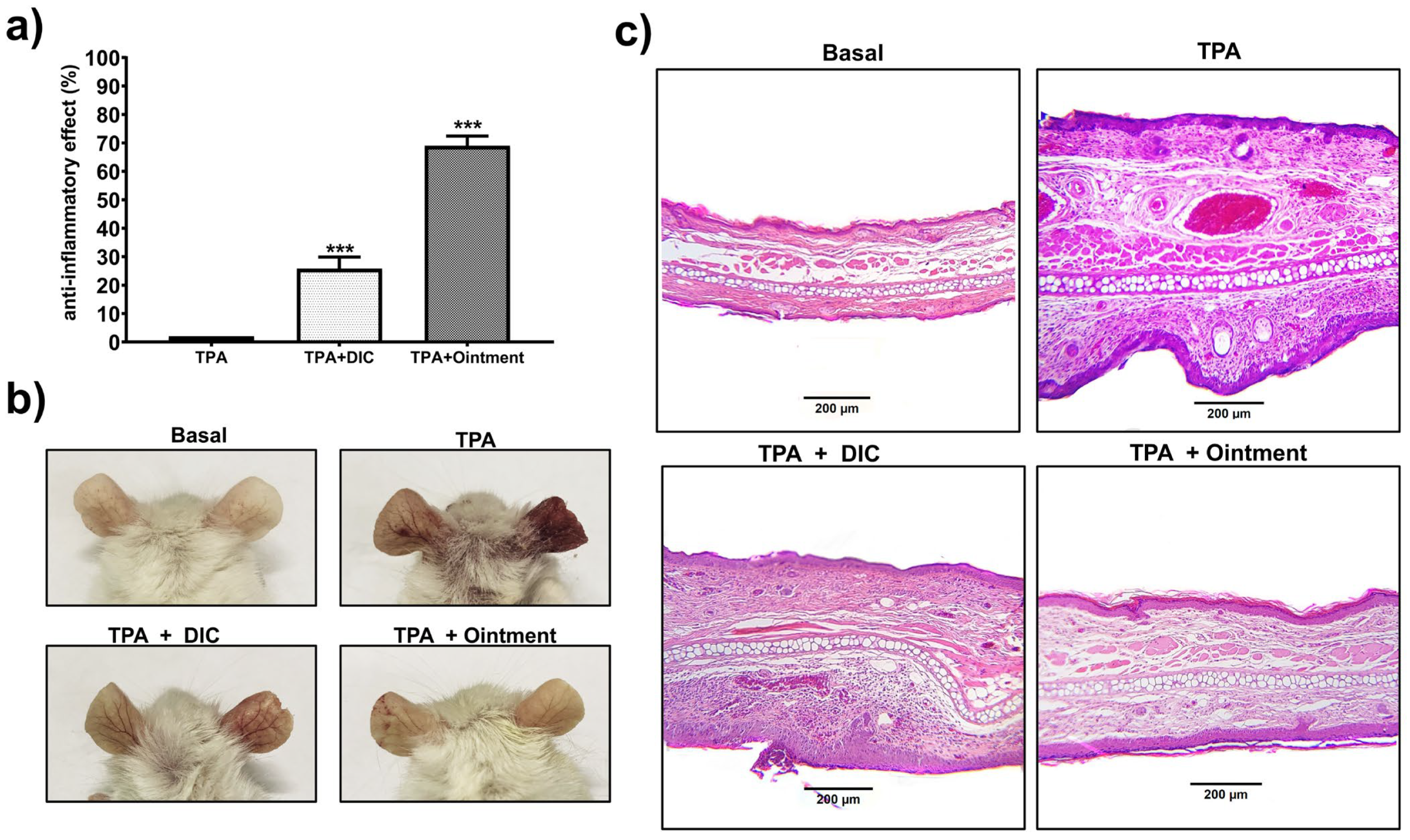
2.12. TPA-Induced Acute and Chronic Mouse Ear Edema
2.13. Tissue Homogenization
2.14. Protein Quantification by Bradford Assay
2.15. Enzyme-Linked Immunosorbent Assay (ELISA)
2.16. Myeloperoxidase (MPO) Assay
2.17. Nitric Oxide (NO) Estimation
2.18. Western Blot Analysis
2.19. In-Silico Study, Computational Details
2.20. Statistical Analysis
3. Results
3.1. Chemical Composition of the Ointment
3.2. Organoleptic and Physicochemical Characterization
3.3. Skin Irritation Test
3.4. Antinociceptive Response
3.5. Protective Effects of the Ointment on Acute and Chronic TPA-Induced Ear Edema
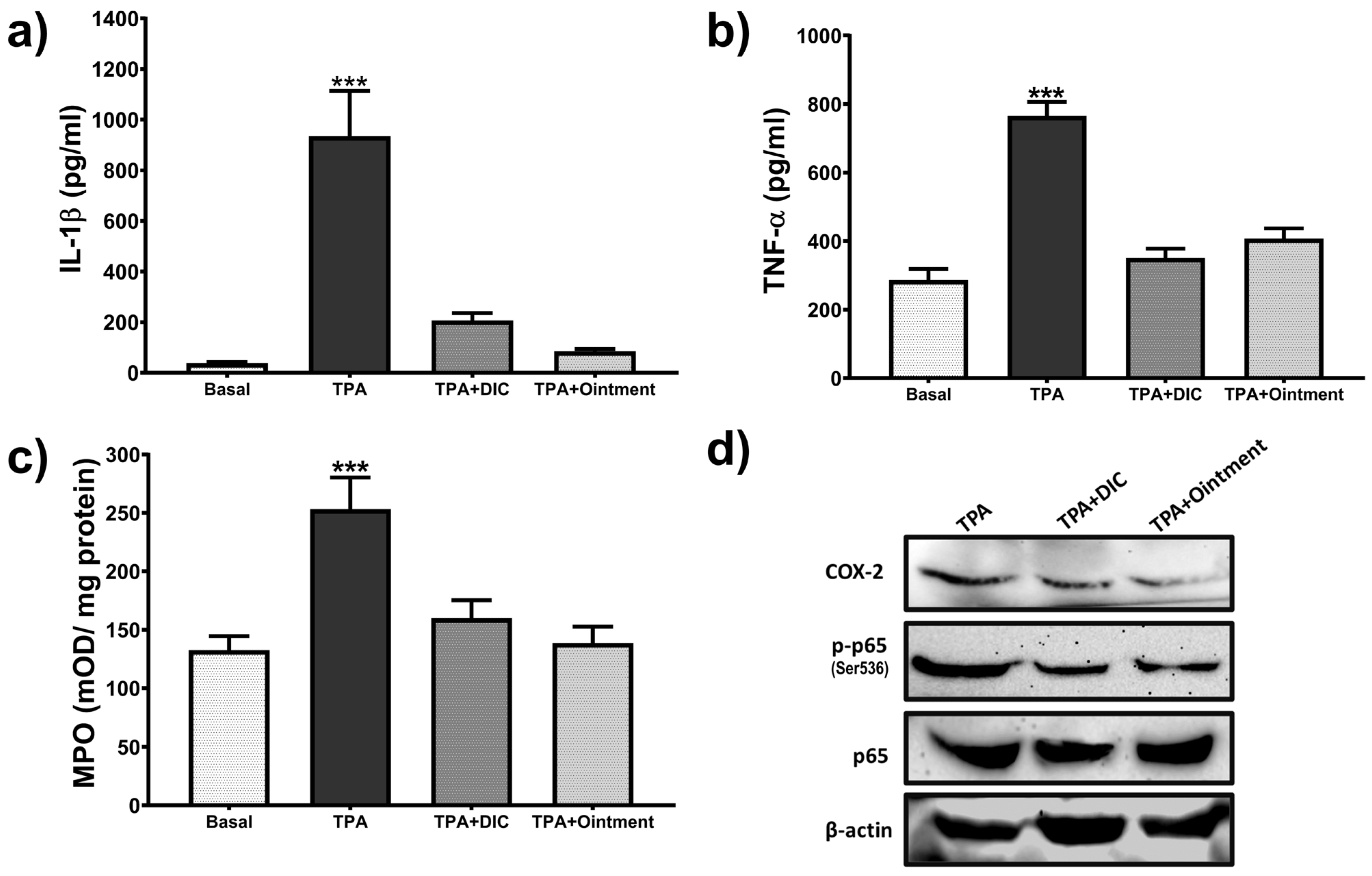
3.6. Effect of the Ointment on Inflammatory Mediators
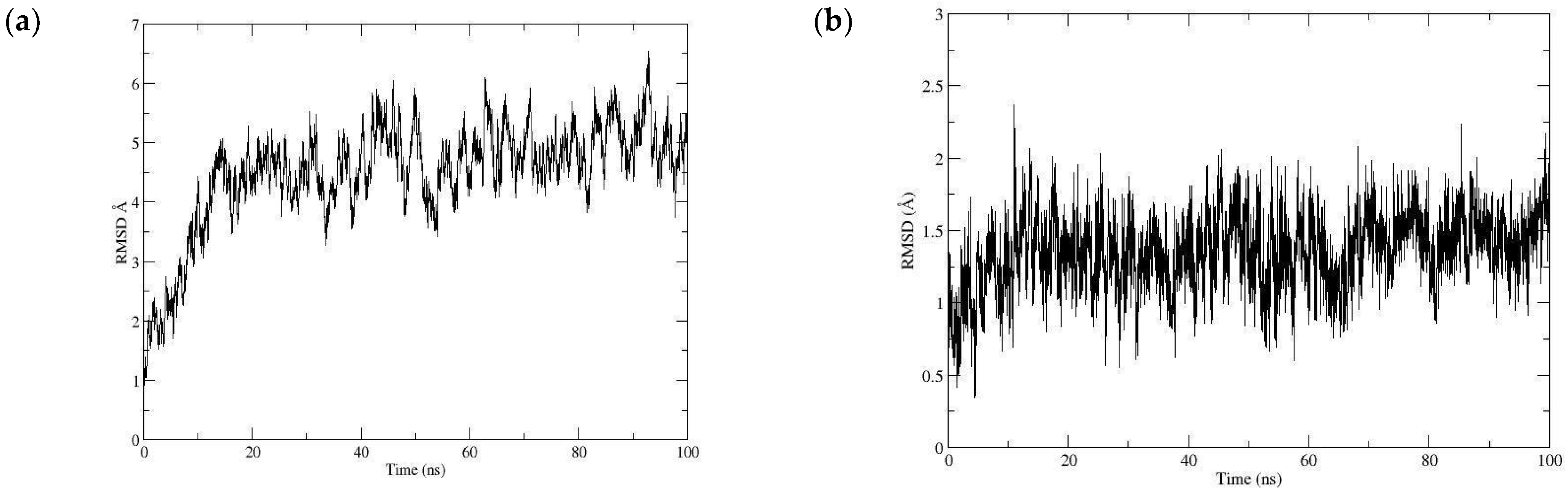
3.7. Docking Study
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. Side Effects of Commonly Prescribed Analgesic Medications. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Scheidt, C.; Otuki, M.; Santos, A.R. Biological Activity of Plant Extracts: Novel Analgesic Drugs. Expert Opin. Emerg. Drugs 2001, 6, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chandra, H. Suppression of Inflammatory and Infection Responses in Lung Macrophages by Eucalyptus Oil and Its Constituent 1,8-Cineole: Role of Pattern Recognition Receptors TREM-1 and NLRP3, the MAP Kinase Regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, J.; Kim, M.S.; Seol, G.H.; Min, S.S. Analgesic Effects of Eucalyptus Essential Oil in Mice. Korean J. Pain 2019, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis virginiana Bark in Human Keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef]
- Gu, D.; Wang, H.; Yan, M.; Li, Y.; Yang, S.; Shi, D.; Guo, S.; Wu, L.; Liu, C. Echinacea purpurea (L.) Moench Extract Suppresses Inflammation by Inhibition of C3a/C3aR Signaling Pathway in TNBS-Induced Ulcerative Colitis Rats. J. Ethnopharmacol. 2023, 307, 116221. [Google Scholar] [CrossRef]
- Abdi, T.; Mahmoudabady, M.; Marzouni, H.Z.; Niazmand, S.; Khazaei, M. Ginger (Zingiber Officinale Roscoe) Extract Protects the Heart Against Inflammation and Fibrosis in Diabetic Rats. Can. J. Diabetes 2021, 45, 220–227. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Alba-Betancourt, C.; Yáñez-Barrientos, E.; Luna-Rocha, C.; Páramo-Castillo, A.S.; Aragón-Martínez, O.H.; Zapata-Morales, J.R.; Cruz-Jiménez, G.; Gasca-Martínez, D.; González-Ibarra, A.A.; et al. Diuretic Activity and Neuropharmacological Effects of an Ethanol Extract from Senna septemtrionalis (Viv.) H.S. Irwin & Barneby (Fabaceae). J. Ethnopharmacol. 2019, 239, 111923. [Google Scholar] [CrossRef]
- NORMA Oficial Mexicana NOM-073-SSA1-2005 (Mexican Official Standard NOM-073-SSA1-2005); Estabilidad de Fármacos y Medicamentos (Stability of Drugs and Medications); Secretaría de Salud de México: Mexico City, Mexico. 2005. Available online: https://salud.gob.mx/unidades/cdi/nom/073ssa105.html (accessed on 3 June 2024).
- OECD. Guideline for Testing of Chemicals: Draft Updated Test Guideline 434 on Acute Dermal Toxicity; OECD: Paris, France, 2015; pp. 1–12. [Google Scholar]
- Hunskaar, S.; Hole, K. The Formalin Test in Mice: Dissociation between Inflammatory and Non-Inflammatory. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Serrano-Vega, R.; Pérez Gutiérrez, S.; Isiordia-Espinoza, M.A.; Solorio-Alvarado, C.R. Myristic acid reduces skin inflammation and nociception. J. Food Biochem. 2022, 46, e14013. [Google Scholar] [CrossRef]
- Minett, M.S.; Quick, K.; Wood, J.N. Behavioral Measures of Pain Thresholds. Curr. Protoc. Mouse Biol. 2011, 1, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Pinardi, G.; Sierralta, F.; Miranda, H.F. Adrenergic Mechanisms in Antinociceptive Effects of Non Steroidal Anti-Inflammatory Drugs in Acute Thermal Nociception in Mice. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2002, 51, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.A. Chapter 8—Analgesics. In Screening Methods in Pharmacology; Turner, R.A., Ed.; Academic Press: Cambridge, MA, USA, 1965; pp. 100–117. ISBN 978-1-4832-3266-9. [Google Scholar]
- De Young, L.M.; Kheifets, J.B.; Ballaron, S.J.; Young, J.M. Edema and Cell Infiltration in the Phorbol Ester-Treated Mouse Ear Are Temporally Separate and Can Be Differentially Modulated by Pharmacologic Agents. Agents Actions 1989, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse Skin Inflammation Induced by Multiple Topical Applications of 12-O-Tetradecanoylphorbol-13-Acetate. Skin Pharmacol. 1991, 4, 262–271. [Google Scholar] [CrossRef]
- González-Velasco, H.E.; Pérez-Gutiérrez, M.S.; Alonso-Castro, Á.J.; Zapata-Morales, J.R.; Niño-Moreno, P.D.C.; Campos-Xolalpa, N.; González-Chávez, M.M. Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes parryi A. Gray (Asteraceae) and Verbenone. Molecules 2022, 27, 2612. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay Method for Myeloperoxidase in Human Polymorphonuclear Leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Spartan’20, Version 20.1.3; Wavefunction, Inc.: Irvine, CA, USA; Q-CHEM: Pleasanton, CA, USA, 2020.
- Frisch, M.E.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Visualizer; Accelrys Software Inc.: San Diego, CA, USA, 2019. [Google Scholar]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hitzenberger, M.; Rieger, M.; Kern, N.R.; Zacharias, M.; Im, W. CHARMM-GUI supports the Amber force fields. J. Chem. Phys. 2020, 153, 035103. [Google Scholar] [CrossRef]
- Vanommeslaeghe, E.; Hatcher, C.; Acharya, S.; Kundu, S.; Zhong, J.; Shim, E.; Darian, O.; Guvench, P.; Lopes, I.; Vorobyov, A.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Jacob, J.J.; Ramabadran, K. Enhancement of a Nociceptive Reaction by Opioid Antagonists in Mice. Br. J. Pharmacol. 1978, 64, 91–98. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent Development on COX-2 Inhibitors as Promising Anti-Inflammatory Agents: The Past 10 Years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of Autophagy by Polyphenolic Compounds as a Potential Therapeutic Strategy for Cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.J. The Development of COX2 Inhibitors. Nat. Rev. Drug Discov. 2003, 2, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Afrin, R.; Arumugam, S.; Rahman, A.; Wahed, M.I.I.; Karuppagounder, V.; Harima, M.; Suzuki, H.; Miyashita, S.; Suzuki, K.; Yoneyama, H. Curcumin Ameliorates Liver Damage and Progression of NASH in NASH-HCC Mouse Model Possibly by Modulating HMGB1-NF-ΚB Translocation. Int. Immunopharmacol. 2017, 44, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhou, H.; Wang, Y.; Gurley, E.C.; Feng, B.; Chen, L.; Xiao, J.; Yang, S.; Li, X. Inhibition of LPS-induced Production of Inflammatory Factors in the Macrophages by Mono-carbonyl Analogues of Curcumin. J. Cell. Mol. Med. 2009, 13, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Alexa, I.-D.; Ilie, A.C.; Prada, G.; Herghelegiu, A.M.; Luca, A.; Rotaru, T.S.; Dondaș, A.; Rusu-Zota, G.; Alexa-Stratulat, T.; Bohotin, C.-R. A Comprehensive Behavioural Assessment of Curcumin’s Effect on Inflammatory and Non-Inflammatory Pain in Mice. Farmacia 2020, 68, 829–834. [Google Scholar] [CrossRef]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Niknejad, A.H.; Nouri, K.; Momtaz, S.; Gumpricht, E.; Jamialahmadi, T.; Abdolghaffari, A.H.; Barreto, G.E.; et al. Protective Effects of Curcumin against Traumatic Brain Injury. Biomed. Pharmacother. 2022, 154, 113621. [Google Scholar] [CrossRef]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea Purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Baugé, C.; Lhuissier, E.; Girard, N.; Quesnelle, C.; Ewert, G.; Boumediene, K. Anti-Inflammatory Effects of an Injectable Copolymer of Fatty Acids (Ara 3000 Beta®) in Joint Diseases. J. Inflamm. 2015, 12, 17. [Google Scholar] [CrossRef][Green Version]
- Toupet, K.; Jorgensen, C.; Noël, D. An Injectable Copolymer of Fatty Acids (ARA 3000 BETA) as a Promising Treatment for Osteoarthritis. Sci. Rep. 2023, 13, 7783. [Google Scholar] [CrossRef]
- Charlet, R.; Le Danvic, C.; Sendid, B.; Nagnan-Le Meillour, P.; Jawhara, S. Oleic Acid and Palmitic Acid from Bacteroides Thetaiotaomicron and Lactobacillus Johnsonii Exhibit Anti-Inflammatory and Antifungal Properties. Microorganisms 2022, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Prasath, K.G.; Alexpandi, R.; Parasuraman, R.; Pavithra, M.; Ravi, A.V.; Pandian, S.K. Anti-Inflammatory Potential of Myristic Acid and Palmitic Acid Synergism against Systemic Candidiasis in Danio Rerio (Zebrafish). Biomed. Pharmacother. 2021, 133, 111043. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.-H.; Lin, S.-Y.; Ou, Y.-C.; Chen, W.-Y.; Chuang, Y.-H.; Yen, Y.-J.; Liao, S.-L.; Raung, S.-L.; Chen, C.-J. Stearic Acid Attenuates Cholestasis-Induced Liver Injury. Biochem. Biophys. Res. Commun. 2010, 391, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, A.; Ramachandran, J.; Lakshmanan, D.K.; Ravichandran, G.; Thilagar, S. Ethnomedical, Phytochemical and Pharmacological Insights on an Indian Medicinal Plant: The Balloon Vine (Cardiospermum Halicacabum Linn.). J. Ethnopharmacol. 2022, 291, 115143. [Google Scholar] [CrossRef] [PubMed]
- Acid, L. Final Report on the Safety Assessment of Oleic Acid, Laurie Acid, Palmitic Acid, Myristic Acid, and Stearic Acid. J. Am. Coll. Toxicol 1987, 6, 321–401. [Google Scholar]
- Famurewa, A.C.; Aja, P.M.; Maduagwuna, E.K.; Ekeleme-Egedigwe, C.A.; Ufebe, O.G.; Azubuike-Osu, S.O. Antioxidant and Anti-Inflammatory Effects of Virgin Coconut Oil Supplementation Abrogate Acute Chemotherapy Oxidative Nephrotoxicity Induced by Anticancer Drug Methotrexate in Rats. Biomed. Pharmacother. 2017, 96, 905–911. [Google Scholar] [CrossRef]
- Barda, C.; Grafakou, M.-E.; Kalpoutzakis, E.; Heilmann, J.; Skaltsa, H. Chemical Composition of Crepis foetida L. and C. rubra L. Volatile Constituents and Evaluation of the in Vitro Anti-Inflammatory Activity of Salicylaldehyde Rich Volatile Fraction. Biochem. Syst. Ecol. 2021, 96, 104256. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Wakode, S.R.; Ali, M. Chemical Composition, Antimicrobial and Topical Anti-Inflammatory Activity of Essential Oil of Amomum Subulatum Fruits. Acta Pol. Pharm. 2012, 69, 1177–1181. [Google Scholar] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.L.; Kobilka, B.K.; Granier, S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McCurdy, C.R.; Metzger, T.G.; Portoghese, P.S. Specific cross-linking of Lys233 and Cys235 in the mu opioid receptor by a reporter affinity label. Biochemistry 2005, 44, 2271–2275. [Google Scholar] [CrossRef]
- Kaserer, T.; Lantero, A.; Schmidhammer, H.; Spetea, M.; Schuster, D. μ Opioid receptor: Novel antagonists and structural modeling. Sci. Rep. 2016, 6, 21548. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sessa, W.C. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J. Biol. Chem. 1994, 269, 11691–11694. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Jia, Y.; Fukuyama, J.; Switzer, C.H.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J. Biol. Chem. 2007, 282, 15404–15415. [Google Scholar] [CrossRef]
- Khalil, A.S.M.; Giribabu, N.; Yelumalai, S.; Shahzad, H.; Kilari, E.K.; Salleh, N. Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci. 2021, 278, 119605. [Google Scholar] [CrossRef]
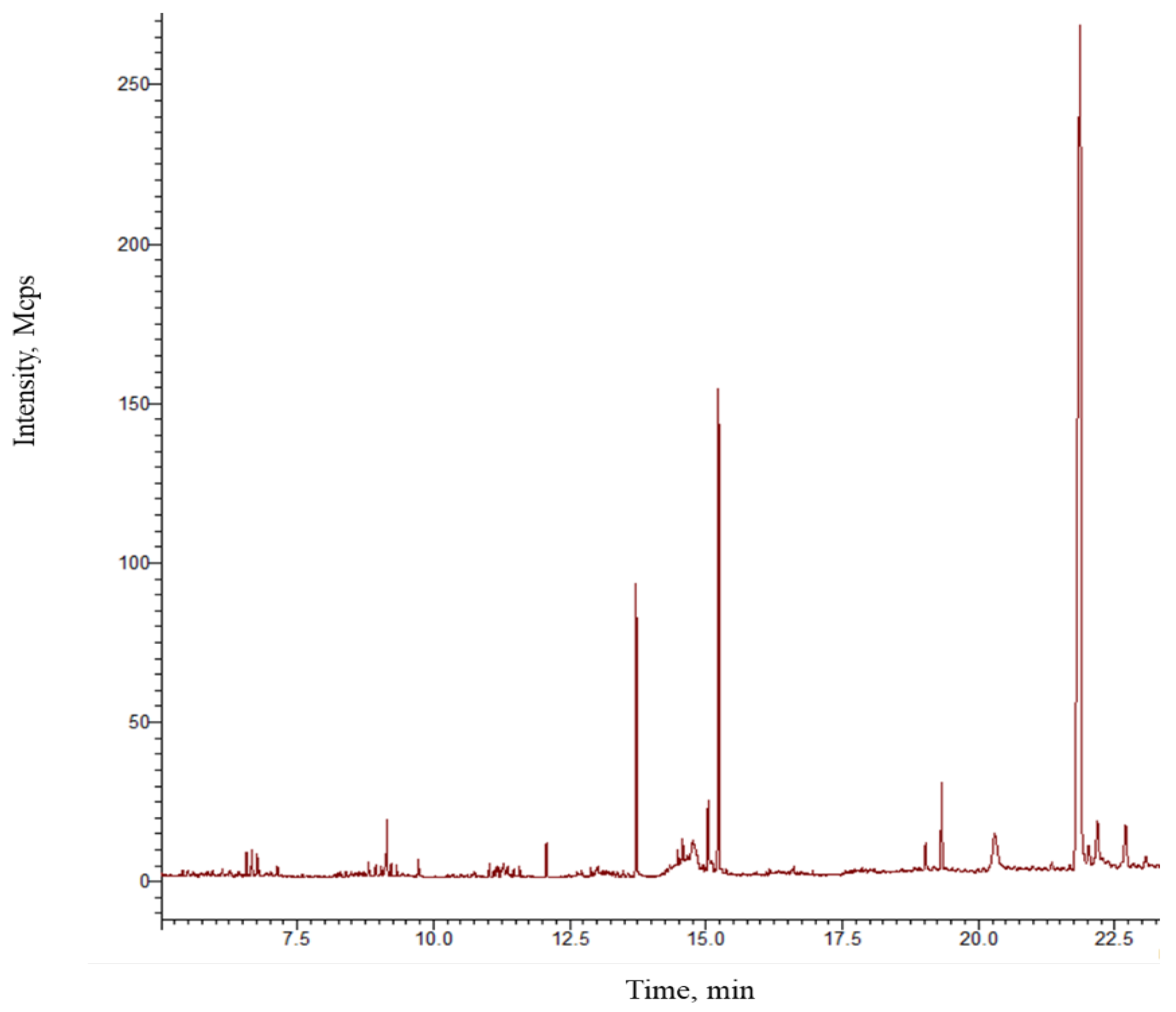





| No. | Compound | * RT | TRI | ERI | %RE |
|---|---|---|---|---|---|
| 1 | 1-Ethoxy-1-(trimethylsiloxy)octane | 7.59 | 1311 | 1269 | 3.20 |
| 2 | 5-(Cyclohexylmethyl)-2-pyrrolidinone | 8.64 | 1484 | 1485 | 0.07 |
| 3 | 3,4-Dimethylbenzoic acid, trimethylsilyl ester | 8.68 | 1493 | 1489 | 0.22 |
| 4 | Trimethyl-[2-[2-(2-trimethylsilyloxyethoxy)ethoxy]ethoxy]silane | 8.72 | 1489 | 1493 | 0.27 |
| 5 | α-[3′-(Trifluoromethyl)benzyl]-γ-butyrolactone | 8.81 | 1507 | 1501 | 0.40 |
| 6 | Ethisolide | 8.94 | 1540 | 1514 | 1.69 |
| 7 | 2-Hexyldecanol | 9.21 | 1504 | 1540 | 2.39 |
| 8 | Ethyl phthalate | 9.72 | 1578 | 1588 | 0.63 |
| 9 | 2-Methyl-6-(4-methylphenyl)-2-hepten-4-one | 10.48 | 1660 | 1667 | 0.42 |
| 10 | 2-Methylhexadecane | 10.69 | 1664 | 1689 | 1.50 |
| 11 | Myristic acid, trimethylsilyl ester | 12.07 | 1842 | 1840 | 0.11 |
| 12 | n-Nonadecane | 12.63 | 1900 | 1906 | 0.32 |
| 13 | D-Mannitol, 1,2,3,4,5,6-hexakis-O-(trimethylsilyl)- | 12.71 | 1979 | 1916 | 3.18 |
| 14 | n-Pentanoic acid, trimethylsilyl ester | 12.90 | 1942 | 1939 | 0.15 |
| 15 | Sulfurous acid, butyl nonyl ester | 12.99 | 1937 | 1948 | 0.57 |
| 16 | 1-Trimethylsiloxyhexadecane | 10.01 | 1965 | 1951 | 0.71 |
| 17 | Palmitic acid, trimethylsilyl ester | 13.71 | 2039 | 2022 | 0.83 |
| 18 | 2-Hydroxy-2-methyl-N-phenyl-1,4-dioxane-3-carboxamide | 14.32 | 2043 | 2071 | 1.37 |
| 19 | Trimethylsilyl heptadecanoate | 14.40 | 2087 | 2083 | 0.19 |
| 20 | n-Heneicosane | 14.64 | 2100 | 2096 | 0.19 |
| 21 | Docos-1-ene | 14.94 | 2192 | 2187 | 0.23 |
| 22 | cis-9-Octadecenoic acid, trimethylsilyl ester | 15.03 | 2208 | 2217 | 0.41 |
| 23 | Stearic acid, trimethylsilyl ester | 15.20 | 2236 | 2284 | 2.15 |
| 24 | 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene | 19.02 | 3548 | 3587 | 1.10 |
| 25 | Methyl 9-desoxomesopyrochlorophllide | 19.32 | 3698 | 3690 | 0.22 |
| 26 | 3β-ACETOXY-CHOLEST-6-ENO-[7,6-D]-2′-PHENYLOXAZOL | 21.89 | 4598 | 4573 | 0.54 |
| Protein | Ligand | Affinity Energy (kcal/mol) | Type of Interactions |
|---|---|---|---|
| 4DKL Morphine (reference drug) −10.56 kcal/mol | Stearic acid | −6.13 | π-sigma Trp293, His297, Tyr226 Alkyl/pi-alkyl: Met151, Ile296, Ile322, Val300 |
| Palmitic acid | −5.60 | H-bond: TYR148 π-Sigma: Trp293 Alkyl/pi-alkyl: Met151, Val236, Ile296, His297, Val300 | |
| Valeric acid | −3.26 | H-bond: TYR148 Alkyl/pi-alkyl: Val236, His297, Val300 | |
| Myristic acid | −5.61 | H-bond: Lys233 π-Sigma: Tyr326 Alkyl/pi-alkyl: Met151, Trp293, Ile296, His297, Val300, Ile322 |
| Protein | Ligand | Binding Energy (kcal/mol) | Interactions |
|---|---|---|---|
| 4NOS Ethilisothiourea (referencia) −4.75 kcal/mol | Stearic acid | −6.69 | H-bond: Tyr347 Alkyl/π-Alkil: Pro350, Val352, Phe369, Tyr372, HEM510 |
| Palmitic acid | −6.60 | H-bond: Tyr347, Ala351 Alkyl/π-Alkil: Pro350, Val352, HEM510 | |
| Valeric acid | −3.92 | H-bond: Phe369 Alkyl/π-Alkil: Pro350, HEM510 | |
| Myristic acid | −6.34 | H-bond: Tyr347, Arg488 Alkyl/π-Alkil: Pro350, Val352, Phe369, Tyr373, HEM510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragan-Galvez, J.C.; Gonzalez-Rivera, M.L.; Jiménez-Cruz, J.C.; Hernandez-Flores, A.; de la Rosa, G.; Lopez-Moreno, M.L.; Yañez-Barrientos, E.; Romero-Hernández, M.; Deveze-Alvarez, M.A.; Navarro-Santos, P.; et al. A Patent-Pending Ointment Containing Extracts of Five Different Plants Showed Antinociceptive and Anti-Inflammatory Mechanisms in Preclinical Studies. Pharmaceutics 2024, 16, 1215. https://doi.org/10.3390/pharmaceutics16091215
Barragan-Galvez JC, Gonzalez-Rivera ML, Jiménez-Cruz JC, Hernandez-Flores A, de la Rosa G, Lopez-Moreno ML, Yañez-Barrientos E, Romero-Hernández M, Deveze-Alvarez MA, Navarro-Santos P, et al. A Patent-Pending Ointment Containing Extracts of Five Different Plants Showed Antinociceptive and Anti-Inflammatory Mechanisms in Preclinical Studies. Pharmaceutics. 2024; 16(9):1215. https://doi.org/10.3390/pharmaceutics16091215
Chicago/Turabian StyleBarragan-Galvez, Juan Carlos, Maria Leonor Gonzalez-Rivera, Juan C. Jiménez-Cruz, Araceli Hernandez-Flores, Guadalupe de la Rosa, Martha L. Lopez-Moreno, Eunice Yañez-Barrientos, Michelle Romero-Hernández, Martha Alicia Deveze-Alvarez, Pedro Navarro-Santos, and et al. 2024. "A Patent-Pending Ointment Containing Extracts of Five Different Plants Showed Antinociceptive and Anti-Inflammatory Mechanisms in Preclinical Studies" Pharmaceutics 16, no. 9: 1215. https://doi.org/10.3390/pharmaceutics16091215
APA StyleBarragan-Galvez, J. C., Gonzalez-Rivera, M. L., Jiménez-Cruz, J. C., Hernandez-Flores, A., de la Rosa, G., Lopez-Moreno, M. L., Yañez-Barrientos, E., Romero-Hernández, M., Deveze-Alvarez, M. A., Navarro-Santos, P., Acosta-Mata, C., Isiordia-Espinoza, M. A., & Alonso-Castro, A. J. (2024). A Patent-Pending Ointment Containing Extracts of Five Different Plants Showed Antinociceptive and Anti-Inflammatory Mechanisms in Preclinical Studies. Pharmaceutics, 16(9), 1215. https://doi.org/10.3390/pharmaceutics16091215









