Evaluation of Biologics ACE2/Ang(1–7) Encapsulated in Plant Cells for FDA Approval: Safety and Toxicology Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry, Manufacturing, and Control Studies for CTB-ACE2 and CTB-Ang(1–7) Drug Substances (DSs) and Drug Product (DP)—ACE2 Gum
2.1.1. Intactness Assessment of CTB-ACE2 and CTB-Ang(1–7) Drug Substances
2.1.2. Stability Assessment of CTB-ACE2 and CTB-Ang(1–7) DSs and DP (ACE2 Gum)
2.1.3. Bioburden Assessment
2.1.4. Moisture Content Analysis by Karl Fisher Coulometry
2.1.5. Content Uniformity Testing for ACE2 Gum
2.1.6. Potency Study for ACE2 Gum Using Pseudo-Virus Neutralization Assays
2.2. Preclinical Toxicology Assessment
2.2.1. Animals and Husbandry
| Species: | Rat |
| Strain: | Crl:CD(SD) Sprague Dawley rat |
| Condition: | Purpose-bred, naive |
| Source: | Charles River Kingston, Stone Ridge, NY |
| Number of Males: | 81 (plus at least 8 alternates) |
| Number of Females: | 81 (plus at least 8 alternates) |
| Age at the Initiation of Dosing: | 6 to 9 weeks |
| Weight at the Initiation of Dosing: | 175 to 350 g (males)/175 to 250 g (females) |
2.2.2. Preparation of CTB-ACE2 and CTB-Ang(1–7) Dose Formulations for Oral Gavage
2.2.3. Toxicokinetic Assessment of CTB-ACE2 and CTB-Ang(1–7)
2.3. Parametric/Non-Parametric
3. Results
3.1. Chemistry, Manufacturing, and Control (CMC) Data for CTB-ACE2 and CTB-Ang(1–7) Drug Substances (DSs) and Drug Product (DP)—ACE2 Gum
3.2. Stability Assessment of CTB-ACE2 and CTB-Ang(1–7) Drug Substances
3.3. Intactness Assessment of Plant Cells Expressing Protein Drugs
3.4. Bioburden and Moisture Content as per USP Guidelines
3.5. Release Criteria for ACE2 Gum
3.6. In Vivo Safety Assessment of CTB-ACE2 and CTB-Ang(1–7)
3.6.1. Clinical Observations
3.6.2. Toxicokinetics
3.6.3. Gross Pathology
3.6.4. Histopathology
3.6.5. Ophthalmology
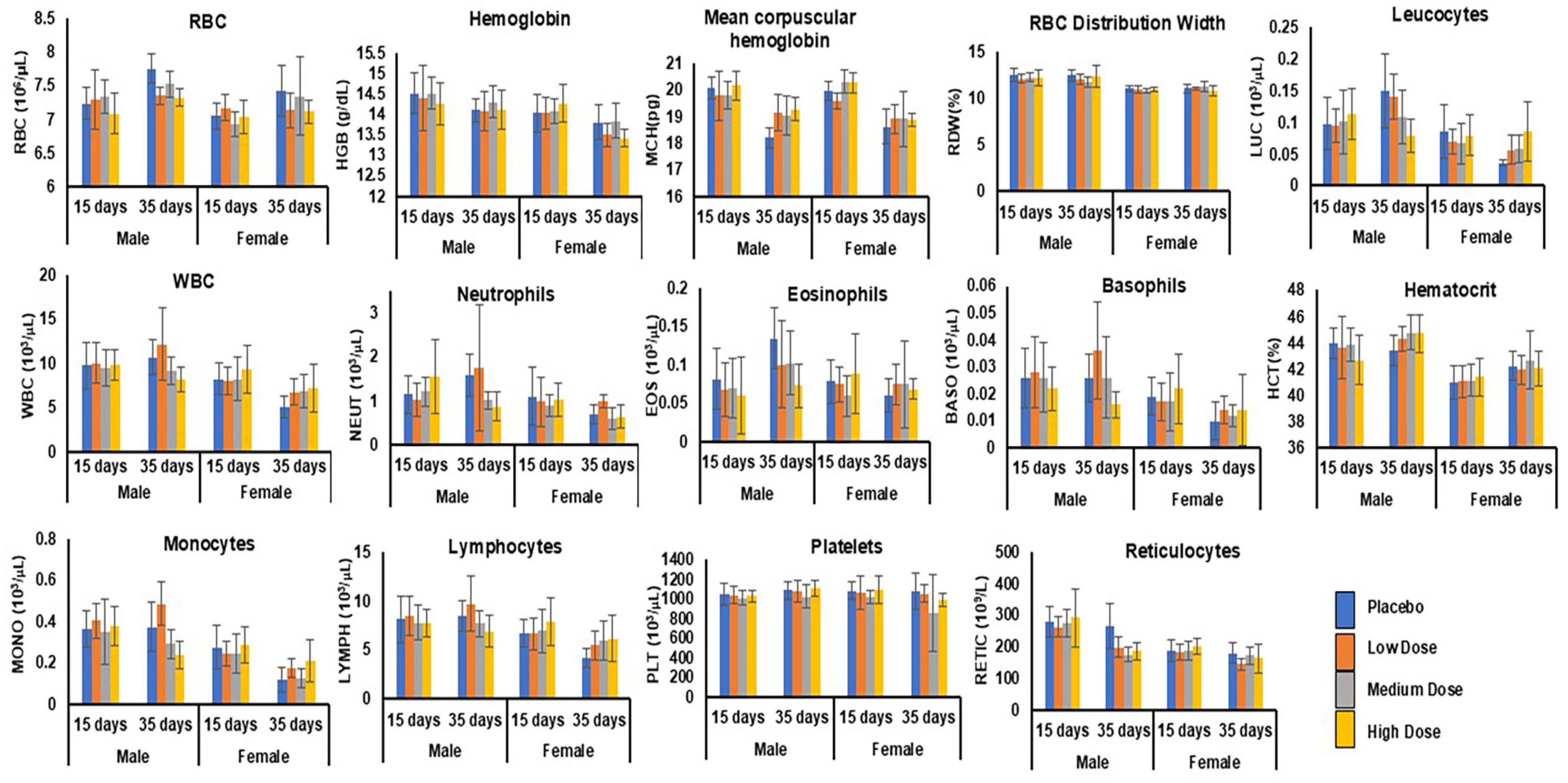
3.6.6. Hematology
3.6.7. Clinical Chemistry
Liver Function Test
Renal Function Test
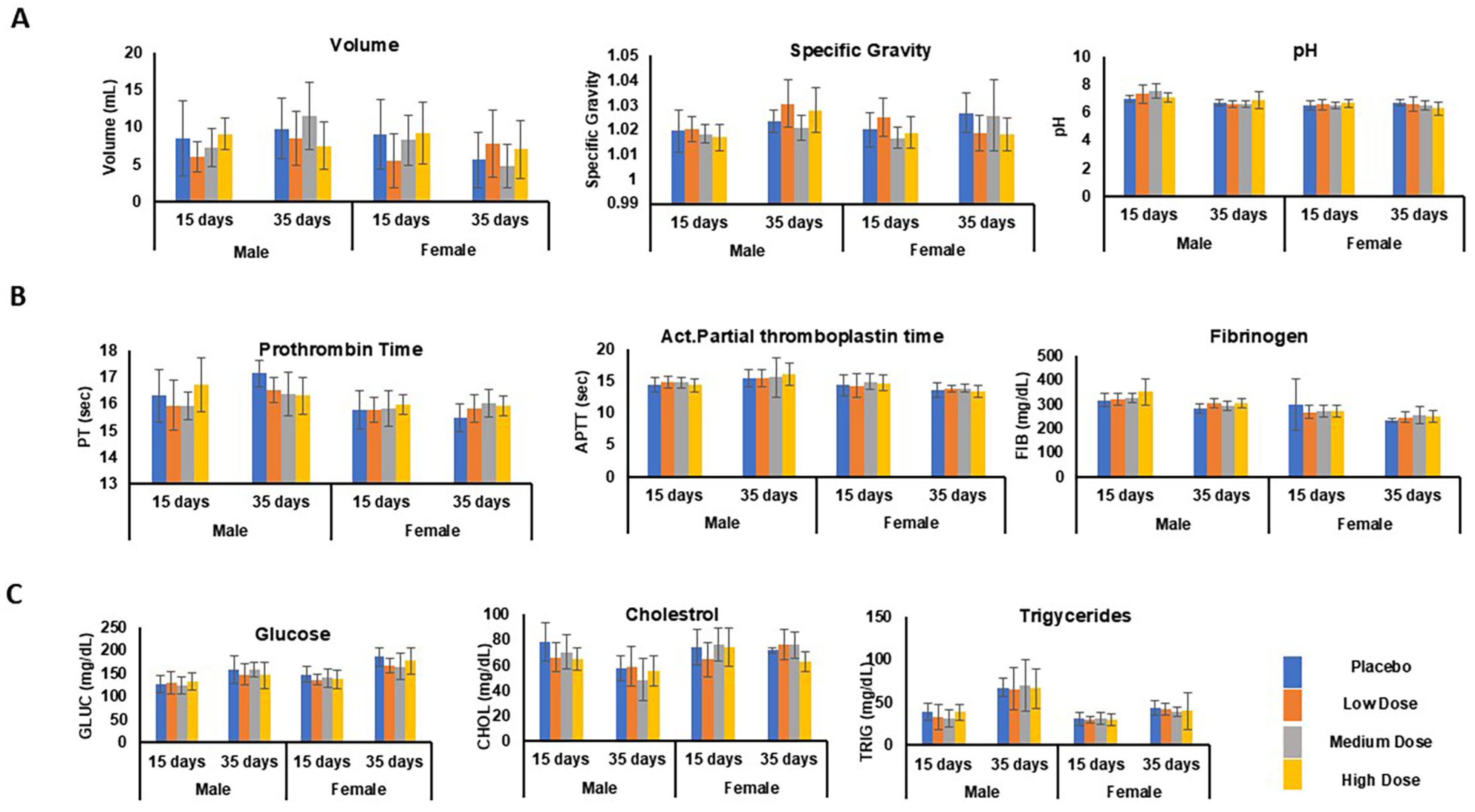
3.6.8. Urinalysis
3.6.9. Coagulation
3.6.10. Metabolic Syndrome Tests
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Global Protein Drugs Market (2021 to 2026)—Featuring Abbvie, Amgen and CSL Among Others. Available online: https://www.businesswire.com/news/home/20210824005462/en/Global-Protein-Drugs-Market-2021-to-2026---Featuring-Abbvie-Amgen-and-CSL-Among-Others---ResearchAndMarkets.com (accessed on 23 April 2023).
- Kintzing, J.R.; Interrante, M.V.F.; Cochran, J.R. Emerging strategies for developing next-generation protein therapeutics for cancer treatment. Trends Pharmacol. Sci. 2016, 37, 993–1008. [Google Scholar] [CrossRef]
- Daniell, H.; Kulchar, R.J.; Herzog, R.W.; Kulis, M.; Leong, K.W. Plant cell-based drug delivery enhances affordability of biologics. Nat. Biotechnol. 2023, 41, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Kulchar, R.J.; Singh, R.; Ding, S.; Alexander, E.; Leong, K.W.; Daniell, H. Delivery of biologics: Topical administration. Biomaterials 2023, 302, 122312. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Martinez, R.; Festini, T.; Peris, J.E. Subcutaneous injection of drugs: Literature review of factors influencing pain sensation at the injection site. Adv. Ther. 2019, 36, 2986–2996. [Google Scholar] [CrossRef]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.S.; Sánchez-Félix, M.; Badkar, A.V.; Mrsny, R. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J. Control. Release 2020, 321, 475–482. [Google Scholar] [CrossRef]
- Jing, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Xiu, P.Z.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N.; et al. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol. Ther. 2022, 30, 1966–1978. [Google Scholar] [CrossRef]
- Greenhawt, M.; Sindher, S.B.; Wang, J.; O’Sullivan, M.; Du Toit, G.; Kim, E.H.; Albright, D.; Anvari, S.; Arends, N.; Arkwright, P.D.; et al. Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy. N. Engl. J. Med. 2023, 388, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Söderling, E.; Pienihäkkinen, K. Effects of xylitol chewing gum and candies on the accumulation of dental plaque: A systematic review. Clin. Oral Investig. 2022, 26, 119–129. [Google Scholar] [CrossRef]
- Round, E.K.; Chen, P.; Taylor, A.K.; Schmidt, E. Biomarkers of tobacco exposure decrease after smokers switch to an e-cigarette or nicotine gum. Nicotine Tob. Res. 2019, 21, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Rogers, S.; Franzen, A.; Gentry, R. A critical review of the literature to conduct a toxicity assessment for oral exposure to methyl salicylate. Crit. Rev. Toxicol. 2017, 47, 98–120. [Google Scholar] [CrossRef]
- Ryan, E.J.; Kim, C.H.; Fickes, E.J.; Williamson, M.; Muller, M.D.; Barkley, J.E.; Gunstad, J.; Glickman, E.L. Caffeine gum and cycling performance: A timing study. J. Strength Cond. Res. 2013, 27, 259–264. [Google Scholar] [CrossRef]
- Brown, R.; Sam, C.H.; Green, T.; Wood, S. Effect of GutsyGum(tm), A novel gum, on subjective ratings of gastro esophageal reflux following a refluxogenic meal. J. Diet. Suppl. 2015, 12, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Nair, S.K.; Guan, H.; Guo, Y.; Kulchar, R.J.; Torres, M.D.; Shahed-Al-Mahmud, M.; Wakade, G.; Liu, Y.M.; Marques, A.D.; et al. Debulking different Corona (SARS-CoV-2 delta, omicron, OC43) and Influenza (H1N1, H3N2) virus strains by plant viral trap proteins in chewing gums to decrease infection and transmission. Biomaterials 2022, 288, 121671. [Google Scholar] [CrossRef] [PubMed]
- Brian Chia, C.S. A review on the metabolism of 25 peptide drugs. Int. J. Pept. Res. Ther. 2021, 27, 1397–1418. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Karidi, K.; Bourganis, V.; Kontonikola, K.; Kammona, O.; Kiparissides, C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2015, 97, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, E.J.; Na, D.H. Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals. Pharmaceuticals 2022, 15, 1585. [Google Scholar] [CrossRef]
- Abramson, A.; Frederiksen, M.R.; Vegge, A.; Jensen, B.; Poulsen, M.; Mouridsen, B.; Jespersen, M.O.; Kirk, R.K.; Windum, J.; Hubálek, F.; et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol. 2022, 40, 103–109. [Google Scholar] [CrossRef]
- Brayden, D.J.; Hill, T.A.; Fairlie, D.P.; Maher, S.; Mrsny, R.J. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Vickery, B.P.; Hourihane, J.O.; Adelman, D.C. Oral Immunotherapy for Peanut Allergy. N. Engl. J. Med. 2019, 380, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Spergel, J.M.; Jones, S.M.; Rachid, R.; Assa’ad, A.H.; Wang, J.; Leonard, S.A.; Laubach, S.S.; Kim, E.H.; Vickery, B.P.; et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 476–485.e3. [Google Scholar] [CrossRef] [PubMed]
- Shaaltiel, Y.; Gingis-Velitski, S.; Tzaban, S.; Fiks, N.; Tekoah, Y.; Aviezer, D. Plant-based oral delivery of β-glucocerebrosidase as an enzyme replacement therapy for Gaucher’s disease. Plant Biotechnol. J. 2015, 13, 1033–1040. [Google Scholar] [CrossRef]
- Park, J.; Yan, G.; Kwon, K.C.; Liu, M.; Gonnella, P.A.; Yang, S.; Daniell, H. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 2020, 233, 119591. [Google Scholar] [CrossRef]
- Daniell, H.; Singh, R.; Mangu, V.; Nair, S.K.; Wakade, G.; Balashova, N. Affordable oral proinsulin bioencapsulated in plant cells regulates blood sugar levels similar to natural insulin. Biomaterials 2023, 298, 122142. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.; Kwon, K.C.; Rathinasabapathy, A.; Lin, S.; Jin, G.; Song, C.; Shil, P.; Nair, A.; Qi, Y.; Li, Q.; et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 2014, 64, 1248–1259. [Google Scholar] [CrossRef]
- Daniell, H.; Mangu, V.; Yakubov, B.; Park, J.; Habibi, P.; Shi, Y.; Gonnella, P.A.; Fisher, A.; Cook, T.; Zeng, L.; et al. Investigational new drug enabling angiotensin oral-delivery studies to attenuate pulmonary hypertension. Biomaterials 2020, 233, 119750. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.W.; Nichols, T.C.; Su, J.; Zhang, B.; Sherman, A.; Merricks, E.P.; Raymer, R.; Perrin, G.Q.; Häger, M.; Wiinberg, B.; et al. Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Herzog, R.W.; Khan, I.; Sherman, A.; Bertolini, T.; Wynn, T.; Daniell, H. Preclinical development of plant-based oral immune modulatory therapy for haemophilia B. Plant Biotechnol. J. 2021, 19, 1952–1966. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.P.; Wang, X.; Avuthu, N.; Bertolini, T.B.; Terhorst, C.; Guda, C.; Daniell, H.; Herzog, R.W. Role of Small Intestine and Gut Microbiome in Plant-Based Oral Tolerance for Hemophilia. Front. Immunol. 2020, 11, 844. [Google Scholar] [CrossRef]
- Xiao, Y.; Kwon, K.C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low-cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed]
- Rome, B.N.; Egilman, A.C.; Kesselheim, A.S. Trends in prescription drug launch prices, 2008-2021. JAMA 2022, 327, 2145–2147. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.C.; Daniell, H. Oral delivery of protein drugs bioencapsulated in plant cells. Mol. Ther. 2016, 24, 1342–1350. [Google Scholar] [CrossRef]
- Ganesan, P.K.; Kulchar, R.J.; Kaznica, P.; Montoya-Lopez, R.; Green, B.J.; Streatfield, S.J.; Daniell, H. Optimization of biomass and target protein yield for Phase III clinical trial to evaluate Angiotensin Converting Enzyme 2 expressed in lettuce chloroplasts to reduce SARS-CoV-2 infection and transmission. Plant Biotechnol. J. 2023, 21, 244–246. [Google Scholar] [CrossRef]
- Kwon, K.C.; Sherman, A.; Chang, W.J.; Kamesh, A.; Biswas, M.; Herzog, R.W.; Daniell, H. Expression and assembly of largest foreign protein in chloroplasts: Oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol. J. 2018, 16, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hejnaes, K.R.; Ransohoff, T.C. Biopharmaceutical Processing: Development, Design, and Implementation of Manufacturing Processes; Chemistry, manufacture and control; Elsevier Press: Amsterdam, The Netherlands, 2018; pp. 1105–1136. ISBN 978-0-08-100623-8. [Google Scholar]
- DiPaolo, M.; Locher, M. Biopharmaceuticals: CMC Development “Points to Consider” from a Regulatory Perspective. In Regulatory Toxicology; Springer: Cham, Switzerland, 2021; pp. 1275–1294. ISBN 978-3-030-57498-7. [Google Scholar]
- Su, J.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low-cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials 2015, 70, 84–93. [Google Scholar] [CrossRef]
- Herzog, R.W.; Sherman, A.; Kwon, K.C.; Chang, W.J.; Daniell, H. Lettuce Plants Expressing High Levels of Factor VIII Antigen in the Chloroplast for Oral Tolerance in Hemophilia a. Blood 2017, 130, 362. [Google Scholar]
- Lionte, C.; Bologa, C.; Sorodoc, V.; Petris, O.R.; Puha, G.; Stoica, A.; Ceasovschih, A.; Jaba, E.; Sorodoc, L. Biomarkers of inflammation and inflammation-related indexes upon emergency department admission are predictive for the risk of intensive care unit hospitalization and mortality in acute poisoning: A 6-year prospective observational study. Dis. Markers 2021, 19, 4696156. [Google Scholar] [CrossRef]
- Petterino, C.; Argentino-Storino, A. Clinical chemistry and hematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lillie, L.E.; Temple, N.J.; Florence, L.Z. Reference values for young normal Sprague-Dawley rats: Weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1996, 15, 612–616. [Google Scholar] [CrossRef]
- He, Q.; Su, G.; Liu, K.; Zhang, F.; Jiang, Y.; Gao, J.; Liu, L.; Jiang, Z.; Jin, M.; Xie, H. Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method. PLoS ONE 2017, 12, e0189837. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.A.; Do, H.N.; Song, Y.I.; Bae, R.J.; Lee, H.Y.; Park, S.H.; Kang, J.S.; Kang, J.K. Historical control data from 13-week repeated toxicity studies in Crj:CD (SD) rats. Lab. Anim. Res. 2012, 28, 115–121. [Google Scholar] [CrossRef]
- Jenkins, J.R. Rodent Diagnostic Testing. J. Exot. Pet Med. 2008, 17, 16–25. [Google Scholar] [CrossRef]
- Wohlauer, M.V.; Moore, E.E.; Harr, J.; Gonzalez, E.; Fragoso, M.; Silliman, C.C. A standardized technique for performing thrombelastography in rodents. Shock 2011, 36, 524. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 2015, 971453. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Gamboa-Gómez, C.I.; Aradillas-García, C.; Rodríguez-Morán, M.; Guerrero-Romero, F. The triglyceride and glucose index is a useful biomarker to recognize glucose disorders in apparently healthy children and adolescents. Eur. J. Pediatr. 2020, 179, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Delwatta, S.L.; Gunatilake, M.; Baumans, V.; Seneviratne, M.D.; Dissanayaka, M.L.B.; Batagoda, S.S.; Udagedara, A.H.; Walpola, P.B. Reference values for selected hematological, biochemical and physiological parameters of Sprague-Dawley rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Anim. Models Exp. Med. 2018, 1, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Languth, P.; Bohner, V.; Heizmann, J.; Merkle, H.P.; Wolffram, S.; Amidon, G.L.; Yamashita, S. The challenge of proteolytic enzymes in intestinal peptide delivery. J. Control. Release 1997, 46, 39–57. [Google Scholar] [CrossRef]
- Gavhane, Y.N.; Yadav, A.V. Loss of orally administered drugs in GI tract. Saudi Pharm. J. 2012, 20, 331–344. [Google Scholar] [CrossRef]
- Moroz, E.; Matoori, S.; Leroux, J.C. Oral delivery of macromolecular drugs: Where we are after almost 100years of attempts. Adv. Drug Deliv. Rev. 2016, 101, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.S.; Halagali, P.; Johnson, A.P.; Spandana, K.A.; Gangadharappa, H.V. Oral insulin delivery: Barriers, strategies, and formulation approaches: A comprehensive review. Int. J. Biol. Macromol. 2023, 242, 125114. [Google Scholar]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef]
- Twomey, J.D.; Luo, S.; Dean, A.Q.; Bozza, W.P.; Nalli, A.; Zhang, B. COVID-19 update: The race to therapeutic development. Drug Resist. Updates 2020, 53, 100733. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Rathinasabapathy, A.; Austin, E.A.; Brittain, E.L.; Carrier, E.J.; Chen, X.; Fessel, J.P.; Fike, C.D.; Fong, P.; Fortune, N.; et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702638. [Google Scholar] [CrossRef]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21, 234. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Veneziani, A.; Mores, N.; Di Daniele, N.; Cardillo, C. Favorable vascular actions of angiotensin-(1–7) in human obesity. Hypertension 2018, 71, 185–191. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Nixon, P.A.; Chappell, M.C.; Diz, D.I.; Russell, G.B.; Shaltout, H.A.; O’Shea, T.M.; Washburn, L.K. Obesity is associated with higher blood pressure and higher levels of angiotensin II but lower angiotensin-(1-7) in adolescents born preterm. J. Pediatr. 2019, 205, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Nair, S.K.; Shi, Y.; Wang, P.; Montone, K.T.; Shaw, P.A.; Choi, G.H.; Ghani, D.; Weaver, J.; Rader, D.J.; et al. Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment. Mol. Ther.-Methods Clin. Dev. 2022, 26, 266–278. [Google Scholar] [CrossRef]
- Sandle, T. A new standard for bioburden testing: USP chapter in development. GMP Rev. 2013, 12, 10–12. [Google Scholar]
- Ojha, T.; Pathak, V.; Drude, N.; Weiler, M.; Rommel, D.; Rütten, S.; Geinitz, B.; van Steenbergen, M.J.; Storm, G.; Kiessling, F.; et al. Shelf-Life Evaluation and Lyophilization of PBCA-Based Polymeric Microbubbles. Pharmaceutics 2018, 11, 433. [Google Scholar] [CrossRef]
- Patel, A.; Jin, C.; Handzo, B.; Kalyanaraman, R. Measurement of Moisture Content in Pharmaceutical Tablets by Handheld Near-Infrared Spectrometer: Adopting Quality by Design Approach to Analytical Method Lifecycle Management. J. Pharm. Biomed. Anal. 2023, 229, 115381. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010, 74, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yang, J.; Zhang, Y.; Dong, M.; Wang, S.; Zhang, Q.; Fang, F.L.; Zhang, K.; Zhang, C. Angiotensin-converting enzyme 2 and angiotensin 1–7: Novel therapeutic targets. Nat. Rev. Cardiol. 2014, 11, 413–426. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.A.; Fattah, C.; Loughrey, C.M.; Milligan, G.; Nicklin, S.A. Angiotensin-(1–7) and angiotensin-(1–9): Function in cardiac and vascular remodelling. Clin. Sci. 2014, 126, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Peiró, C.; Moncada, S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation 2020, 141, 1665–1666. [Google Scholar] [CrossRef]
- Hughes, S.; Neumiller, J.J. Oral Semaglutide. Clin. Diabetes Publ. Am. Diabetes Assoc. 2020, 38, 109–111. [Google Scholar] [CrossRef]
- Labadzhyan, A.; Nachtigall, L.B.; Fleseriu, M.; Gordon, M.B.; Molitch, M.; Kennedy, L.; Samson, S.L.; Greenman, Y.; Biermasz, N.; Bolanowski, M.; et al. Oral octreotide capsules for the treatment of acromegaly: Comparison of 2 phase 3 trial results. Pituitary 2021, 24, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, J.D.; Pamulapati, L.G.; Carter, N.; Malloy, K.; Dixon, D.L.; Sisson, E.M. Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist. Diabetes Technol. Ther. 2020, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, M.; Sampson, H.A.; Kim, E.H.; Bee, K.J.; Green, T.D.; Burks, A.W. Epicutaneous immunotherapy for the treatment of peanut allergy. Allergy, 2024; early view. [Google Scholar] [CrossRef]
- The COVID Sales Boom Is Over for Pfizer. Available online: https://www.cnn.com/2023/01/31/investing/pfizer-earnings-covid-vaccine-paxlovid/index.html (accessed on 25 November 2024).
- Sun, J.B.; Holmgren, J.; Czerkinsky, C. Cholera toxin B subunit: An efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 10795–10799. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Guo, Y.; Singh, R.; Karki, U.; Kulchar, R.J.; Wakade, G.; Pihlava, J.-M.; Khazaei, H. Debulking Influenza and Herpes Simplex virus strains by a wide-spectrum anti-viral protein formulated in clinical-grade chewing gum. Mol. Ther. 2024; in press. [Google Scholar]
- Singh, R.; Ren, Z.; Shi, Y.; Lin, S.; Kwon, K.C.; Balamurugan, S.; Rai, V.; Mante, F.; Koo, H.; Daniell, H. Affordable oral health care: Dental biofilm disruption using chloroplast made enzymes with chewing gum delivery. Plant Biotechnol. J. 2021, 19, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Loembe, M.M.; Nkengasong, J.N. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity 2021, 54, 1353–1362. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Knop, F.K.; Aroda, V.R.; do Vale, R.D.; Holst-Hansen, T.; Laursen, P.N.; Rosenstock, J.; Rubino, D.M.; Garvey, W.T.; OASIS 1 Investigators. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J.; Mahmood, T.A. Re-assessing PK/PD issues for oral protein and peptide delivery. Pharmaceutics 2021, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Han, L.; Sun, X.; Yu, H.; Yu, Y. Time-action profile of an oral enteric insulin formulation in healthy Chinese volunteers. Clin. Ther. 2012, 34, 2333–2338. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S.A. Simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a nasal spray containing iota-carrageenan in the postexposure prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Buchkov, A.; Kolev, E.; Klein, P.; Schoop, R. Reduction of Viral Load in Patients with Acute Sore Throats: Results from an Observational Clinical Trial with Echinacea/Salvia Lozenges. Complement. Med. Res. 2023, 30, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sumer, J.; Keckeis, K.; Scanferla, G.; Frischknecht, M.; Notter, J.; Steffen, A.; Kohler, P.; Schmid, P.; Roth, B.; Wissel, K.; et al. Novel Echinacea formulations for the treatment of acute respiratory tract infections in adults—A randomized blinded controlled trial. Front. Med. 2023, 10, 948787. [Google Scholar] [CrossRef]
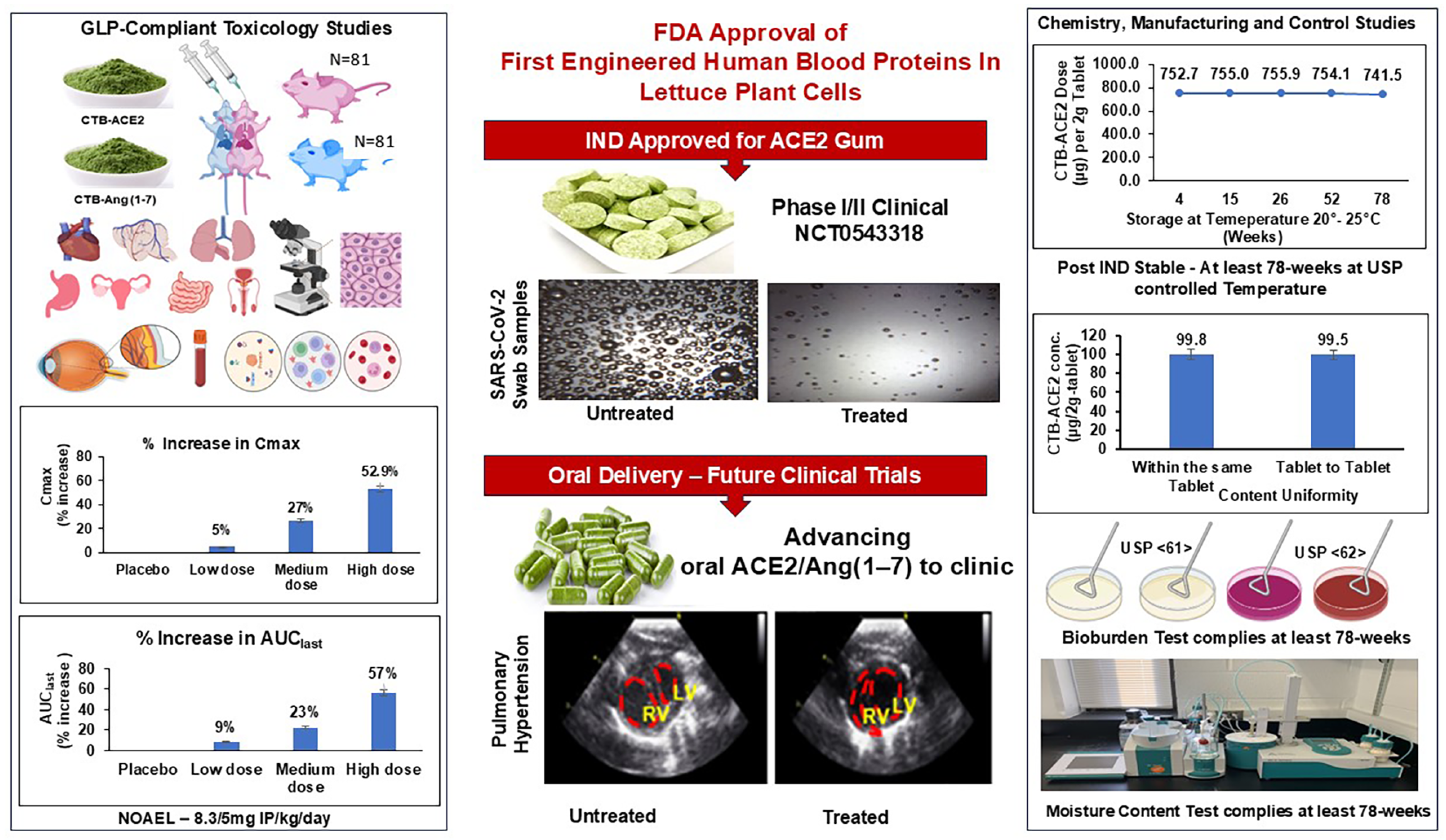





| Drug Substance | CTB-ACE2 | CTB-Ang(1–7) |
|---|---|---|
| Age | 17 weeks, 14 days | 16 months |
| Appearance—light- to dark-green powder | Pass | Pass |
| Total aerobic microbial count, 103 cfu/g | 0 | 0 |
| Total yeast, 103 cfu/g | 0 | 0 |
| Total molds, 104 cfu/g | 0 | 0 |
| Absence of Salmonella | Absent | Absent |
| Absence of Escherichia coli | Absent | Absent |
| Assay (mg/g Dry wt.) | 18.37 ± 0.89 | 15.4 ± 0.9 |
| Moisture content | 5.5% | 5.8% |
| Specifications | 4 Weeks | 12 Weeks | 26 Weeks | 52 Weeks | 78 Weeks |
|---|---|---|---|---|---|
| Total aerobic microbial count, <103 CFU/g | 0 | 0 | 0 | 0 | 0 |
| Total yeast count, <103 CFU/g | 0 | 0 | 0 | 0 | 0 |
| Total mold count, <103 CFU/g | 0 | 0 | 0 | 0 | 0 |
| Absence of Salmonella | Absent | Absent | Absent | Absent | Absent |
| Absence of Escherichia coli | Absent | Absent | Absent | Absent | Absent |
| Drug dose—250–1000 µg CTB-ACE2 per 2 g dry weight of gum tablet | 752.6 ± 3.4 µg | 755 ± 3.1 µg | 755.9 ± 2.3 µg | 754.1 ± 2.6 µg | 741.5 ± 8.9 µg |
| Moisture content—<10% | 1.2 | 1.24 | 1.25 | 1.3 | 1.33 |
| Potency—80–110% neutralization at 50 mg ACE2 gum | 100% | 100% | 100% | 100% | 100% |
| Test | Parameters | Results | Reference |
|---|---|---|---|
| Hematology | RBC, Hb, MCH, RBW, LEUC, WBC, NEUT, EOS, BASO, HEMAT, MONO, LYMPHO, PLAT, RETIC | No statistically significant differences observed across dose groups compared to control animals. | Figure 5 |
| Liver function | AST, ALT, ALP, GGT, TBIL, TIPRO, ALB, GLOB, A/G | Figure 6 | |
| Renal function | CK, UREA, CREAT, CA, PHOS, NA, K, CL. | Figure 7 | |
| Urinalysis | VOL, sp. gravity, pH | Figure 8A | |
| Coagulation | PT, PTT, FIB | Figure 8B | |
| Metabolic syndrome test | GLUC, CHOL, TRIG | Figure 8C | |
| Body weights/body weight gain/food consumption | Weight measurement, food consumption in g | Figures S1–S3 | |
| Gross pathology | Full battery of organs and tissues of all animals | No abnormalities detected. | Table 4 and Table 5 |
| Histopathology | Table 6 | ||
| Ophthalmology | Eyelids, conjunctiva, cornea, lens, iris, cortex, nucleus, indirect ophthalmoscopy, biomicroscopic examinations | Table 7 | |
| Clinical observations | Respiratory rate, breathing, posture, fur, activity | No abnormalities detected. | Table S5 |
| (A) | |||||
| Sex: Male Rat Body/Organs | Weight (g) and % Difference | Control | Low Dose | Medium Dose | High Dose |
| Original Body Weight | Mean | 264.6 | 262.3 | 262.3 | 269.3 |
| Terminal Body [G] | Mean | 351.5 | 340.9 | 342.8 | 350.6 |
| %Diff a | 26.7 | 24.4 | 25.0 | 24.8 | |
| Brain [G] | Mean | 2.0041 | 1.9748 | 1.9921 | 1.9582 |
| %Diff | - | −1.4620 | −0.5988 | −2.2903 | |
| Epididymis [G] | Mean | 0.7873 | 0.7877 | 0.7666 | 0.7764 |
| %Diff | - | 0.0508 | −2.6292 | −1.3845 | |
| Gland, Adrenal [G] | Mean | 0.05786 | 0.05175 | 0.05435 | 0.052 |
| %Diff | - | −10.5 | −6.0 | −9.4 | |
| Gland, Pituitary [G] | Mean | 0.01225 | 0.01098 | 0.01209 | 0.01273 |
| %Diff | - | −10.36735 | −1.30612 | 3.91837 | |
| Gland, Prostate [G] | Mean | 0.6615 | 0.7103 | 0.6787 | 0.6408 |
| %Diff | - | 7.3772 | 2.6002 | −3.1293 | |
| Thyroid/Parathyroid [G] | Mean | 0.02235 | 0.02090 | 0.02084 | 0.01977 |
| %Diff | - | −6.48770 | −6.756 | −11.543 | |
| Heart [G] | Mean | 1.3533 | 1.3433 | 1.35 | 1.27 |
| %Diff | - | −0.74 | 0.33 | −5.7 | |
| Kidney [G] | Mean | 2.5699 | 2.5686 | 2.4617 | 2.4654 |
| %Diff | - | −0.0506 | −4.2103 | −4.0663 | |
| Liver [G] | Mean | 10.43 | 10.4256 | 10.2 | 10.50 |
| %Diff | - | −0.1035 | −2.2604 | 0.6602 | |
| Spleen [G] | Mean | 0.7858 | 0.7347 | 0.7894 | 0.7910 |
| %Diff | - | −6.50 | 0.45 | 0.66 | |
| Testis [G] | Mean | 3.2570 | 2.9544 | 3.1996 | 3.1390 |
| %Diff | - | −9.29 | −1.76 | −3.62 | |
| Thymus [G] | Mean | 0.5277 | 0.4706 | 0.5240 | 0.5391 |
| %Diff | - | −10.82 | −0.70 | 2.16 | |
| (B) | |||||
| Sex: Male Rat Body/Organs | Weight (g) and % Difference | Control | Low Dose | Medium Dose | High Dose |
| Original Body Weight | Mean | 264.6 | 262.3 | 262.3 | 269.3 |
| Terminal Body [G] | Mean | 445.6 | 483.6 | 465.4 | 469.8 |
| %Diff a | 68.4 | 84.4 | 77.4 | 74.5 | |
| Brain Weight [G] | Mean | 2.0512 | 2.0706 | 2.0748 | 2.1232 |
| %Diff a | - | 0.9458 | 1.1505 | 3.5101 | |
| Epididymis [G] | Mean | 1.0636 | 1.1960 | 1.0742 | 1.1946 |
| %Diff | - | 12.4483 | 0.9966 | 12.3167 | |
| Gland, Adrenal [G] | Mean | 0.05968 | 0.07038 | 0.06108 | 0.07020 |
| %Diff | 0.00831 | 0.00481 | 0.00768 | 0.01486 | |
| Gland, Pituitary [G] | Mean | 0.01332 | 0.01588 | 0.01388 | 0.01442 |
| %Diff | - | 19.21922 | 4.20420 | 8.25826 | |
| Gland, Prostate [G] | Mean | 0.8154 | 1.0784 | 0.9630 | 0.9886 |
| %Diff | - | 32.2541 | 18.1015 | 21.2411 | |
| Thyroid/Parathyroid [G] | Mean | 0.02654 | 0.02652 | 0.02366 | 0.02280 |
| %Diff | - | −0.07536 | −10.85154 | −14.09194 | |
| Heart [G] | Mean | 1.4710 | 1.5446 | 1.5538 | 1.5792 |
| %Diff | - | 5.0034 | 5.6288 | 7.3555 | |
| Kidney [G] | Mean | 2.9388 | 2.9740 | 3.0762 | 3.0624 |
| %Diff | - | 1.1978 | 4.6754 | 4.2058 | |
| Liver [G] | Mean | 12.8086 | 14.3502 | 13.2068 | 13.1266 |
| %Diff | - | 12.0357 | 3.1088 | 2.4827 | |
| Spleen [G] | Mean | 0.9742 | 0.9222 | 0.9046 | 0.8174 |
| %Diff | - | −5.3377 | −7.1443 | −16.0953 | |
| Testis [G] | Mean | 3.1518 | 3.6024 | 3.2626 | 3.5478 |
| %Diff | - | 14.2966 | 3.5155 | 12.5642 | |
| Thymus [G] | Mean | 0.5102 | 0.4798 | 0.5662 | 0.4630 |
| %Diff | - | −5.9584 | 10.9761 | −9.2513 | |
| (A) | |||||
| Sex: Female Rat Body/Organs | Weight (g) and % Difference | Control | Low Dose | Medium Dose | High Dose |
| Original Body Weight | Mean | 205.9 | 201.5 | 201.5 | 207.3 |
| Terminal Body [G] | Mean | 247.2 | 232.4 | 240.8 | 245.6 |
| %Diff a | 20.1 | 15.3 | 19.5 | 18.5 | |
| Brain [G] | Mean | 1.88 | 1.86 | 1.89 | 1.89 |
| %Diff | - | −1.0710 | 0.4719 | 0.6732 | |
| Gland, Adrenal [G] | Mean | 0.06737 | 0.06959 | 0.06589 | 0.06988 |
| %Diff | - | 3.29524 | −2.19847 | 3.72240 | |
| Gland, Pituitary [G] | Mean | 0.015 | 0.015 | 0.015 | 0.015 |
| %Diff | - | −2.27716 | −5.44350 | −2.40729 | |
| Thyroid/Parathyroid [G] | Mean | 0.01646 | 0.01672 | 0.01863 | 0.01828 |
| %Diff | - | 1.57959 | 13.20373 | 11.04361 | |
| Heart [G] | Mean | 1.02 | 0.97 | 1.01 | 1.03 |
| %Diff | - | −4.8668 | −1.0750 | 1.1990 | |
| Kidney [G] | Mean | 1.88 | 1.79 | 1.83 | 1.83 |
| %Diff | - | −4.6767 | −2.6135 | −2.3235 | |
| Liver [G] | Mean | 7.5775 | 7.4375 | 7.5021 | 7.6938 |
| %Diff | - | −1.8476 | −0.9949 | 1.5345 | |
| Ovary [G] | Mean | 0.0902 | 0.0910 | 0.0996 | 0.0958 |
| %Diff | - | 0.8869 | 10.3720 | 6.1838 | |
| Spleen [G] | Mean | 0.5747 | 0.5769 | 0.5737 | 0.5967 |
| %Diff | - | 0.3828 | −0.1798 | 3.8223 | |
| Thymus [G] | Mean | 0.5813 | 0.5211 | 0.5647 | 0.6487 |
| %Diff | - | −10.3561 | −2.8614 | 11.5890 | |
| Uterus/Cervix [G] | Mean | 0.5512 | 0.5227 | 0.4920 | 0.6197 |
| %Diff | - | −5.1705 | −10.7402 | 12.4214 | |
| (B) | |||||
| Sex: Female Rat Body/Organs | Weight (g) and % Difference | Control | Low Dose | Medium Dose | High Dose |
| Original Body Weight | Mean | 205.9 | 201.5 | 201.5 | 207.3 |
| Terminal Body [G] | Mean | 288.4 | 295.0 | 280.0 | 298.2 |
| %Diff a | 40.1 | 46.4 | 39.0 | 43.8 | |
| Brain Weight [G] | Mean | 1.9774 | 1.9784 | 1.9508 | 1.9702 |
| %Diff | - | 0.0506 | −1.3452 | −0.3641 | |
| Gland, Adrenal [G] | Mean | 0.07008 | 0.07348 | 0.06602 | 0.07878 |
| %Diff | - | 4.85160 | −5.79338 | 12.41438 | |
| Gland, Pituitary [G] | Mean | 0.01750 | 0.01696 | 0.01640 | 0.01738 |
| %Diff | - | −3.08571 | −6.28571 | −0.68571 | |
| Thyroid/Parathyroid [G] | Mean | 0.01808 | 0.02090 | 0.02420 | 0.01866 |
| %Diff | - | 15.59735 | 33.84956 | 3.20796 | |
| Heart [G] | Mean | 1.1000 | 1.1160 | 0.9948 | 1.1102 |
| %Diff | - | 1.4545 | −9.5636 | 0.9273 | |
| Kidney [G] | Mean | 1.8836 | 1.9534 | 1.9060 | 1.9436 |
| %Diff | - | 3.7057 | 1.1892 | 3.1854 | |
| Liver [G] | Mean | 8.3656 | 8.6844 | 7.7492 | 8.3342 |
| %Diff | - | 3.8108 | −7.3683 | −0.3753 | |
| Ovary [G] | Mean | 0.0998 | 0.1076 | 0.0984 | 0.1058 |
| %Diff | - | 7.8156 | −1.4028 | 6.0120 | |
| Spleen [G] | Mean | 0.5928 | 0.6336 | 0.6162 | 0.7262 |
| %Diff | - | 6.8826 | 3.9474 | 22.5034 | |
| Thymus [G] | Mean | 0.5154 | 0.4606 | 0.5362 | 0.5540 |
| %Diff | - | −10.6325 | 4.0357 | 7.4893 | |
| Uterus/Cervix (g)—[G] | Mean | 0.6672 | 0.5628 | 0.7350 | 0.6720 |
| %Diff | - | −15.6475 | 10.1619 | 0.7194 | |
| Organs Systems (Male and Female—n = 120) | Histopathological Observations in All Groups | |
|---|---|---|
| Integumentary | Skin | No visible lesions |
| Musculoskeletal | Bone marrow, sternum, bone, femur, bone, sternum, joint, femorotibial, muscle, skeletal | |
| Lymphatic | Galt, mandibular, mesenteric, spleen, mediastinal | Minimal increase in plasma cell, cellularity, no visible lesions |
| Endocrine | Adrenal, harderian, mammary, parathyroid, thymus, pituitary, salivary, mandibular, thyroid, prostate, seminal vesicle | No aberrant craniopharyngeal structures, inflammation, hypertrophy, acinar cells, cyst, ultimobranchial lesions |
| Nervous | Brain, nerves—optic, sciatic, spinal cord | No visible lesions |
| Eye | No visible lesions, retina folds | |
| Respiratory | Lung, trachea | Mild inflammation, mixed-cell infiltrate, perivascular, alveolar macrophages, alveoli/interstitium, no visible lesions |
| Cardiovascular | Heart, artery, aorta | Absence of necrosis/inflammatory infiltrate, mixed cells, myocardial fiber degeneration, epicardial and lesions in tissue and adventitia |
| Gastrointestinal | Tongue; large intestine—cecum, colon, rectum; small intestine—duodenum, ileum, jejunum | No fibrosis or visible lesions |
| Esophagus | No degeneration/regeneration | |
| Liver | Minimal vacuolation, hepatocyte, periportal and mononuclear cell infiltration, necrosis focal/multifocal, no visible lesions | |
| Pancreas | No atrophy, acinar cell abnormality, no visible lesions | |
| Stomach | Minimal erosion, pylorus, glandular | |
| Reproductive | Ovary, vagina, uterus/cervix, testis, epididymis | No visible lesions, cyst, squamous, absence of atrophy, degeneration in tubules |
| Urinary | Kidney, urinary bladder | Absence of mineralization, basophilia, dilatation, cyst in medulla, no inflammation, mixed cells in pelvis |
| Ophthalmology Parameters | Observations |
|---|---|
| Eyelids, conjunctiva cornea, lens, iris, cortex, nucleus | No observable lesions |
| Ophthalmoscopic (indirect ophthalmoscopy) examination using a 60-diopter lens following dilation of the pupils with 0.5% tropicamide ophthalmic solution | No abnormalities in the anterior segment of the eye |
| Biomicroscopic (slit lamp) examination using a Kowa SL-17 biomicroscope following dilation of the pupils with 0.5% tropicamide ophthalmic solution | No abnormalities in the posterior segment of the eye |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniell, H.; Wakade, G.; Nair, S.K.; Singh, R.; Emanuel, S.A.; Brock, B.; Margulies, K.B. Evaluation of Biologics ACE2/Ang(1–7) Encapsulated in Plant Cells for FDA Approval: Safety and Toxicology Studies. Pharmaceutics 2025, 17, 12. https://doi.org/10.3390/pharmaceutics17010012
Daniell H, Wakade G, Nair SK, Singh R, Emanuel SA, Brock B, Margulies KB. Evaluation of Biologics ACE2/Ang(1–7) Encapsulated in Plant Cells for FDA Approval: Safety and Toxicology Studies. Pharmaceutics. 2025; 17(1):12. https://doi.org/10.3390/pharmaceutics17010012
Chicago/Turabian StyleDaniell, Henry, Geetanjali Wakade, Smruti K. Nair, Rahul Singh, Steven A. Emanuel, Barry Brock, and Kenneth B. Margulies. 2025. "Evaluation of Biologics ACE2/Ang(1–7) Encapsulated in Plant Cells for FDA Approval: Safety and Toxicology Studies" Pharmaceutics 17, no. 1: 12. https://doi.org/10.3390/pharmaceutics17010012
APA StyleDaniell, H., Wakade, G., Nair, S. K., Singh, R., Emanuel, S. A., Brock, B., & Margulies, K. B. (2025). Evaluation of Biologics ACE2/Ang(1–7) Encapsulated in Plant Cells for FDA Approval: Safety and Toxicology Studies. Pharmaceutics, 17(1), 12. https://doi.org/10.3390/pharmaceutics17010012







