Dual-Mechanism Gastroretentive Tablets with Encapsulated Gentian Root Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid Gentian Extract Preparation
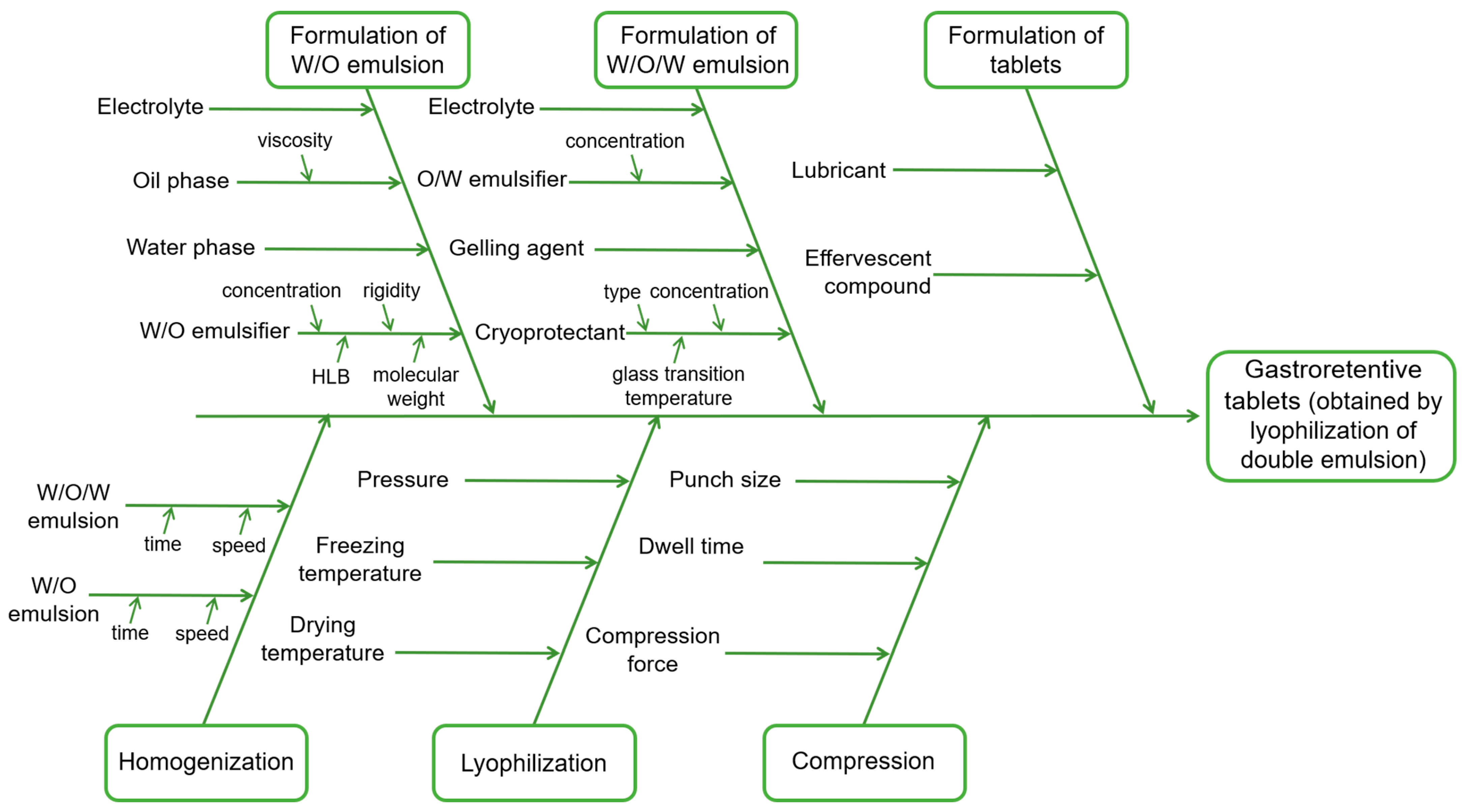
2.3. Risk Assessment Analysis
2.4. Preparation of W/O/W Emulsions
2.5. Characterization of W/O/W Emulsions
2.6. Powder Preparation
2.7. Scanning Electron Microscopy (SEM)
2.8. Differential Scanning Calorimetry
2.9. Fourier Transform Infrared (FTIR) Spectroscopy
2.10. Yield
2.11. Determination of the Encapsulation Efficiency
2.12. High-Performance Liquid Chromatography
2.13. Moisture Content Analyze
2.14. Floating Behavior
2.15. Evaluation of Powder Flowability
2.16. Antioxidant Activity
2.17. FRAP Assay
2.18. DPPH Assay
2.19. Beta-Carotene Bleaching Assay
2.20. Tablet Preparation
2.21. Friability Testing
2.22. Mucoadhesion Evaluation
2.23. In Vitro Gentiopicroside Release Testing
2.24. Assessment of Dispersibility During In Vitro Release Testing
2.25. In Vitro Digestion
2.26. Statistics
3. Results and Discussion
3.1. Identification of QTPP and CQAs
3.2. Risk Assessment
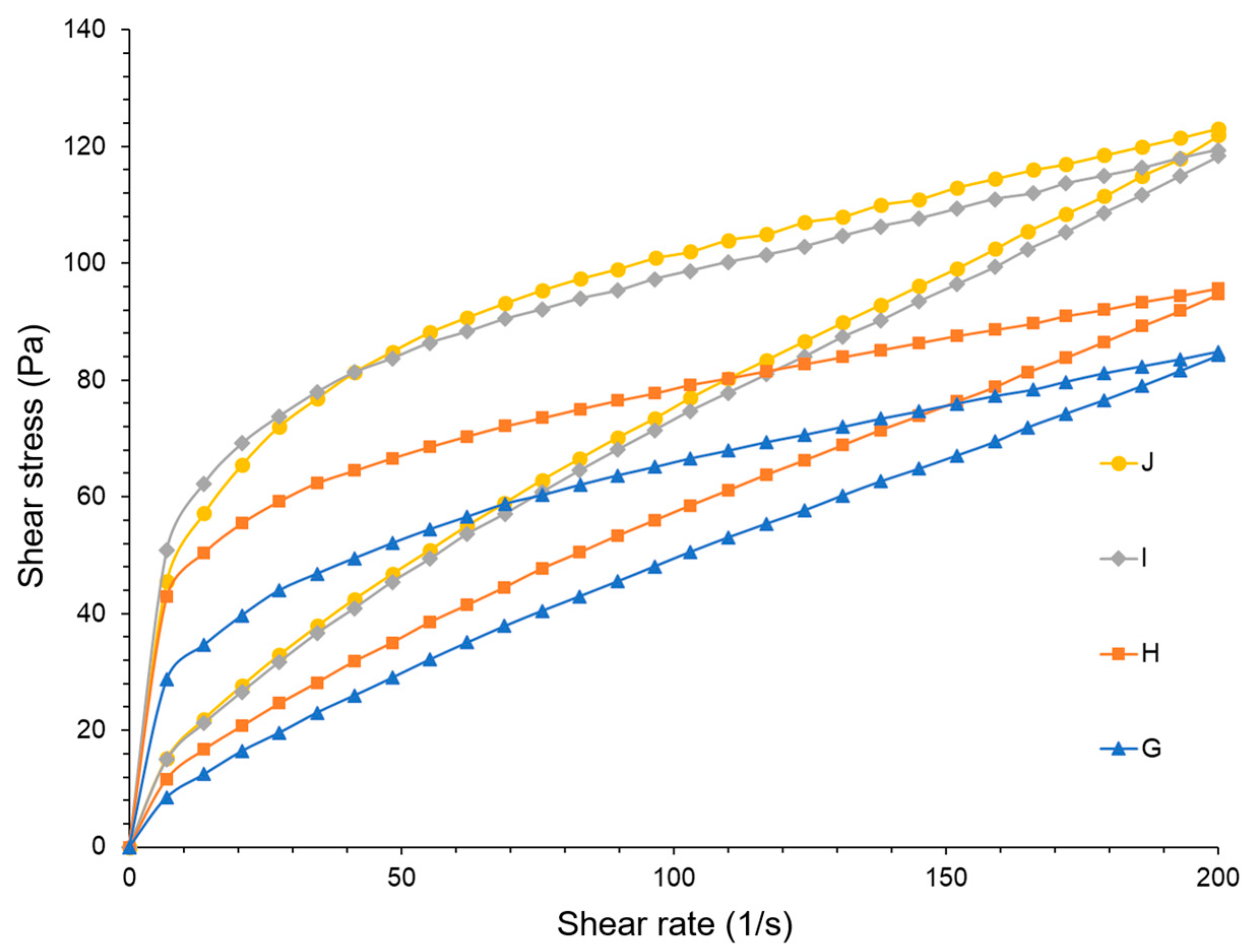
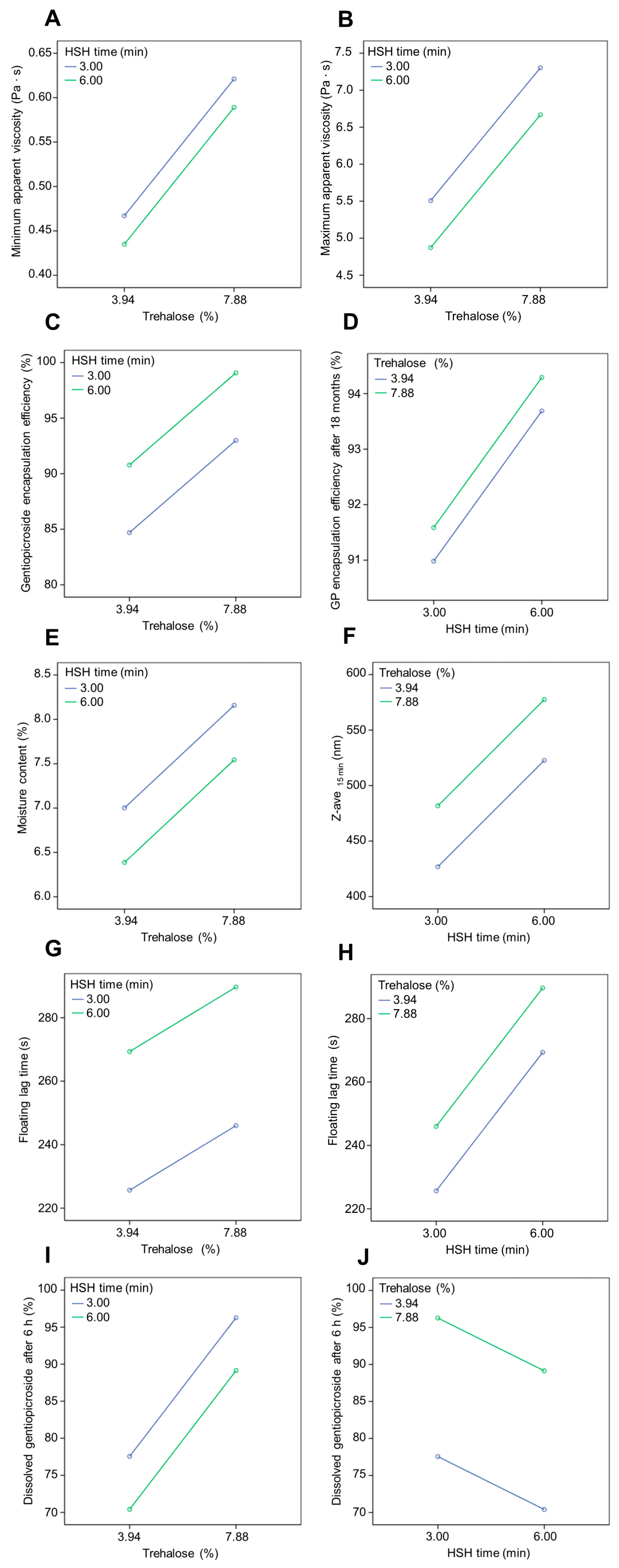
3.3. Emulsions
3.4. Powders
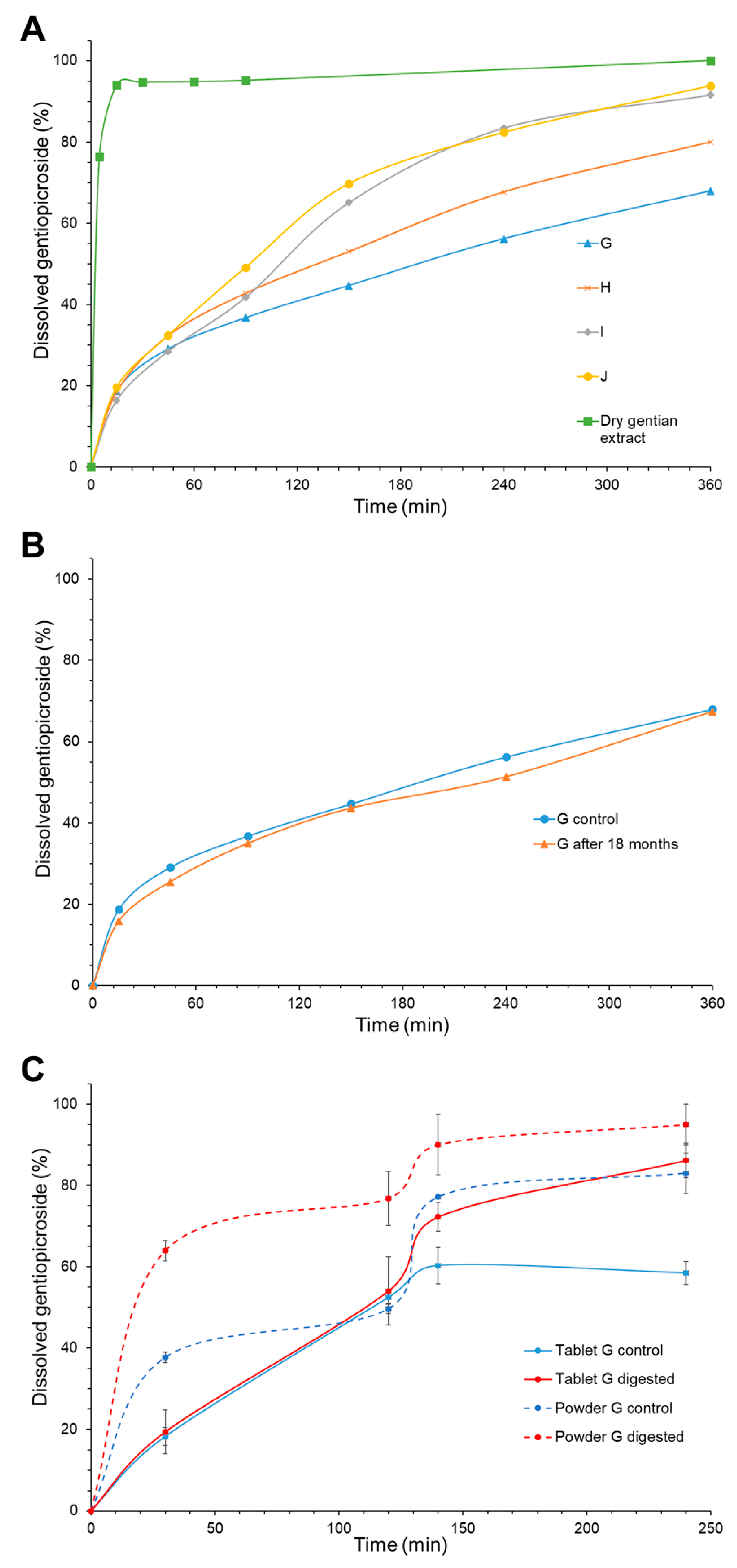
3.5. Tablets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on Gastroretentive Drug Delivery Systems for Improving Drug Bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Waqar, M.A.; Mubarak, N.; Khan, A.M.; Khan, R.; Shaheen, F.; Shabbir, A. Advanced Polymers and Recent Advancements on Gastroretentive Drug Delivery System; a Comprehensive Review. J. Drug Target. 2024, 32, 655–671. [Google Scholar] [CrossRef]
- Vrettos, N.N.; Roberts, C.J.; Zhu, Z. Gastroretentive technologies in tandem with controlled-release strategies: A potent answer to oral drug bioavailability and patient compliance implications. Pharmaceutics 2021, 13, 1591. [Google Scholar] [CrossRef]
- Karemore, M.N.; Bali, N.R. Gellan gum based gastroretentive tablets for bioavailability enhancement of cilnidipine in human volunteers. Int. J. Biol. Macromol. 2021, 174, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Gao, Y.; Zhu, J. Preparation and evaluation of glyceryl monooleate-coated hollow-bioadhesive microspheres for gastroretentive drug delivery. Int. J. Pharm. 2011, 413, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Q.; Li, J.; Ren, Y.; Wu, G.; Liu, Y.; Shi, Y. Development and evaluation of a new gastroretentive drug delivery system: Nanomicelles-loaded floating mucoadhesive beads. J. Drug Deliv. Sci. Technol. 2019, 51, 485–492. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.F.; Ismail, S.; Tadros, M.I.; Elnabarawi, M.A. Alfuzosin hydrochloride-loaded low-density gastroretentive sponges: Development, in vitro characterization and gastroretentive monitoring in healthy volunteers via MRI. Pharm. Dev. Technol. 2020, 25, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Yousuf, R.I.; Ahmed, F.R.; Shoaib, M.H.; Irshad, A.; Saleem, M.T.; Qazi, F.; Sarfaraz, S.; Rizvi, S.A.; Mahmood, Z.A. Cellulose ether and carbopol 971 based gastroretentive controlled release formulation design, optimization and physiologically based pharmacokinetic modeling of ondansetron hydrochloride minitablets. Int. J. Biol. Macromol. 2024, 276, 133841. [Google Scholar] [CrossRef] [PubMed]
- Lalge, R.; Thipsay, P.; Shankar, V.K.; Maurya, A.; Pimparade, M.; Bandari, S.; Zhang, F.; Murthy, S.N.; Repka, M.A. Preparation and Evaluation of Cefuroxime Axetil Gastro-Retentive Floating Drug Delivery System via Hot Melt Extrusion Technology. Int. J. Pharm. 2019, 566, 520–531. [Google Scholar] [CrossRef]
- Mudrić, J.; Šavikin, K.; Đekić, L.; Pavlović, S.; Kurćubić, I.; Ibrić, S.; Đuriš, J. Development of Lipid-Based Gastroretentive Delivery System for Gentian Extract by Double Emulsion–Melt Dispersion Technique. Pharmaceutics 2021, 13, 2095. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Sutariya, B.; Lokras, A.; Thamm, J.; Saraf, M.; Warawdekar, U.; Fahr, A.; Nagarsenker, M. Lipid Nanoconstructs for Superior Hepatoprotection: In Vitro Assessments as Predictive Tool for in Vivo Translation. Int. J. Pharm. 2020, 579, 119176. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-gentiana-lutea-l-radix-revision-1_en.pdf (accessed on 1 June 2023).
- Wang, C.H.; Wang, Z.T.; Annie Bligh, W.W.; White, K.N.; White, C.J.B. Pharmacokinetics and Tissue Distribution of Gentiopicroside Following Oral and Intravenous Administration in Mice. Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Xia, P.-F.; Peng, X.J.; Wu, X.Y.; Jin, H.; Zhang, J.; Zhao, L. Synthesis, and Anti-Inflammatory Activities of Gentiopicroside Derivatives. Chin. J. Nat. Med. 2022, 20, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf (accessed on 10 July 2024).
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S.; Gupta, M.K. QbD Based Approach for Formulation Development of Spray Dried Microparticles of Erlotinib Hydrochloride for Sustained Release. J. Drug Deliv. Sci. Technol. 2020, 57, 101684. [Google Scholar] [CrossRef]
- Bansal, S.; Beg, S.; Asthana, A.; Garg, B.; Asthana, G.S.; Kapil, R.; Singh, B. QbD-Enabled Systematic Development of Gastroretentive Multiple-Unit Microballoons of Itopride Hydrochloride. Drug Deliv. 2016, 23, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.B.; Vilmann, P.; Bar-Shalom, D.; Müllertz, A.; Baldursdottir, S.G. Polymer selection for simulation of rheological properties of human gastric fluid. Nord. Rheol. Soc. Annu. Trans. 2013, 21, 241–247. [Google Scholar]
- European Pharmacopoeia, 11th ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2022.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study between in Vivo and in Vitro Samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. Disintegration and Dissolution of Dietary Supplements. In The United States Pharmacopeia and National Formulary USP 36–NF 31; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2013; pp. 1111–1116. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of Curves with an Emphasis on in Vitro Dissolution Profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Peña, J.; Veiga, M.-D. Tenofovir Hot-Melt Granulation Using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV. Pharmaceutics 2019, 11, 137. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Aziz-Kalbhenn, H.; Ammar, R.M.; Rabini, S.; Moissl-Eichinger, C.; Bauer, R. Application of an in Vitro Digestion Model to Study the Metabolic Profile Changes of an Herbal Extract Combination by UHPLC–HRMS. Phytomedicine 2020, 71, 153221. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, M.; Lela, L.; Moles, M.; Mangieri, C.; Bisaccia, D.; Faraone, I.; Falabella, R.; Milella, L. The Healing Bitterness of Gentiana Lutea L., Phytochemistry and Biological Activities: A Systematic Review. Phytochemistry 2023, 206, 113518. [Google Scholar] [CrossRef] [PubMed]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-Retentive Drug Delivery Systems and Their in Vivo Success: A Recent Update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef]
- Lamba, H.; Sathish, K.; Sabikhi, L. Double emulsions: Emerging delivery system for plant bioactives. Food Bioprocess Technol. 2015, 8, 709–728. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Sharma, O.P.; Shah, M.V.; Parikh, D.C.; Mehta, T.A. Formulation Optimization of Gastroretentive Drug Delivery System for Allopurinol Using Experimental Design. Expert Opin. Drug Deliv. 2015, 12, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Aboul-Einien, M.H. In vitro and in vivo evaluation of a fast-disintegrating lyophilized dry emulsion tablet containing griseofulvin. Eur. J. Pharm. Sci. 2007, 32, 58–68. [Google Scholar] [CrossRef]
- Čerpnjak, K.; Pobirk, A.Z.; Vrečer, F.; Gašperlin, M. Tablets and minitablets prepared from spray-dried SMEDDS containing naproxen. Int. J. Pharm. 2015, 495, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, J.; Đuriš, J.; Cvijić, S.; Dobričić, V.; Montepietra, A.; Lombardi, C.; Ibrić, S.; Rossi, A. Development of Solid Lipid Microparticles by Melt-Emulsification/Spray-Drying Processes as Carriers for Pulmonary Drug Delivery. Eur. J. Pharm. Sci. 2021, 156, 105588. [Google Scholar] [CrossRef]
- Thapa, P.; Jeong, S.H. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics 2018, 10, 161. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Molet-Rodríguez, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Formation of Double (W1/O/W2) Emulsions as Carriers of Hydrophilic and Lipophilic Active Compounds. Food Bioprocess Technol. 2019, 12, 422–435. [Google Scholar] [CrossRef]
- Hedoux, A.; Paccou, L.; Achir, S.; Guinet, Y. Mechanism of Protein Stabilization by Trehalose During Freeze-Drying Analyzed by In Situ Micro-Raman Spectroscopy. J. Pharm. Sci. 2013, 102, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Abla, K.K.; Mehanna, M.M. Freeze-Drying: A Flourishing Strategy to Fabricate Stable Pharmaceutical and Biological Products. Int. J. Pharm. 2022, 628, 122233. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yang, G.; Zhou, Z.; Gao, Y. Exploring Trehalose on the Release of Levonorgestrel from Implantable PLGA Microneedles. Polymers 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; McGarry, K.; Chaw, C.; Elkordy, A. Feasibility of Using Gluconolactone, Trehalose and Hydroxy-Propyl Gamma Cyclodextrin to Enhance Bendroflumethiazide Dissolution Using Lyophilisation and Physical Mixing Techniques. Pharmaceutics 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Dolinina, E.S.; Vlasenkova, M.I.; Parfenyuk, E.V. Effect of Trehalose on Structural State of Bovine Serum Albumin Adsorbed onto Mesoporous Silica and the Protein Release Kinetics in Vitro. Colloids Surf. A Physicochem. Eng. Asp. 2017, 527, 101–108. [Google Scholar] [CrossRef]
- Choi, M.J.; Briançon, S.; Bazile, D.; Royere, A.; Min, S.G.; Fessi, H. Effect of cryoprotectant and freeze-drying process on the stability of W/O/W emulsions. Dry. Technol. 2007, 25, 809–819. [Google Scholar] [CrossRef]
- Chen, A.; Tapia, H.; Goddard, J.M.; Gibney, P.A. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef]
- Schuch, A.; Wrenger, J.; Schuchmann, H.P. Production of W/O/W Double Emulsions. Part II: Influence of Emulsification Device on Release of Water by Coalescence. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 344–351. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, B. Optimization of Encapsulation Efficiency and Average Particle Size of Hohenbuehelia Serotina Polysaccharides Nanoemulsions Using Response Surface Methodology. Food Chem. 2017, 229, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Gardouh, A.R.; Ghorab, M.M.; Abdel-Rahman, S.G. Effect of viscosity, method of preparation and homogenization speed on physical characteristics of solid lipid nanoparticles. ARPN J. Sci. Technol. 2012, 2, 996–1006. [Google Scholar]
- Liu, F.; Wang, Y.; Li, X.; Zhang, Z.; Dai, X.; Wang, X.; Xin, Y.; Liu, K.; Gao, L.; Du, D.; et al. The Phase Inversion Mechanism of the pH-Sensitive Reversible Invert Emulsion from w/o to o/w. Open Phys. 2020, 18, 380–390. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double Emulsions Prepared by Two–Step Emulsification: History, State-of-the-Art and Perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Magalhães, W.V.; Sufi, B.D.S.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; Lannes, S.C.D.S.; Baby, A.R. Vitamin E-Loaded Bigels and Emulsions: Physicochemical Characterization and Potential Biological Application. Colloids Surf. B Biointerfaces 2021, 201, 111651. [Google Scholar] [CrossRef]
- Kanouni, M.; Rosano, H.L.; Naouli, N. Preparation of a Stable Double Emulsion (W1/O/W2): Role of the Interfacial Films on the Stability of the System. Adv. Colloid Interface Sci. 2002, 99, 229–254. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256, 319–327. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Oleksza, K. Rheological Properties and Physical Stability of o/w Emulsions Stabilized by OSA Starch with Trehalose. Food Hydrocoll. 2015, 44, 49–58. [Google Scholar] [CrossRef]
- Álvarez Cerimedo, M.S.; Cerdeira, M.; Candal, R.J.; Herrera, M.L. Microencapsulation of a Low- Trans Fat in Trehalose as Affected by Emulsifier Type. J. Americ. Oil Chem. Soc. 2008, 85, 797–807. [Google Scholar] [CrossRef]
- Drusch, S.; Berg, S. Extractable Oil in Microcapsules Prepared by Spray-Drying: Localisation, Determination and Impact on Oxidative Stability. Food Chem. 2008, 109, 17–24. [Google Scholar] [CrossRef]
- Olgenblum, G.I.; Sapir, L.; Harries, D. Properties of Aqueous Trehalose Mixtures: Glass Transition and Hydrogen Bonding. J. Chem. Theory Comput. 2020, 16, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S.; Scherer, R.; Castilho, R.O.; Faraco, A.A.G.; Ruas, F.G.; Oliveira, J.P.; Guimarães, M.C.C.; De Andrade, T.U.; et al. Maltodextrin and Gum Arabic-Based Microencapsulation Methods for Anthocyanin Preservation in Juçara Palm (Euterpe Edulis Martius) Fruit Pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dolly, P.; Anishaparvin, A.; Joseph, G.S.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus Plantarum (Mtcc 5422) by Spray-Freeze-Drying Method and Evaluation of Survival in Simulated Gastrointestinal Conditions. J. Microencapsul. 2011, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, X.; Wu, X.; Diao, Y.; Liu, Y.; Liu, P. Rapid Identification of Wild Gentiana Genus in Different Geographical Locations Based on FT-IR and an Improved Neural Network Structure Double-Net. Molecules 2022, 27, 5979. [Google Scholar] [CrossRef]
- Coelho, C.; Figueredo, G.; Lafarge, C.; Bou-Maroun, E.; Flahaut, S. Mid-Infrared Spectroscopy Combined with Multivariate Analysis and Machine-Learning: A Powerful Tool to Simultaneously Assess Geographical Origin, Growing Conditions and Bitter Content in Gentiana Lutea Roots. Ind. Crops Prod. 2022, 187, 115349. [Google Scholar] [CrossRef]
- Windholz, M. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 10th ed.; Merck: Rahway, NJ, USA, 1983. [Google Scholar]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of Iridoids, Secoiridoids and Xanthones in Centaurium Erythraea, Frasera Caroliniensis and Gentiana Lutea Using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- El Assasy, A.E.-H.I.; Younes, N.F.; Makhlouf, A.I.A. Enhanced Oral Absorption of Amisulpride Via a Nanostructured Lipid Carrier-Based Capsules: Development, Optimization Applying the Desirability Function Approach and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 82. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Qian, Y.; Chen, Y. The Effects of Cryoprotectants on the Freeze-Drying of Ibuprofen-Loaded Solid Lipid Microparticles (SLM). Eur. J. Pharm. Biopharm. 2008, 69, 750–759. [Google Scholar] [CrossRef]
- Almukainzi, M.; A El-Masry, T.; A Negm, W.; Elekhnawy, E.; Saleh, A.; E Sayed, A.; A Khattab, M.; H Abdelkader, D. Gentiopicroside PLGA Nanospheres: Fabrication, in Vitro Characterization, Antimicrobial Action, and in Vivo Effect for Enhancing Wound Healing in Diabetic Rats. IJN 2022, 17, 1203–1225. [Google Scholar] [CrossRef]
- Almukainzi, M.; El-Masry, T.A.; Negm, W.A.; Elekhnawy, E.; Saleh, A.; Sayed, A.E.; Ahmed, H.M.; Abdelkader, D.H. Co-Delivery of Gentiopicroside and Thymoquinone Using Electrospun m-PEG/PVP Nanofibers: In-Vitro and In Vivo Studies for Antibacterial Wound Dressing in Diabetic Rats. Int. J. Pharm. 2022, 625, 122106. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Bligh, S.W.A.; White, K.N.; Branford-White, C.J.; Wang, Z. Pharmacokinetics and Bioavailability of Gentiopicroside from Decoctions of Gentianae and Longdan Xiegan Tang after Oral Administration in Rats—Comparison with Gentiopicroside Alone. J. Pharm. Biomed. Anal. 2007, 44, 1113–1117. [Google Scholar] [CrossRef]
- Canonica, L.; Pelizzoni, F.; Manitto, P.; Jommi, G. Structure of gentiopicroside. Tetrahedron 1961, 16, 192–200. [Google Scholar] [CrossRef]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the storage-related oxidative/hydrolytic degradation of olive oil secoiridoids by liquid chromatography and high-resolution Fourier transform mass spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Dumoulin, E.D.; Richard, H.M.J.; Noleau, I.; Lebert, A.M. Flavor Encapsulation by Spray Drying: Application to Citral and Linalyl Acetate. J. Food Sci. 1992, 57, 217–221. [Google Scholar] [CrossRef]
- Rautenberg, A.; Lamprecht, A. Spray-Freeze-Dried Lyospheres: Solid Content and the Impact on Flowability and Mechanical Stability. Powder Technol. 2022, 411, 117905. [Google Scholar] [CrossRef]
- Sulistiawati; Saka Dwipayanti, K.; Azhar, M.; Rahman, L.; Pakki, E.; Himawan, A.; Permana, A.D. Enhanced Skin Localization of Metronidazole Using Solid Lipid Microparticles Incorporated into Polymeric Hydrogels for Potential Improved of Rosacea Treatment: An Ex Vivo Proof of Concept Investigation. Int. J. Pharm. 2022, 628, 122327. [Google Scholar] [CrossRef]
- Rosiaux, Y.; Jannin, V.; Hughes, S.; Marchaud, D. Solid lipid excipients as matrix agents for sustained drug delivery. J. Control. Release 2014, 188, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Hens, B.; Vertzoni, M.; Brouwers, J.; Berben, P.; Dressman, J.; Andreas, C.J.; Schaefer, K.J.; Mann, J.; McAllister, M.; et al. In Vitro Models for the Prediction of in Vivo Performance of Oral Dosage Forms: Recent Progress from Partnership through the IMI OrBiTo Collaboration. Eur. J. Pharm. Biopharm. 2019, 136, 70–83. [Google Scholar] [CrossRef] [PubMed]
| Emulsion | Powder | |||||
|---|---|---|---|---|---|---|
| I and J | G and H | I and J | G and H | |||
| W1 | Liquid gentian extract (%) | 13.13 | 13.13 | Dry gentian extract (%) | 19.35 | 23.26 |
| Sodium chloride (%) | 0.06 | 0.06 | Sodium chloride (%) | 0.25 | 0.30 | |
| Sodium alginate (%) | 0.26 | 0.26 | Sodium alginate (%) | 1.12 | 1.35 | |
| O | Gelucire® 43/01 (%) | 5.32 | 5.32 | Gelucire® 43/01 (%) | 22.73 | 27.33 |
| PGPR (%) | 0.98 | 0.98 | PGPR (%) | 4.21 | 5.06 | |
| W2 | Purified water (%) | 68.00 | 71.93 | Purified water (%) | 0 | 0 |
| Sodium chloride (%) | 0.23 | 0.23 | Sodium chloride (%) | 0.99 | 1.19 | |
| Sodium alginate (%) | 1.58 | 1.58 | Sodium alginate (%) | 6.73 | 8.09 | |
| Trehalose (%) | 7.88 | 3.94 | Trehalose (%) | 33.66 | 20.20 | |
| Lecithin (%) | 1.58 | 1.58 | Lecithin (%) | 6.73 | 8.09 | |
| Sylysia® 350 (%) | 1.00 | 1.00 | Sylysia® 350 (%) | 4.23 | 5.09 | |
| QTPP | Target | Explanation |
|---|---|---|
| Route of administration | Oral | According to the EMA monograph [13], gentian root extract is administered via the oral route. |
| Indication | Dyspeptic symptoms/gastrointestinal disorders: heartburn, vomiting, stomach pain, nausea, loss of appetite, constipation, flatulence | Furthermore, antioxidant, anti-inflammatory, anti-microbial, anti-obesogenic, anti-atherosclerotic, gastroprotective, neurotrophic, anti-genotoxic effects are reported for gentian extracts, and isolated bioactive components [13,29]. |
| Dosage form type | Gastroretentive tablets | The prolonged residence time of gentian extract in the stomach is desired due to the low bioavailability and short elimination half-life of gentiopicroside in conventional forms, as well as due to the local gastric effect of gentian extract [13,14]. |
| Delivery type | Combined floating–mucoadhesive lipid-based system | The absorption of bioactive compounds is enhanced when triglyceride-based formulations are in contact with intestinal membranes. Also, the combination of the floating and mucoadhesive approach could improve gastroretention [2,12,30]. |
| Dissolution profile | Modified gentiopicroside release | The release of an initial, effective dose of gentiopicroside, followed by a further sustained release at the place of action (stomach), is required in order to achieve and maintain the therapeutic effect of the gentian extract. |
| Stability | At least an 18-month shelf life | Store at room temperature. |
| Target | Is This CQA? | Justification | |
|---|---|---|---|
| Color Odor Appearance | Acceptable to patients | No | This parameter is not critical for efficacy and safety. |
| Emulsion viscosity | As low as possible minimal and maximal apparent viscosity | Yes | To encapsulate active compounds effectively in a dehydrated emulsion, an encapsulating matrix should have low viscosity [31]. |
| Physical stability | No separation or change in consistency after the centrifugation test of double emulsion | No | It is essential that the double emulsions are physically stable. This parameter was not considered a critical parameter, since the tested formulations during the preliminary phase were stable. |
| Encapsulation efficiency | ≥80% | Yes | As high as possible. A higher encapsulation efficiency is crucial since the manufacturing costs are reduced in this way. Furthermore, higher gentiopicroside content in the formulation can result in a reduced dosing frequency and consequently improved patient compliance. |
| Encapsulation efficiency after 18 months | ≥90% | Yes | It is important to retain high content of gentiopicroside during storage. |
| Yield | ≥80% | No | It is essential to develop powder with high yield in order to decrease production costs. This parameter is not considered critical since during preliminary studies, the yield of the investigated formulations was high. |
| Antioxidant potency composite index (ACI)—after 12 months | ≥70% | No | It is vital to retain the high antioxidant activity of the extract during production and storage. However, this result could be affected by a number of factors, such as the type and amount of solvent used, the presence and concentration of hydrogen and metal ions, and the turbidity of the sample [32]. |
| Moisture content | As low as possible | Yes | Moisture content is a critical attribute, since elevated water content could influence physico-chemical and microbiological stability, as well as flowability. |
| Flowability | Carr index ≤ 15 Hausner ratio ≤ 1.18 | Yes | Free-flowing powders are more suitable for tablet production. |
| Floating duration (tablets) | ≥6 h | No | As long as possible. This parameter is not considered critical since during preliminary studies, the investigated formulations floated for more than 6 h. |
| Floating lag time (tablets) | 0–5 min | Yes | As minimum as possible, since increase in the floating lag time increases the chance of the gastric emptying of the tablet before the release of gentiopicroside in the stomach. |
| Gentiopicroside release | 20% ≤ Q 45 min ≤ 40% 60% ≤Q 6 h ≤ 100% | Yes | It is reported that gentiopicroside elimination half-life is short (2.8 h) [14]. Therefore, it is important to achieve an initial, effective dose and, after that, to provide sustained release in order to increase bioavailability and to reduce the dosing frequency. |
| Friability of tablets | According to Ph. Eur. 11.0 (2023) requirements—less than 1% | No | This parameter is not considered critical since during preliminary studies, all investigated formulations accomplished the requirements. |
| Force of adhesion | More than 0.94 N | Yes | It is reported that mucoadhesive–floating tablets with a force of adhesion of 0.94 N enabled gastroretention in vivo [33]. |
| Z average | ≤1000 nm | Yes | The presence of smaller droplets during the in vitro release study may accelerate the release and improve the bioavailability of the encapsulated bioactive compounds [34,35]. |
| CQA | Material Atributes | Process Parameters | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid Content | W/O Emulsifier Content | O/W Emulsifier Content | Trehalose Content | Sylysia® 350 Content | Sodium Alginate Content | Salt Content | Sirring Time | Stirring Speed | HSH Speed | HSH Time | Lyophilization Time | Lyophilization Pressure | Lyophilization Temperature | Compression Force | Dwell Time | |
| Emulsion viscosity | Medium | Medium | Low | Medium | Low | High | Low | Low | Low | Medium | Medium | |||||
| Floatation lag time | Medium | Low | Low | Medium | Medium | Low | Low | Low | Low | Medium | Medium | Low | Medium | Low | High | Medium |
| Gentiopicroside release | High | Medium | Medium | High | Medium | Medium | Low | Low | Medium | Medium | High | Medium | Medium | Low | Medium | Medium |
| Force of adhesion | Low | Medium | Low | Low | Low | High | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Encapsulation efficiency | High | High | High | High | Low | Low | Medium | Medium | Medium | High | High | Medium | Medium | Low | Low | Low |
| Encapsulation efficiency after 18 months | Medium | High | High | High | Low | Medium | Medium | Medium | Medium | High | High | Medium | Medium | Medium | Low | Low |
| Flowability | Medium | Low | Low | Medium | Medium | Low | Low | Low | Low | Low | Low | Medium | Low | Low | Low | Low |
| Moisture content | Low | Low | Low | High | Medium | Low | Low | Low | Low | Low | Medium | High | Medium | Medium | Low | Low |
| Z-ave | Low | Medium | Medium | Medium | Low | Low | Low | Low | Medium | Medium | Medium | Medium | Medium | Low | Medium | Medium |
| pH | Conductivity (µS/cm) | ηmax (Pa·s) | ηmin (Pa·s) | HA (Pa/s) | |
|---|---|---|---|---|---|
| G | 4.46 ± 0.18 | 4.25 ± 0.32 | 4.17 ± 0.02 | 0.424 ± 0.007 | 2963.45 |
| H | 4.39 ± 0.11 | 5.54 ± 0.28 | 6.21 ± 0.07 | 0.478 ± 0.008 | 3997.54 |
| I | 4.39 ± 0.09 | 5.21 ± 0.24 | 7.37 ± 0.30 | 0.600 ± 0.044 | 4932.91 |
| J | 4.40 ± 0.13 | 4.78 ± 0.17 | 6.60 ± 0.06 | 0.610 ± 0.006 | 4815.73 |
| Encapsulation Efficiency (%) | Encapsulation Efficiency- 18 Months (%) | Yield (%) | Carr Index (%) | Hausner Ratio | Moisture Content (%) | Floating Lag Time (s) | Floating Duration (h) | ACI- 12 Months (%) | |
|---|---|---|---|---|---|---|---|---|---|
| G | 95.13 ± 0.68 | 94.34 ± 1.31 | 92.31 ± 2.11 | 13.53 ± 0.23 | 1.16 ± 0.01 | 6.23 ± 1.04 | 0 | >6 | 89.82 ± 2.85 |
| H | 80.35 ± 1.36 | 90.33 ± 1.22 | 94.88 ± 3.16 | 12.92 ± 0.42 | 1.14 ± 0.02 | 7.16 ± 0.52 | 0 | >6 | 95.20 ± 3.94 |
| I | 94.72 ± 0.75 | 93.64 ± 1.23 | 90.56 ± 1.79 | 13.50 ± 0.51 | 1.16 ± 0.01 | 7.70 ± 0.41 | 0 | >6 | 89.61 ± 3.01 |
| J | 97.34 ± 0.29 | 92.24 ± 1.40 | 92.64 ± 1.94 | 13.35 ± 0.34 | 1.15 ± 0.02 | 8.00 ± 0.63 | 0 | >6 | 86.45 ± 4.23 |
| Friability (%) | Floating Lag Time (s) | Floating Duration (h) | Force of Adhesion (N) | Z-average (nm)— 15 min | Z-average (nm)— 6 h | |
|---|---|---|---|---|---|---|
| G | 0.51 | 275 ± 12 | >6 | 1.18 ± 0.35 | 521.40 ± 71.99 | 529.70 ± 6.30 |
| H | 0.43 | 220 ± 10 | >6 | 1.23 ± 0.41 | 428.23 ± 45.68 | 642.47 ± 16.93 |
| I | 0.60 | 284 ± 12 | >6 | 1.05 ± 0.33 | 578.90 ± 112.81 | 707.60 ± 24.94 |
| J | 0.61 | 251 ± 7 | >6 | 1.12 ± 0.54 | 480.45 ± 29.77 | 521.27 ± 18.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudrić, J.; Đekić, L.; Krgović, N.; Medarević, Đ.; Šavikin, K.; Radan, M.; Ćujić Nikolić, N.; Ilić, T.; Vidović, B.; Đuriš, J. Dual-Mechanism Gastroretentive Tablets with Encapsulated Gentian Root Extract. Pharmaceutics 2025, 17, 71. https://doi.org/10.3390/pharmaceutics17010071
Mudrić J, Đekić L, Krgović N, Medarević Đ, Šavikin K, Radan M, Ćujić Nikolić N, Ilić T, Vidović B, Đuriš J. Dual-Mechanism Gastroretentive Tablets with Encapsulated Gentian Root Extract. Pharmaceutics. 2025; 17(1):71. https://doi.org/10.3390/pharmaceutics17010071
Chicago/Turabian StyleMudrić, Jelena, Ljiljana Đekić, Nemanja Krgović, Đorđe Medarević, Katarina Šavikin, Milica Radan, Nada Ćujić Nikolić, Tijana Ilić, Bojana Vidović, and Jelena Đuriš. 2025. "Dual-Mechanism Gastroretentive Tablets with Encapsulated Gentian Root Extract" Pharmaceutics 17, no. 1: 71. https://doi.org/10.3390/pharmaceutics17010071
APA StyleMudrić, J., Đekić, L., Krgović, N., Medarević, Đ., Šavikin, K., Radan, M., Ćujić Nikolić, N., Ilić, T., Vidović, B., & Đuriš, J. (2025). Dual-Mechanism Gastroretentive Tablets with Encapsulated Gentian Root Extract. Pharmaceutics, 17(1), 71. https://doi.org/10.3390/pharmaceutics17010071













