Modulating Polyphenol Activity with Metal Ions: Insights into Dermatological Applications
Abstract
:1. Introduction
2. Literature Review Process
3. Wound Healing Aided by Polyphenol–Metal Combinations
4. Polyphenol–Metal Combinations Against Bacteria
5. Polyphenol–Metal Combinations as Future Anticancer Agents
| Combination | Effects | Molecular Pathway | References |
|---|---|---|---|
| Curcumin-Silver NPs | Induction of apoptosis; significant reduction in cell viability | Generation of ROS; activation of caspase pathways; inhibition of NF-κB signaling | [112,113,114] |
| Grape polyphenols + Gold NPs | Reduction in metastatic potential | Disruption of membrane integrity; modulation of MAPK pathways; induction of apoptosis via increased ROS | [115,116,117] |
| Resveratrol + Gold NPs | Inhibition of tumor growth; antiangiogenic effects | Activation of p38 MAPK pathway; suppression of VEGF; enhancement of ROS production leading to cell death | [118,119,120] |
| Quercetin + Zinc NPs | Inhibition of proliferation; enhancement of apoptosis | Inhibition of CDK activity leading to G1 arrest; reduction in pro-inflammatory cytokines | [121,122] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| US | United States |
| ROS | reactive oxygen species |
| VEGF | vascular endothelial growth factor |
| IL | interleukin |
| TNF-α | tumor necrosis factor alpha |
| DNA | deoxyribonucleic acid |
| EGCG | epigallocatechin gallate |
| MRSA | methicillin-resistant Staphylococcus aureus |
| UV | ultraviolet |
| CDK | cyclin-dependent kinases |
| MAPK | mitogen-activated protein kinase |
| MMPs | matrix metalloproteinase |
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, M.H.; Ng, W.L.; Liu, D.; Naing, M.W.; Yeong, W.Y.; Spiller, K.L.; Van Dyke, M.; Ng, K.W. Healing of Chronic Wounds: An Update of Recent Developments and Future Possibilities. Tissue Eng. Part B Rev. 2019, 25, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, J.; Fantuzzi, G.; Minshall, R.; Wu, S.; Oddo, V.M.; Koh, T.J. Racial/Ethnic Disparities in Chronic Wounds: Perspectives on Linking Upstream Factors to Health Outcomes. Wound Repair Regen. 2024, 32, 770–779. [Google Scholar] [CrossRef]
- Cho, S.K.; Mattke, S.; Gordon, H.; Sheridan, M.; Ennis, W. Development of a Model to Predict Healing of Chronic Wounds Within 12 Weeks. Adv. Wound Care 2020, 9, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds: Chronic Wound Care and Management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin Reduces Malondialdehyde and Improves Antioxidants in Humans with Diseased Conditions: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Biomedicine 2019, 9, 23. [Google Scholar] [CrossRef]
- Chen, B.; He, Q.; Chen, C.; Lin, Y.; Xiao, J.; Pan, Z.; Li, M.; Li, S.; Yang, J.; Wang, F.; et al. Combination of Curcumin and Catalase Protects against Chondrocyte Injury and Knee Osteoarthritis Progression by Suppressing Oxidative Stress. Biomed. Pharmacother. 2023, 168, 115751. [Google Scholar] [CrossRef]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The Biomedical Potential of Cellulose Acetate/Polyurethane Nanofibrous Mats Containing Reduced Graphene Oxide/Silver Nanocomposites and Curcumin: Antimicrobial Performance and Cutaneous Wound Healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A Review on Nanoparticle Based Treatment for Wound Healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for Wound Healing: Scope and Advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol. Pharm. 2021, 18, 550–575. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Littig, J.P.B.; Moellmer, R.; Estes, A.M.; Agrawal, D.K.; Rai, V. Increased Population of CD40+ Fibroblasts Is Associated with Impaired Wound Healing and Chronic Inflammation in Diabetic Foot Ulcers. J. Clin. Med. 2022, 11, 6335. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The Physiology of Wound Healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9871718-2-5. [Google Scholar]
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef]
- Arghand, N.; Reiisi, S.; Karimi, B.; Khorasgani, E.M.; Heidari, R. Biosynthesis of Nanocomposite Alginate-Chitosan Loaded with Silver Nanoparticles Coated with Eugenol/Quercetin to Enhance Wound Healing. BioNanoScience 2024, 14, 5149–5166. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Karamian, M.; Abedi, F.; Hanafi-Bojd, M.Y. Topical Treatment of Cutaneous Leishmaniasis Lesions Using Quercetin/Artemisia-Capped Silver Nanoparticles Ointment: Modulation of Inflammatory Response. Acta Trop. 2022, 228, 106325. [Google Scholar] [CrossRef]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin Loaded Silver Nanoparticles in Hydrogel Matrices for Diabetic Wound Healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, A.; Karamian, M.; Abedi, F.; Hanafi-Bojd, M.Y.; Ghatee, M.A.; Hemmati, M.; Alemzadeh, E. Topically Applied Luteolin/Quercetin-Capped Silver Nanoparticle Ointment as Antileishmanial Composite: Acceleration Wound Healing in BALB/c Mice. Adv. Mater. Sci. Eng. 2023, 2023, 1878170. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Zhang, H.; Kang, Y.; Wang, A. Incorporation of Silver Nanoparticles/Curcumin/Clay Minerals into Chitosan Film for Enhancing Mechanical Properties, Antioxidant and Antibacterial Activity. Int. J. Biol. Macromol. 2022, 223, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Z.; Chen, J.; He, K.; Lin, J.; Zhang, H.; Zeng, Y. Synergistic Delivery of Resveratrol and Ultrasmall Copper-Based Nanoparticles by Aptamer-Functionalized Ultrasound Nanobubbles for the Treatment of Nonalcoholic Fatty Liver Disease. Front. Physiol. 2022, 13, 950141. [Google Scholar] [CrossRef]
- Sharma, R.; Basist, P.; Alhalmi, A.; Khan, R.; Noman, O.M.; Alahdab, A. Synthesis of Quercetin-Loaded Silver Nanoparticles and Assessing Their Anti-Bacterial Potential. Micromachines 2023, 14, 2154. [Google Scholar] [CrossRef]
- Chahardoli, A.; Hajmomeni, P.; Ghowsi, M.; Qalekhani, F.; Shokoohinia, Y.; Fattahi, A. Optimization of Quercetin-Assisted Silver Nanoparticles Synthesis and Evaluation of Their Hemocompatibility, Antioxidant, Anti-Inflammatory, and Antibacterial Effects. Glob. Chall. 2021, 5, 2100075. [Google Scholar] [CrossRef]
- Usama, S.; Riaz, M.; Ali, B.; Khan, D.S.; Ahmad, S.; Ahmad, Z. Hydrogel-Plant Extract Composites in Wound Healing. Phytopharm. Res. J. 2024, 3, 26–29. [Google Scholar]
- Wang, J.; Sun, Y.; Liu, X.; Kang, Y.; Cao, W.; Ye, J.; Gao, C. An Antibacterial and Anti-Oxidant Hydrogel Containing Hyperbranched Poly-l-Lysine and Tea Polyphenols Accelerates Healing of Infected Wound. Biomater. Adv. 2024, 157, 213755. [Google Scholar] [CrossRef]
- Lungu, I.I.; Cioanca, O.; Mircea, C.; Tuchilus, C.; Stefanache, A.; Huzum, R.; Hancianu, M. Insights into Catechin–Copper Complex Structure and Biologic Activity Modulation. Molecules 2024, 29, 4969. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper Nanoparticles Promote Rapid Wound Healing in Acute Full Thickness Defect via Acceleration of Skin Cell Migration, Proliferation, and Neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xie, H.; Li, S.; Kang, Y.J. Copper Stimulates Growth of Human Umbilical Vein Endothelial Cells in a Vascular Endothelial Growth Factor-Independent Pathway. Exp. Biol. Med. 2012, 237, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Trincavelli, M.L.; Satriano, C.; Hansson, Ö.; La Mendola, D.; Rizzarelli, E.; Martini, C. ♦Copper (II) Ions Modulate Angiogenin Activity in Human Endothelial Cells. Int. J. Biochem. Cell Biol. 2015, 60, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.-P.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Yaman, I.; Derici, H.; Kara, C.; Kamer, E.; Diniz, G.; Ortac, R.; Sayin, O. Effects of Resveratrol on Incisional Wound Healing in Rats. Surg. Today 2013, 43, 1433–1438. [Google Scholar] [CrossRef]
- Xue, R.; He, L.; Wu, J.; Kong, X.; Wang, Q.; Chi, Y.; Liu, J.; Wang, Z.; Zeng, K.; Chen, W.; et al. Multifunctional Sprayable Carboxymethyl Chitosan/Polyphenol Hydrogel for Wound Healing. Int. J. Biol. Macromol. 2024, 275, 133303. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Li, S.; Huang, L.; Zhang, R.; Zhang, L.; Yu, A.; Duan, B. Polyphenol-Driving Assembly for Constructing Chitin-Polyphenol-Metal Hydrogel as Wound Dressing. Carbohydr. Polym. 2022, 290, 119444. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Wei, Y.; Wang, Y.; Jin, Z.; Ma, G.; Jiang, Y.; Zhang, W.; Hu, Z. Facile Preparation of Polyphenol-Crosslinked Chitosan-Based Hydrogels for Cutaneous Wound Repair. Int. J. Biol. Macromol. 2023, 228, 99–110. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Zhang, H.; Wu, X.; Li, X.; Zhou, C.; Ao, Q. Polyphenol Enhances the Functionality of Borate Hydrogel in Wound Repair by Regulating the Wound Microenvironment. Colloids Surf. B Biointerfaces 2025, 247, 114390. [Google Scholar] [CrossRef]
- Tang, X.; Li, L.; You, G.; Li, X.; Kang, J. Metallic Elements Combine with Herbal Compounds Upload in Microneedles to Promote Wound Healing: A Review. Front. Bioeng. Biotechnol. 2023, 11, 1283771. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ahmad, A.; Mishra, R.K.; Raza, S.S.; Khan, R. Zinc Gluconate-Loaded Chitosan Nanoparticles Reduce Severity of Collagen-Induced Arthritis in Wistar Rats. ACS Biomater. Sci. Eng. 2019, 5, 3380–3397. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Stefanache, A.; Crivoi, F.; Burec, A.-F.; Belei, D.; Cioanca, O.; Hancianu, M. INNOVATIVE SYNTHESIS OF ZINC AND SELENIUM COMPLEXES WITH GALLIC ACID: EXPLORING THEIR ANTIOXIDANT POTENTIAL. Med.-Surg. J. 2024, 128, 177–188. [Google Scholar] [CrossRef]
- Han, B.; Fang, W.H.; Zhao, S.; Yang, Z.; Hoang, B.X. Zinc Sulfide Nanoparticles Improve Skin Regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102263. [Google Scholar] [CrossRef]
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A Cut above the Rest: Oxidative Stress in Chronic Wounds and the Potential Role of Polyphenols as Therapeutics. J. Pharm. Pharmacol. 2022, 74, 485–502. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Li, L.; Guo, F.; Ma, L.; Fu, P.; Wang, Y. Chitosan Coated Bacteria Responsive Metal-Polyphenol Coating as Efficient Platform for Wound Healing. Compos. Part B Eng. 2022, 234, 109665. [Google Scholar] [CrossRef]
- Yu, R.; Chen, H.; He, J.; Zhang, Z.; Zhou, J.; Zheng, Q.; Fu, Z.; Lu, C.; Lin, Z.; Caruso, F.; et al. Engineering Antimicrobial Metal–Phenolic Network Nanoparticles with High Biocompatibility for Wound Healing. Adv. Mater. 2024, 36, 2307680. [Google Scholar] [CrossRef]
- Huang, X.; Fu, M.; Lu, M.; Wu, X.; Hong, W.D.; Wang, X.; Wu, P.; Wu, K. Polyphenol-Based Photothermal Nanoparticles with Sprayable Capability for Self-Regulation of Microenvironment to Accelerate Diabetic Wound Healing. Eng. Regen. 2024, 5, 505–520. [Google Scholar] [CrossRef]
- Ohanyan, N.; Abelyan, N.; Manukyan, A.; Hayrapetyan, V.; Chailyan, S.; Tiratsuyan, S.; Danielyan, K. Tannin–Albumin Particles as Stable Carriers of Medicines. Nanomedicine 2024, 19, 689–708. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver Nanoparticles: Advanced and Promising Technology in Diabetic Wound Therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Choudhary, A.; Kant, V.; Jangir, B.L.; Joshi, V.G. Quercetin Loaded Chitosan Tripolyphosphate Nanoparticles Accelerated Cutaneous Wound Healing in Wistar Rats. Eur. J. Pharmacol. 2020, 880, 173172. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Nakajima, N.; Guo, J.; Mitragotri, S. Engineering of Bioactive Nanocomplexes on Dental Floss for Targeted Gingival Therapy. Bioeng. Transl. Med. 2023, 8, e10452. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of Interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Gowda, B.H.J.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.A.; Ghosh, S.; Ansari, M.J.; Pramanik, S. Nanoparticle-Based Therapeutic Approaches for Wound Healing: A Review of the State-of-the-Art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, F.; Liu, Y.; Yang, M.; Zhang, B.; Bai, Z.; Zhao, B.; Li, X. Recent Advances of Copper-Based Metal Phenolic Networks in Biomedical Applications. Colloids Surf. B Biointerfaces 2024, 244, 114163. [Google Scholar] [CrossRef]
- Flieger, J.; Tatarczak-Michalewska, M.; Blicharska, E.; Świeboda, R.; Banach, T. HPLC Identification of Copper (II)-Trans-Resveratrol Complexes in Ethanolic Aqueous Solution. J. Chromatogr. Sci. 2017, 55, 445–450. [Google Scholar] [CrossRef]
- Moodi, Z.; Bagherzade, G.; Peters, J. Quercetin as a Precursor for the Synthesis of Novel Nanoscale Cu (II) Complex as a Catalyst for Alcohol Oxidation with High Antibacterial Activity. Bioinorg. Chem. Appl. 2021, 2021, 8818452. [Google Scholar] [CrossRef]
- Somturk Yilmaz, B. Anticancer and Antimicrobial Activities of Quercetin-CuhNFs and Quercetin-CohNFs on MDA-MB-231 (Breast Cancer). J. Inorg. Organomet. Polym. 2024. [Google Scholar] [CrossRef]
- Ramzan, N.; Abbas, G.; Mahmood, K.; Aziz, M.; Rasul, S.; Ahmed, N.; Shah, S.; Uzair, M.; Usman, M.; Khan, W.S.; et al. Concomitant Effect of Quercetin and Its Copper Complex in the Development of Sustained-Release Nanoparticles of Polycaprolactone, Used for the Treatment of Skin Infection. Mol. Pharm. 2023, 20, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.M.; Hamza, R.Z. Synthesis, Spectroscopic Characterizations, Conductometric Titration and Investigation of Potent Antioxidant Activities of Gallic Acid Complexes with Ca (II), Cu (II), Zn(III), Cr(III) and Se (IV) Metal Ions. J. Mol. Liq. 2022, 358, 119196. [Google Scholar] [CrossRef]
- Hamedi, H.; Javanbakht, S.; Mohammadi, R. In-Situ Synthesis of Copper-Gallic Acid Metal–Organic Framework into the Gentamicin-Loaded Chitosan Hydrogel Bead: A Synergistic Enhancement of Antibacterial Properties. J. Ind. Eng. Chem. 2024, 133, 454–463. [Google Scholar] [CrossRef]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial Activity of Olive Oil Polyphenol Extract Against Salmonella Typhimurium and Staphylococcus Aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Nayik, G.A.; Gupta, S.D.; Areche, F.O.; Jagdale, Y.D.; Ansari, M.J.; Hemeg, H.A.; AL-Farga, A.; Alotaibi, S.S. Chemical Aspects of Polyphenol-Protein Interactions and Their Antibacterial Activity. Crit. Rev. Food Sci. Nutr. 2023, 63, 9482–9505. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial Activities of Polyphenols against Foodborne Pathogens and Their Application as Antibacterial Agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial Activity of Polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent Advances in the Development and Antimicrobial Applications of Metal–Phenolic Networks. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, X.; Ruan, H.; Yang, J.; Wei, W.; Wu, Y.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Curcumin-Stabilized Silver Nanoparticles Encapsulated in Biocompatible Electrospun Nanofibrous Scaffold for Sustained Eradication of Drug-Resistant Bacteria. J. Hazard. Mater. 2023, 452, 131290. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubaiti, E.H. Antibacterial and Antioxidant Activities of Curcumin/Zn Metal Complex with Its Chemical Characterization and Spectroscopic Studies. Heliyon 2023, 9, e17468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced Antibacterial Activity of Curcumin by Combination With Metal Ions. Colloid Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Syed, H.K.; Iqbal, M.A.; Haque, R.A.; Peh, K.-K. Synthesis, Characterization and Antibacterial Activity of a Curcumin–Silver(I) Complex. J. Coord. Chem. 2015, 68, 1088–1100. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Lin, L.; Jiao, Y.; Zhou, C.; Liu, Z. Polyphenol and Cu2+ Surface-Modified Chitin Sponge Synergizes with Antibacterial, Antioxidant and pro-Vascularization Activities for Effective Scarless Regeneration of Burned Skin. Chem. Eng. J. 2021, 419, 129488. [Google Scholar] [CrossRef]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef]
- Fujie, T.; Murakami, M.; Yoshida, E.; Tachinami, T.; Shinkai, Y.; Fujiwara, Y.; Yamamoto, C.; Kumagai, Y.; Naka, H.; Kaji, T. Copper Diethyldithiocarbamate as an Activator of Nrf2 in Cultured Vascular Endothelial Cells. J. Biol. Inorg. Chem. 2016, 21, 263–273. [Google Scholar] [CrossRef]
- Trickler, W.J.; Lantz, S.M.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Paule, M.G.; Slikker, W.; Biris, A.S.; Hussain, S.M.; et al. Effects of Copper Nanoparticles on Rat Cerebral Microvessel Endothelial Cells. Nanomedicine 2012, 7, 835–846. [Google Scholar] [CrossRef]
- Vatani, E.; Shayestehpour, M.; Motallebi, M.; Razmjoue, D.; Moosavi, G.A.; Khaledi, A.; Rahimi, M. Antimicrobial Effect of Curcumin Nanoparticles and Ferulago Angulate Boiss Extract Against Methicillin-Resistant Staphylococcus Aureus (MRSA) Isolated from Wound Infections. BioNanoScience 2024, 14, 2228–2236. [Google Scholar] [CrossRef]
- El-Kattan, N.; Emam, A.N.; Mansour, A.S.; Ibrahim, M.A.; El-Razik, A.B.A.; Allam, K.A.M.; Youssef Riad, N.; Ibrahim, S.A. Curcumin Assisted Green Synthesis of Silver and Zinc Oxide Nanostructures and Their Antibacterial Activity against Some Clinical Pathogenic Multi-Drug Resistant Bacteria. RSC Adv. 2022, 12, 18022–18038. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-Encapsulated Nanoparticles as Innovative Antimicrobial and Wound Healing Agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhang, L.; Yang, J.; Zhu, X.; Wei, W.; Ji, M.; Jiang, H.; Chen, J. Encapsulating Antibiotic and Protein-Stabilized Nanosilver into Sandwich-Structured Electrospun Nanofibrous Scaffolds for MRSA-Infected Wound Treatment. ACS Appl. Mater. Interfaces 2023, 15, 48978–48995. [Google Scholar] [CrossRef] [PubMed]
- Avila, S.R.R.; Schuenck, G.P.D.; Silva, L.P.C.e.; Keijok, W.J.; Xavier, L.M.; Endringer, D.C.; Oliveira, J.P.; Schuenck, R.P.; Guimarães, M.C.C. High Antibacterial in Vitro Performance of Gold Nanoparticles Synthesized by Epigallocatechin 3-Gallate. J. Mater. Res. 2021, 36, 518–532. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, E.-Y.; Park, Y. Shape-Dependent Cytotoxicity and Cellular Uptake of Gold Nanoparticles Synthesized Using Green Tea Extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef]
- Xu, F.-W.; Lv, Y.-L.; Zhong, Y.-F.; Xue, Y.-N.; Wang, Y.; Zhang, L.-Y.; Hu, X.; Tan, W.-Q. Beneficial Effects of Green Tea EGCG on Skin Wound Healing: A Comprehensive Review. Molecules 2021, 26, 6123. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Lu, K.; Zou, Y.; Jia, D.; Yang, H.; Chen, H.; Zhang, Y.; Yu, Q. Bi-Functional Quercetin/Copper Nanoparticles Integrating Bactericidal and Anti-Quorum Sensing Properties for Preventing the Formation of Biofilms. Biomater. Sci. 2024, 12, 1788–1800. [Google Scholar] [CrossRef]
- Rohatgi, N.; Ganapathy, D.; Sathishkumar, P. Eradication of Pseudomonas Aeruginosa Biofilm Using Quercetin-Mediated Copper Oxide Nanoparticles Incorporated in the Electrospun Polycaprolactone Nanofibrous Scaffold. Microb. Pathog. 2023, 185, 106453. [Google Scholar] [CrossRef]
- Arasoğlu, T.; Derman, S.; Mansuroğlu, B.; Uzunoğlu, D.; Koçyiğit, B.; Gümüş, B.; Acar, T.; Tuncer, B. Preparation, Characterization, and Enhanced Antimicrobial Activity: Quercetin-Loaded PLGA Nanoparticles against Foodborne Pathogens. Turk. J. Biol. 2017, 41, 127–140. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M. del V.; Machado, F. Evaluation of the Antimicrobial, Antioxidant and Physicochemical Properties of Poly(Vinyl Chloride) Films Containing Quercetin and Silver Nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef]
- Rahaiee, S.; Ranjbar, M.; Azizi, H.; Govahi, M.; Zare, M. Green Synthesis, Characterization, and Biological Activities of Saffron Leaf Extract-Mediated Zinc Oxide Nanoparticles: A Sustainable Approach to Reuse an Agricultural Waste. Appl. Organomet. Chem. 2020, 34, e5705. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Z.; Hei, Y.; Wei, W.; Chen, D. The Antibacterial Efficacy and Mechanism of Tea Polyphenol Against Drug-Resistant Aeromonas Veronii TH0426 In Vitro. Foodborne Pathog. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, J.; Qiu, Y.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Structure-Dependent Inhibition of Stenotrophomonas Maltophilia by Polyphenol and Its Impact on Cell Membrane. Front. Microbiol. 2019, 10, 2646. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Amini, S.M.; Mostafavi, S.H.; Amini, S.M. An Overview of Antimicrobial Efficacy of Curcumin-Silver Nanoparticles. Nanomed. Res. J. 2021, 6, 105–111. [Google Scholar] [CrossRef]
- Siriphap, A.; Kiddee, A.; Duangjai, A.; Yosboonruang, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Rawangkan, A. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio Cholerae. Antibiotics 2022, 11, 518. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid–Membrane Interactions: Involvement of Flavonoid–Metal Complexes in Raft Signaling. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-Positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.-G.; Yoon, H.; Choi, Y.-S.; Yoon, J.; Lee, J.-C. Polyphenol/FeIII Complex Coated Membranes Having Multifunctional Properties Prepared by a One-Step Fast Assembly. Adv. Mater. Interfaces 2015, 2, 1500298. [Google Scholar] [CrossRef]
- Yao, T.; Zeng, X.; Li, H.; Luo, T.; Tao, X.; Xu, H. Metal-Polyphenol Coordination Nanosheets with Synergistic Peroxidase-like and Photothermal Properties for Efficient Antibacterial Treatment. Int. J. Biol. Macromol. 2024, 269, 132115. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Craythorne, E.; Al-Niami, F. Skin Cancer. Medicine 2017, 45, 431–434. [Google Scholar] [CrossRef]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. In Sunlight, Vitamin D and Skin Cancer; Reichrath, J., Ed.; Springer: New York, NY, USA, 2014; pp. 120–140. ISBN 978-1-4939-0437-2. [Google Scholar]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A Systematic Review of Worldwide Incidence of Nonmelanoma Skin Cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-Melanoma Skin Cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J.-N. Curcumin Induces Apoptosis through Mitochondrial Pathway and Caspases Activation in Human Melanoma Cells. Mol. Biol. Rep. 2015, 42, 267–275. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, Y.Q.; Lv, Y.T.; Wang, J.M. Effect of Curcumin on the Proliferation, Apoptosis, Migration, and Invasion of Human Melanoma A375 Cells. Genet. Mol. Res. 2015, 14, 1056–1067. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, Y.-S. Synergistic Apoptosis-Inducing Effects on A375 Human Melanoma Cells of Natural Borneol and Curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef]
- Lungu, I.I. SYNTHESIS AND BIOACTIVITY OF A NOVEL DANDELION-LIKE SELENIUM AND CATECHIN COMPLEX. FARMACIA 2024, 72, 346–357. [Google Scholar] [CrossRef]
- Karan, T.; Erenler, R.; Bozer, B.M. Synthesis and Characterization of Silver Nanoparticles Using Curcumin: Cytotoxic, Apoptotic, and Necrotic Effects on Various Cell Lines. Z. Naturforschung C 2022, 77, 343–350. [Google Scholar] [CrossRef]
- Ali, I.; Ahmed, S.B.M.; Elhaj, B.M.; Ali, H.S.; Alsubaie, A.; Almalki, A.S.A. Enhanced Anticancer Activities of Curcumin-Loaded Green Gum Acacia-Based Silver Nanoparticles against Melanoma and Breast Cancer Cells. Appl. Nanosci. 2021, 11, 2679–2687. [Google Scholar] [CrossRef]
- Sheikh, E.; Bhatt, M.L.; Tripathi, M. Bio-Based Synthesised and Characterized Monodispersed Curcuma Longa Silver Nanoparticles Induces Targeted Anticancer Activity in Breast Cancer Cells. Pharmacogn. Mag. 2018, 14, s340–s345. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Akila, S.; Narendhirakannan, R.T.; Chatterjee, S. Vitis Vinifera Peel Polyphenols Stabilized Gold Nanoparticles Induce Cytotoxicity and Apoptotic Cell Death in A431 Skin Cancer Cell Lines. Adv. Powder Technol. 2017, 28, 1170–1184. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Akila, S.; Nadar, M.S.A.M.; Narendhirakannan, R.T.; Chatterjee, S. Biosynthesized Vitis Vinifera Seed Gold Nanoparticles Induce Apoptotic Cell Death in A431 Skin Cancer Cells. RSC Adv. 2016, 6, 82205–82218. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Narendhirakannan, R.T. Vitis Vinifera Peel and Seed Gold Nanoparticles Exhibit Chemopreventive Potential, Antioxidant Activity and Induce Apoptosis through Mutant P53, Bcl-2 and Pan Cytokeratin down-Regulation in Experimental Animals. Biomed. Pharmacother. 2017, 89, 902–917. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Hua, L.-H.; Chen, B.-H. Comparative Study on Inhibition of Pancreatic Cancer Cells by Resveratrol Gold Nanoparticles and a Resveratrol Nanoemulsion Prepared from Grape Skin. Pharmaceutics 2021, 13, 1871. [Google Scholar] [CrossRef]
- Yang, T.; Ren, H.; Zhang, W.; Rong, L.; Zhang, D. Resveratrol-Coated Gold Nanoflowers for CT Imaging and Apoptosis/Photothermal Synergistic Therapy of Malignant Melanoma. ACS Omega 2023, 8, 34629–34639. [Google Scholar] [CrossRef]
- Thipe, V.C.; Panjtan Amiri, K.; Bloebaum, P.; Raphael Karikachery, A.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of Resveratrol-Conjugated Gold Nanoparticles: Interrelationship of Increased Resveratrol Corona on Anti-Tumor Efficacy against Breast, Pancreatic and Prostate Cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef]
- Nisar, M.F.; Yousaf, M.; Saleem, M.; Khalid, H.; Niaz, K.; Yaqub, M.; Waqas, M.Y.; Ahmed, A.; Abaid-Ullah, M.; Chen, J.; et al. Development of Iron Sequester Antioxidant Quercetin@ZnO Nanoparticles with Photoprotective Effects on UVA-Irradiated HaCaT Cells. Oxidative Med. Cell. Longev. 2021, 2021, 6072631. [Google Scholar] [CrossRef]
- Kalam, M.A.; Ali, R.; Alhowyan, A.; Ahmad, A.; Iqbal, M.; Raish, M. Quercetin-Loaded Transliposomal Gel for Effective Management of Skin Cancer: In Vitro and Cell Line Efficacy Studies. J. Drug Deliv. Sci. Technol. 2024, 96, 105659. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro Polyphenol Effects on Apoptosis: An Update of Literature Data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 Signaling Pathways Collaboratively Link Inflammation to Cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Monych, N.K.; Ghosh, S.; Turner, D.L.; Turner, R.J. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef]
- Tabrez, S.; Priyadarshini, M.; Urooj, M.; Shakil, S.; Ashraf, G.M.; Khan, M.S.; Kamal, M.A.; Alam, Q.; Jabir, N.R.; Abuzenadah, A.M.; et al. Cancer Chemoprevention by Polyphenols and Their Potential Application as Nanomedicine. J. Environ. Sci. Health Part C 2013, 31, 67–98. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Shamsnia, H.S.; Roustaei, M.; Ahmadvand, D.; Butler, A.E.; Amirlou, D.; Soltani, S.; Momtaz, S.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Impact of Curcumin on P38 MAPK: Therapeutic Implications. Inflammopharmacol 2023, 31, 2201–2212. [Google Scholar] [CrossRef]
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; Alarcón de la Lastra, C. Curcumin, a Curcuma Longa Constituent, Acts on MAPK P38 Pathway Modulating COX-2 and iNOS Expression in Chronic Experimental Colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef]
- Jeong, C.-W.; Yoo, K.Y.; Lee, S.H.; Jeong, H.J.; Lee, C.S.; Kim, S.J. Curcumin Protects Against Regional Myocardial Ischemia/Reperfusion Injury Through Activation of RISK/GSK-3β and Inhibition of P38 MAPK and JNK. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 387–394. [Google Scholar] [CrossRef]
- Li, G.; Duan, L.; Yang, F.; Yang, L.; Deng, Y.; Yu, Y.; Xu, Y.; Zhang, Y. Curcumin Suppress Inflammatory Response in Traumatic Brain Injury via P38/MAPK Signaling Pathway. Phytother. Res. 2022, 36, 1326–1337. [Google Scholar] [CrossRef]
- Korkina, L.G.; De Luca, C.; Kostyuk, V.A.; Pastore, S. Plant Polyphenols and Tumors: From Mechanisms to Therapies, Prevention, and Protection Against Toxicity of Anti-Cancer Treatments. Curr. Med. Chem. 2009, 16, 3943–3965. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Neupane, N.P.; Otuechere, C.A.; Yadav, J.P.; Bhat, M.A.; Al-Omar, M.A.; Yadav, P.; Verma, A. Cutaneous Wound-Healing Activity of Quercetin-Functionalized Bimetallic Nanoparticles. Chem. Biodivers. 2024, e202401551. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Tuyet, P.-T. Synthesis and Biological Evaluation of Quercetin–Zinc (II) Complex for Anti-Cancer and Anti-Metastasis of Human Bladder Cancer Cells. In Vitro Cell. Dev. Biol.-Anim. 2019, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.B.; Nagaarudkumaran, N.; Kalyaanamoorthy, S.; Ngo, W. In Silico and In Vitro Approach for Validating the Inhibition of Matrix Metalloproteinase-9 by Quercetin. Eye Contact Lens 2023, 49, 193. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic Potential of Nutraceuticals: Mechanisms and Prospects for Futuristic Medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef]
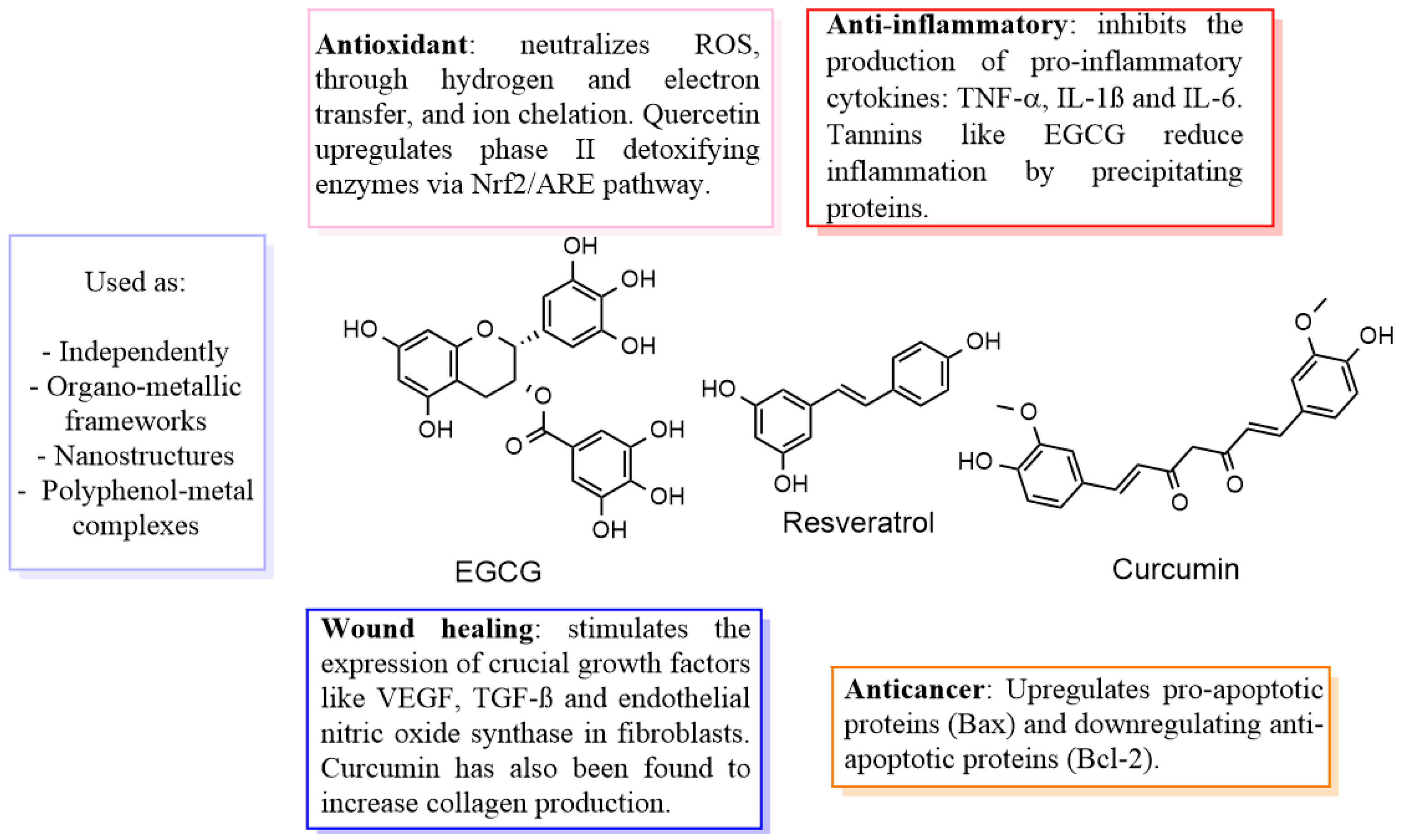
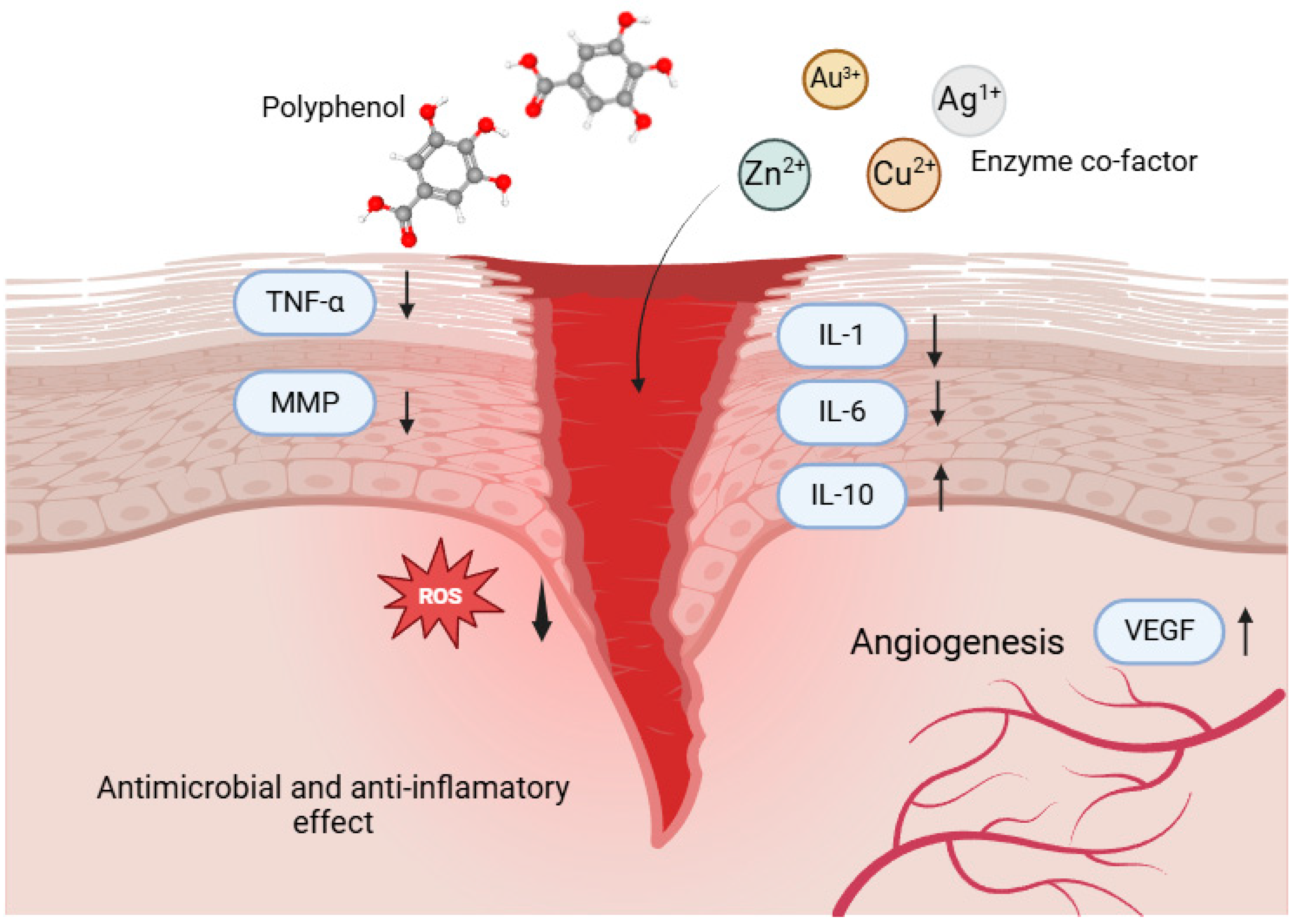

| Combination | Properties | Mechanisms | Reference |
|---|---|---|---|
| Silver NPs + Curcumin | Enhances fibroblast activity | Scavenges ROS; enhances cell migration and proliferation | [24] |
| Copper NPs + Resveratrol | Essential for angiogenesis, promotes collagen synthesis | Stimulates endothelial cell proliferation; supports VEGF signaling for new blood vessel formation | [25,26] |
| Quercetin-Stabilized silver NPs | Provides dual antibacterial and anti-inflammatory benefits | Scavenges free radicals; modulates inflammatory pathways; enhances fibroblast activity | [22,27,28] |
| Polyphenol-Infused hydrogels | Biocompatible; maintains moist environment | Sustained release of bioactive compounds; modulates inflammation; facilitates localized delivery | [29,30] |
| Combination | Target Bacteria | Effects | References |
|---|---|---|---|
| Curcumin-Stabilized silver NPs | MRSA | Significant reduction in viability; inhibition of biofilm formation | [82,83,84,85] |
| Epigallocatechin gallate (EGCG) + Gold NPs | Escherichia coli | Broad-spectrum antibacterial activity; enhanced stability | [86,87,88] |
| Quercetin + Copper NPs | Pseudomonas aeruginosa | Increase in bioavailability; enhanced anti-inflammatory effects; biofilm inhibition | [89,90] |
| Quercetin + Zinc/Silver NPs | Listeria monocytogenes | Significant bacterial inhibition; membrane disfunction | [14,91,92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioanca, O.; Lungu, I.-I.; Batir-Marin, D.; Lungu, A.; Marin, G.-A.; Huzum, R.; Stefanache, A.; Sekeroglu, N.; Hancianu, M. Modulating Polyphenol Activity with Metal Ions: Insights into Dermatological Applications. Pharmaceutics 2025, 17, 194. https://doi.org/10.3390/pharmaceutics17020194
Cioanca O, Lungu I-I, Batir-Marin D, Lungu A, Marin G-A, Huzum R, Stefanache A, Sekeroglu N, Hancianu M. Modulating Polyphenol Activity with Metal Ions: Insights into Dermatological Applications. Pharmaceutics. 2025; 17(2):194. https://doi.org/10.3390/pharmaceutics17020194
Chicago/Turabian StyleCioanca, Oana, Ionut-Iulian Lungu, Denisa Batir-Marin, Andreea Lungu, George-Alexandru Marin, Riana Huzum, Alina Stefanache, Nazim Sekeroglu, and Monica Hancianu. 2025. "Modulating Polyphenol Activity with Metal Ions: Insights into Dermatological Applications" Pharmaceutics 17, no. 2: 194. https://doi.org/10.3390/pharmaceutics17020194
APA StyleCioanca, O., Lungu, I.-I., Batir-Marin, D., Lungu, A., Marin, G.-A., Huzum, R., Stefanache, A., Sekeroglu, N., & Hancianu, M. (2025). Modulating Polyphenol Activity with Metal Ions: Insights into Dermatological Applications. Pharmaceutics, 17(2), 194. https://doi.org/10.3390/pharmaceutics17020194








