Iodinated Copper–Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cu-Cy-I Nanoparticles
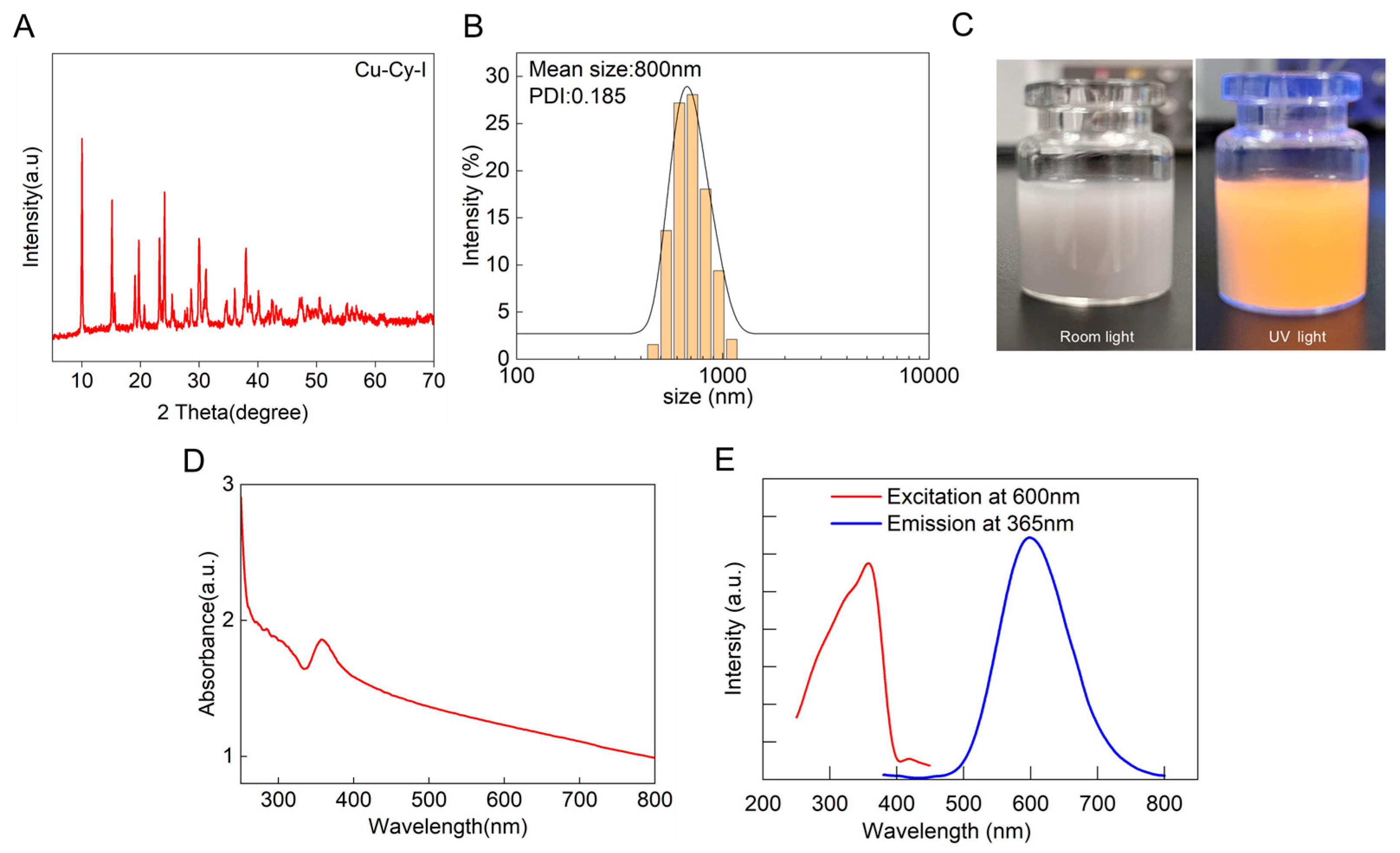
2.3. Characterization and Optical Properties of Cu-Cy-I Nanoparticles
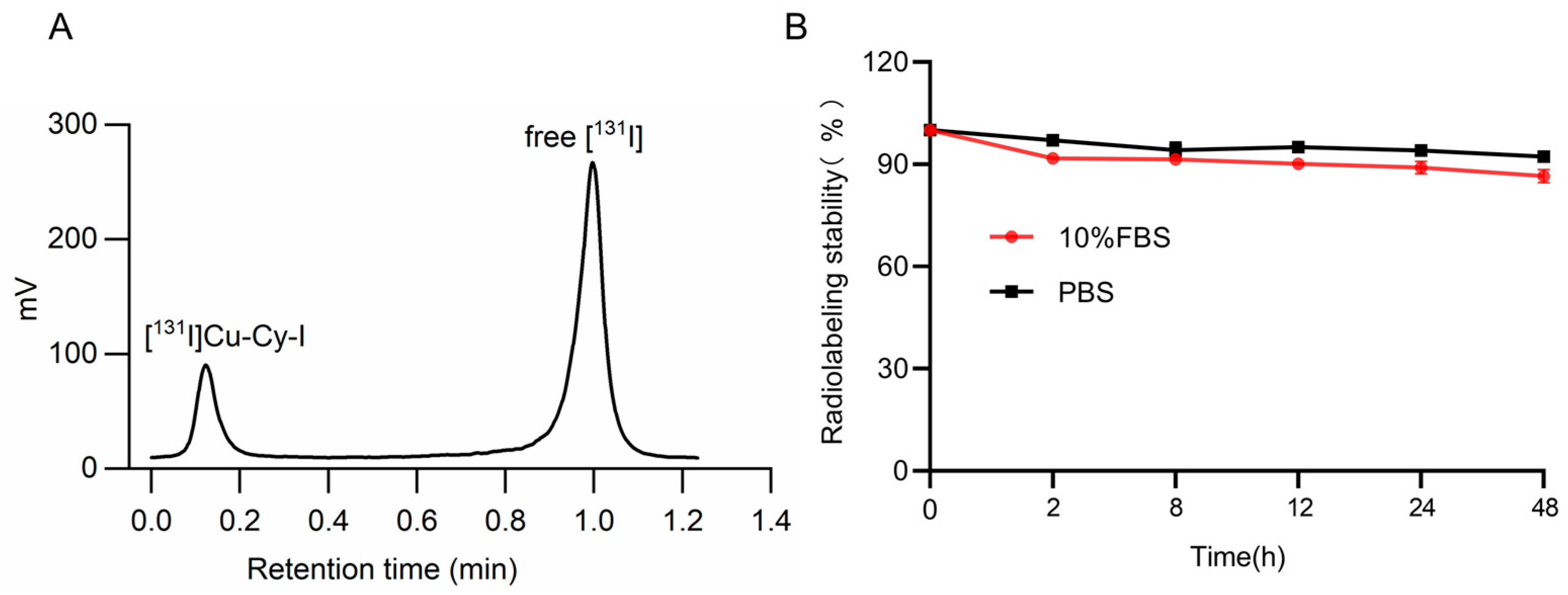
2.4. Preparation of [131I]Cu-Cy-I Nanoparticles
2.5. Tumor Cell Culture
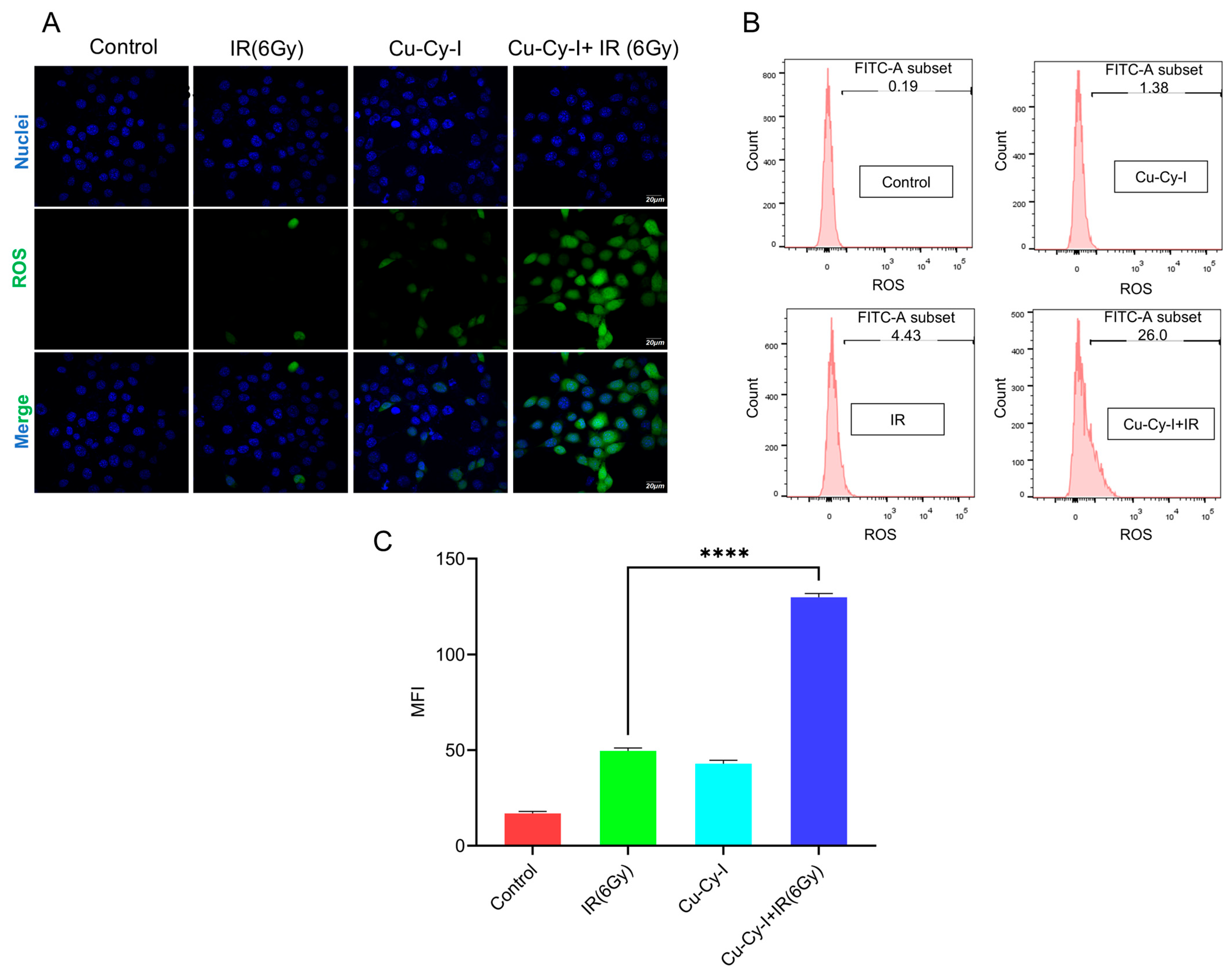
2.6. Detection of ROS in Tumor Cells
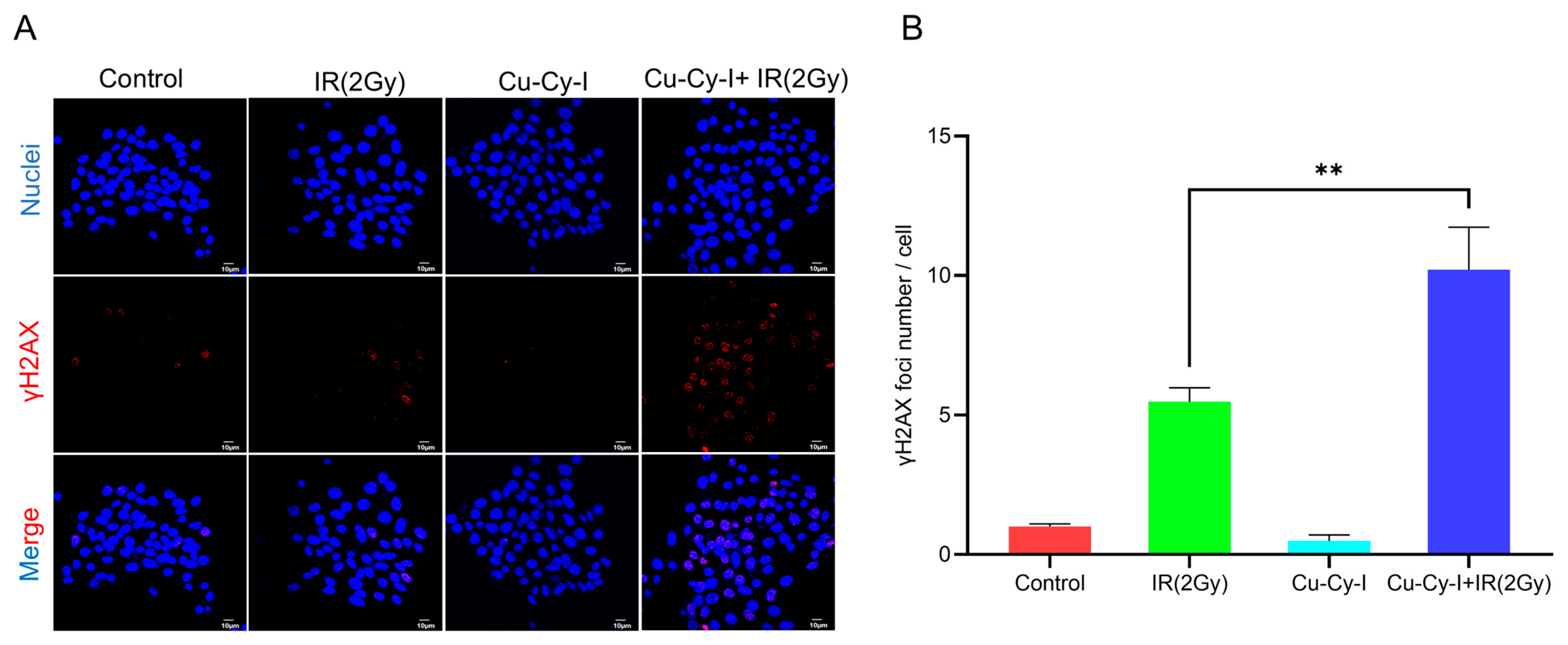
2.7. γH2AX Immunofluorescence of Tumor Cells
2.8. In Vitro Cytotoxicity Analysis
2.9. In Vivo Antitumor Assessment
2.10. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Cu-Cy-I Nanoparticles
3.2. Synthesis of [131I]Cu-Cy-I Nanoparticles and the Radiolabeling Stability
3.3. In Vitro ROS Generation in Tumor Cells Caused by Cu-Cy-I Nanoparticles and X-Ray Irradiation
3.4. Formation of γH2AX Foci in Tumor Cells Caused by Cu-Cy-I Nanoparticles and X-Ray Irradiation
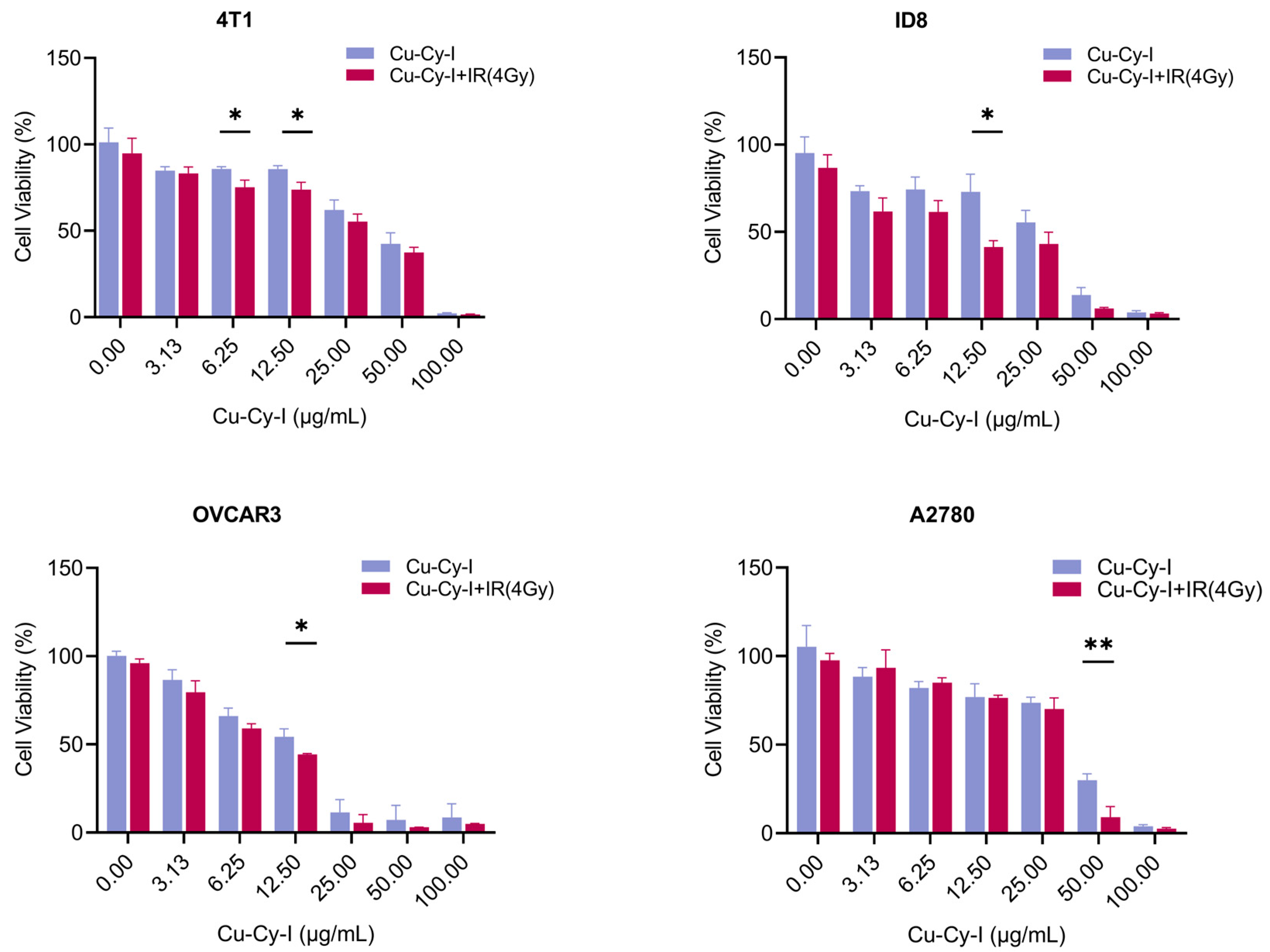
3.5. In Vitro Tumor Cell Toxicity of Cu-Cy-I Nanoparticles with X-Ray Irradiation
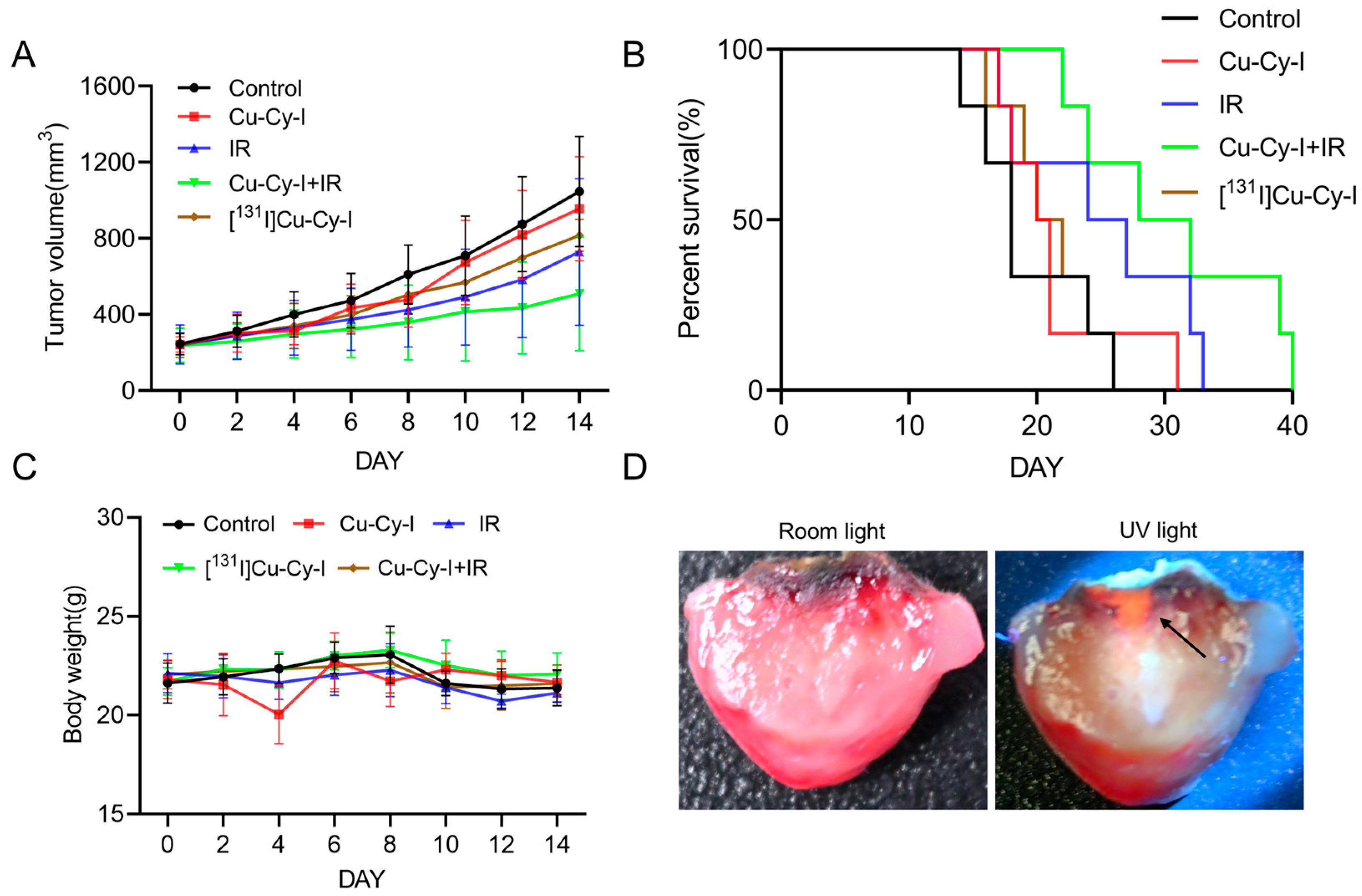
3.6. In Vivo Antitumor Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Chua, M.L.K.; Chitapanarux, I.; Kaidar-Person, O.; Mwaba, C.; Alghamdi, M.; Rodríguez Mignola, A.; Amrogowicz, N.; Yazici, G.; Bourhaleb, Z.; et al. Global radiotherapy demands and corresponding radiotherapy-professional workforce requirements in 2022 and predicted to 2050: A population-based study. Lancet Glob. Health 2024, 12, e1945–e1953. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 12, 791–803. [Google Scholar] [CrossRef]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Chen, Z.; Shen, X.C.; Ji, L.; Chao, H. Recent progress in metal complexes functionalized nanomaterials for photodynamic therapy. Chem. Commun. 2023, 59, 6956–6968. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhao, Y.; Sun, Y.; Cao, J. Recent Progress of Metal-Organic Framework-Based Photodynamic Therapy for Cancer Treatment. Int. J. Nanomed. 2022, 17, 2367–2395. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, J.; Li, X.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Li, S. Progress of Nanomaterials in Photodynamic Therapy Against Tumor. Front. Bioeng. Biotechnol. 2022, 10, 920162. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lo, Y.; Song, H.; Lu, J. Development of a Self-Luminescent Living Bioreactor for Enhancing Photodynamic Therapy in Breast Cancer. Bioconjug. Chem. 2024, 35, 1269–1282. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, N.; Hou, Z.; Shi, J.; Su, X.; Sun, X. Radioiodinated Persistent Luminescence Nanoplatform for Radiation-Induced Photodynamic Therapy and Radiotherapy. Adv. Healthc. Mater. 2021, 10, e2000802. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Daouk, J.; Dhaini, B.; Petit, J.; Frochot, C.; Barberi-Heyob, M.; Schohn, H. Can Cerenkov Light Really Induce an Effective Photodynamic Therapy? Radiation 2020, 1, 5–17. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ni, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Wei, H.; Ferreira, C.A.; Lan, X.; Engle, J.W.; He, Q.; Yu, F.; et al. A “Missile-Detonation” Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv. Mater. 2019, 31, e1904894. [Google Scholar] [CrossRef]
- Lioret, V.; Bellaye, P.S.; Bernhard, Y.; Moreau, M.; Guillemin, M.; Drouet, C.; Collin, B.; Decréau, R.A. Cherenkov Radiation induced photodynamic therapy—Repurposing older photosensitizers, and radionuclides. Photodiagn. Photodyn. Ther. 2023, 44, 103816. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In vivo photoactivation without “light”: Use of Cherenkov radiation to overcome the penetration limit of light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef]
- Liu, N.; Su, X.; Sun, X. Cerenkov radiation-activated probes for deep cancer theranostics: A review. Theranostics 2022, 12, 7404–7419. [Google Scholar] [CrossRef]
- Guo, J.; Feng, K.; Wu, W.; Ruan, Y.; Liu, H.; Han, X.; Shao, G.; Sun, X. Smart 131 I-Labeled Self-Illuminating Photosensitizers for Deep Tumor Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 21884–21889. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, F.; Rashidzadeh, H.; Ghaffarlou, M.; Salehiabar, M.; Ertas, Y.N.; Ramazani, A.; Abazari, M.; Rahmati, M.A.; Javed, Y.; Sharma, S.K.; et al. ROS-Based Cancer Radiotherapy. In Harnessing Materials for X-Ray Based Cancer Therapy and Imaging; Sharma, S.K., Nosrati, H., Kavetskyy, T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 265–309. [Google Scholar]
- Ma, L.; Zou, X.; Chen, W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef]
- Pandey, N.K.; Chudal, L.; Phan, J.; Lin, L.; Johnson, O.; Xing, M.; Liu, J.P.; Li, H.; Huang, X.; Shu, Y.; et al. A facile method for the synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment. J. Mater. Chem. B 2019, 7, 6630–6642. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Chudal, L.; Pandey, N.K.; Phan, J.; Ran, X.; Amador, E.; Huang, X.; Johnson, O.; Ran, Y.; Chen, W.; et al. A powerful combination of copper-cysteamine nanoparticles with potassium iodide for bacterial destruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110659. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Wu, J.; Vanasse, A.; Pandey, N.K.; Chudal, L.; Huang, Z.; Song, W.; Yu, H.; Ma, L.; Chen, W.; et al. Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-ray Induced Photodynamic Therapy. Nanomaterials 2020, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiong, L.; Ouyang, G.; Ma, L.; Sahi, S.; Wang, K.; Lin, L.; Huang, H.; Miao, X.; Chen, W.; et al. Investigation of Copper Cysteamine Nanoparticles as a New Type of Radiosensitiers for Colorectal Carcinoma Treatment. Sci. Rep. 2017, 7, 9290. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhaldi, N.; Pandey, N.; Chudal, L.; Wang, L.; Lin, L.; Zhang, M.; Yong, Y.; Amador, E.; Huda, M.; et al. A new type of cuprous-cysteamine sensitizers: Synthesis, optical properties and potential applications. Mater. Today Phys. 2021, 19, 100435. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.; Schatte, G.; Wang, W.; Joly, A.G.; Huang, Y.; Sammynaiken, R.; Hossu, M. A new Cu–cysteamine complex: Structure and optical properties. J. Mater. Chem. C 2014, 2, 4239–4246. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct. Target. Ther. 2020, 5, 58. [Google Scholar] [CrossRef]
- Alias, M.; Alkhaldi, N.D.; Reguero, M.; Ma, L.; Zhang, J.; De Graaf, C.; Huda, M.N.; Chen, W. Theoretical studies on the energy structures and optical properties of copper cysteamine—A novel sensitizer. Phys. Chem. Chem. Phys. 2019, 21, 21084–21093. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Santos, G.B.D.; Chiu, S.; Xue, L.; Berlin, J.C.; Peng, X.; Wu, H.; Lam, M.; et al. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef]
- Emfietzoglou, D.; Kostarelos, K.; Papakostas, A.; Yang, W.H.; Ballangrud, A.; Song, H.; Sgouros, G. Liposome-mediated radiotherapeutics within avascular tumor spheroids: Comparative dosimetry study for various radionuclides, liposome systems, and a targeting antibody. J. Nucl. Med. 2005, 46, 89–97. [Google Scholar]
- Phillips, W.T.; Bao, A.; Sou, K.; Li, S.; Goins, B. Chapter 10: Radiolabeled liposomes as drug delivery nano-theranostics. In Drug Delivery Applications of Noninvasive Imaging: Validation from Biodistribution to Sites of Action, 1st ed.; Jackson, E.F., Tian, M., Li, C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Goins, B.; Phillips, W.T.; Bao, A. Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opin. Drug Deliv. 2016, 13, 873–889. [Google Scholar] [CrossRef]
- Zweit, J. Radionuclides and carrier molecules for therapy. Phys. Med. Biol. 1996, 41, 1905–1914. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yang, Y.; Xu, Y.; Wang, J.; Li, S. Iodinated Copper–Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy. Pharmaceutics 2025, 17, 149. https://doi.org/10.3390/pharmaceutics17020149
Zhang M, Yang Y, Xu Y, Wang J, Li S. Iodinated Copper–Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy. Pharmaceutics. 2025; 17(2):149. https://doi.org/10.3390/pharmaceutics17020149
Chicago/Turabian StyleZhang, Miaomiao, Yu Yang, Ying Xu, Jie Wang, and Shihong Li. 2025. "Iodinated Copper–Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy" Pharmaceutics 17, no. 2: 149. https://doi.org/10.3390/pharmaceutics17020149
APA StyleZhang, M., Yang, Y., Xu, Y., Wang, J., & Li, S. (2025). Iodinated Copper–Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy. Pharmaceutics, 17(2), 149. https://doi.org/10.3390/pharmaceutics17020149





