Impact of Co-Spray Drying with Leucine or Trileucine on Aerosol Performance, In Vitro Dissolution, and Cellular Uptake of Colistin Powder Formulations for Inhalation
Abstract
:1. Introduction
2. Methods and Materials
2.1. Material
2.2. Production of Dry Powder Formulations by Spray Drying
2.3. Storage Stability Test
2.4. Powder X-Ray Diffraction (PXRD)
2.5. Particle Size Distribution
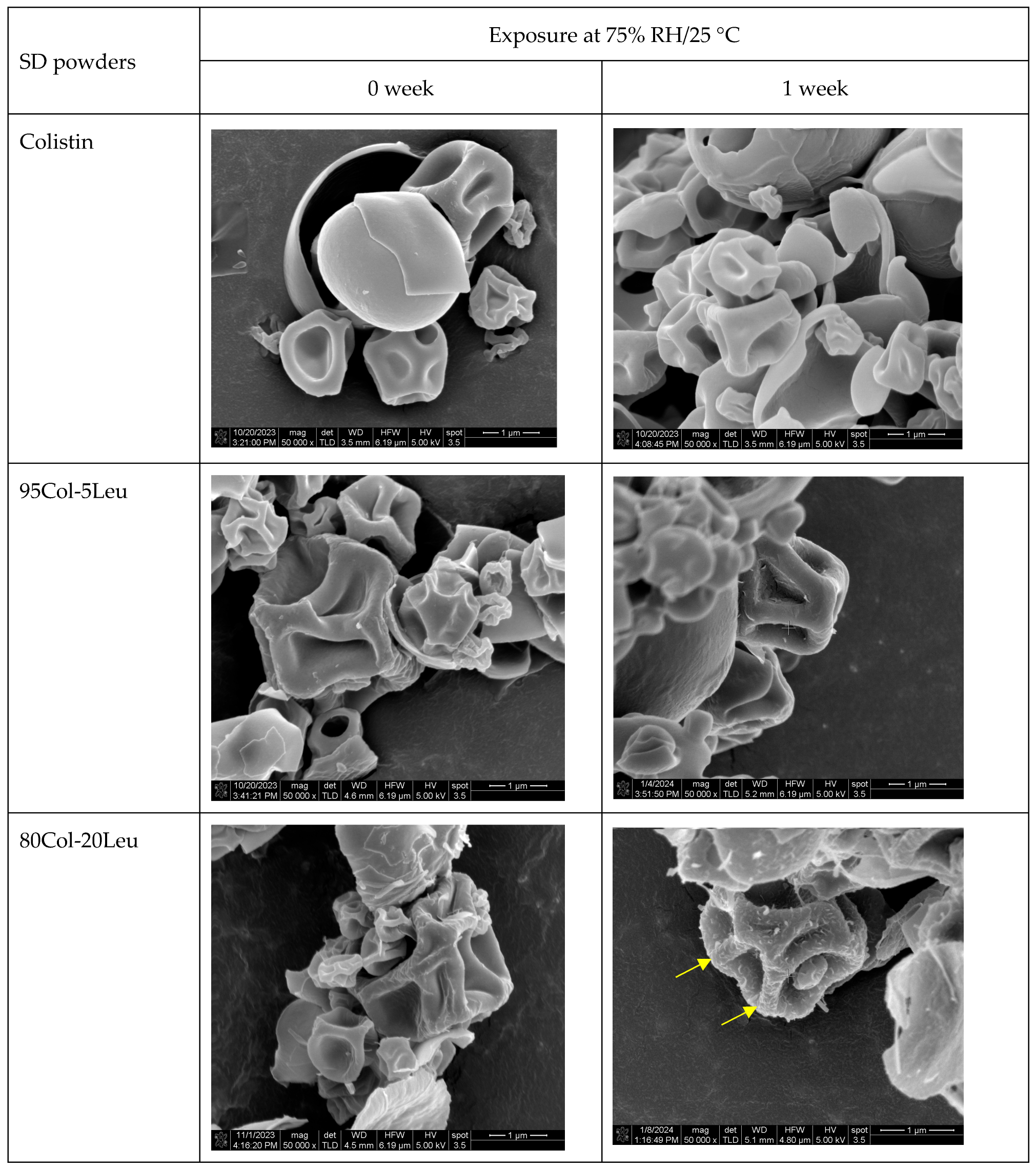
2.6. Scanning Electron Microscopy (SEM)
2.7. Dynamic Vapor Sorption
2.8. X-Ray Photoelectron Spectroscopy
2.9. Assay of Colistin
2.10. In Vitro Aerosolization Performance
2.11. In Vitro Dissolution
2.12. Cell Culture
2.13. In Vitro Cellular Transport
2.14. Statistical Analysis
3. Results
3.1. Particle Size
3.2. Particle Morphology
3.3. Crystallinity
3.4. DVS
3.5. XPS
3.6. In Vitro Aerosol Performance
3.7. In Vitro Dissolution
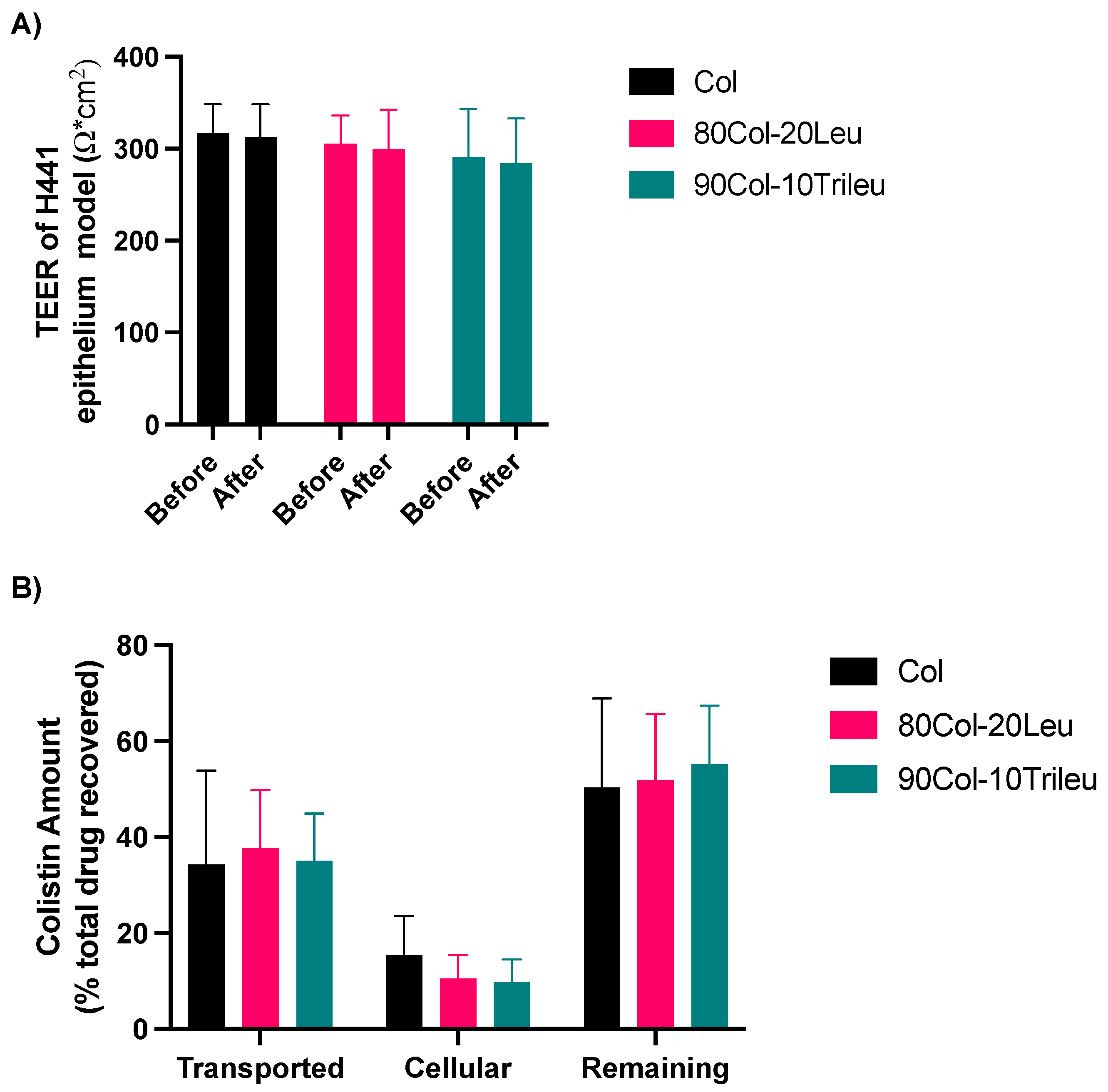
3.8. In Vitro Cell Transport
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, M. Inhalation therapy: An historical review. Prim. Care Respir. J. 2007, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Quon, B.S.; Goss, C.H.; Ramsey, B.W. Inhaled antibiotics for lower airway infections. Ann. Am. Thorac. Soc. 2014, 11, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarevic, I.Z.; Milstone, A.P. Nebulized Therapies in COPD: Past, Present, and the Future. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Leung, S.S.Y.; Tang, P.; Parumasivam, T.; Loh, Z.H.; Chan, H.-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv. Drug Deliv. Rev. 2015, 85, 83–99. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Lin, Y.W.; Wong, J.; Qu, L.; Chan, H.K.; Zhou, Q.T. Powder Production and Particle Engineering for Dry Powder Inhaler Formulations. Curr. Pharm. Des. 2015, 21, 3902–3916. [Google Scholar] [CrossRef]
- Louey, M.D.; Van Oort, M.; Hickey, A.J. Aerosol dispersion of respirable particles in narrow size distributions produced by jet-milling and spray-drying techniques. Pharm. Res. 2004, 21, 1200–1206. [Google Scholar] [CrossRef]
- Zhou, Q.; Morton, D.A.V.; Yu, H.H.; Jacob, J.; Wang, J.; Li, J.; Chan, H.K. Colistin Powders with High Aerosolisation Efficiency for Respiratory Infection: Preparation and In Vitro Evaluation. J. Pharm. Sci. 2013, 102, 3736–3747. [Google Scholar] [CrossRef]
- Shetty, N.; Zeng, L.; Mangal, S.; Nie, H.; Rowles, M.R.; Guo, R.; Han, Y.; Park, J.H.; Zhou, Q. Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations. Pharm. Res. 2018, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Loh, Z.H.; Yu, J.; Sun, S.-p.; Gengenbach, T.; Denman, J.A.; Li, J.; Chan, H.-K. How Much Surface Coating of Hydrophobic Azithromycin Is Sufficient to Prevent Moisture-Induced Decrease in Aerosolisation of Hygroscopic Amorphous Colistin Powder? AAPS J. 2016, 18, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gengenbach, T.; Denman, J.A.; Yu, H.H.; Li, J.; Chan, H.K. Synergistic Antibiotic Combination Powders of Colistin and Rifampicin Provide High Aerosolization Efficiency and Moisture Protection. AAPS J. 2014, 16, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chiou, D.; Langrish, T.A.G. Crystallization of Amorphous Components in Spray-Dried Powders. Dry. Technol. 2007, 25, 1427–1435. [Google Scholar] [CrossRef]
- Maggi, L.; Bruni, R.; Conte, U. Influence of the moisture on the performance of a new dry powder inhaler. Int. J. Pharm. 1999, 177, 83–91. [Google Scholar] [CrossRef]
- Price, R.; Young, P.M.; Edge, S.; Staniforth, J.N. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int. J. Pharm. 2002, 246, 47–59. [Google Scholar] [CrossRef]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.-K. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Lu, J.; Quan, G.; Pan, X.; Liu, P. Effects of L-leucine on the properties of spray-dried swellable microparticles with wrinkled surfaces for inhalation therapy of pulmonary fibrosis. Int. J. Pharm. 2021, 610, 121223. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.P.; Prota, L.; Auriemma, G.; Santoro, A.; Mencherini, T.; Colombo, G.; Russo, P. Dry powder inhalers of gentamicin and leucine: Formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int. J. Pharm. 2012, 426, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Mah, P.T.; O’Connell, P.; Focaroli, S.; Lundy, R.; O’Mahony, T.F.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. The use of hydrophobic amino acids in protecting spray dried trehalose formulations against moisture-induced changes. Eur. J. Pharm. Biopharm. 2019, 144, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Zillen, D.; Beugeling, M.; Hinrichs, W.L.J.; Frijlink, H.W.; Grasmeijer, F. Natural and bioinspired excipients for dry powder inhalation formulations. Curr. Opin. Colloid Interface Sci. 2021, 56, 101497. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Weers, J.G.; Miller, D.P. Formulation Design of Dry Powders for Inhalation. J. Pharm. Sci. 2015, 104, 3259–3288. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Vehring, R. Leucine enhances the dispersibility of trehalose-containing spray-dried powders on exposure to a high-humidity environment. Int. J. Pharm. 2021, 601, 120561. [Google Scholar] [CrossRef]
- Li, L.; Leung, S.S.Y.; Gengenbach, T.; Yu, J.; Gao, G.; Tang, P.; Zhou, Q.; Chan, H.-K. Investigation of L-leucine in reducing the moisture-induced deterioration of spray-dried salbutamol sulfate power for inhalation. Int. J. Pharm. 2017, 530, 30–39. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Wang, W.; Huang, Z.; Zhao, Z.; Wang, G.; Cai, S.; Jing, H.; Huang, Y.; Pan, X.; et al. Moisture-Resistant Co-Spray-Dried Netilmicin with l-Leucine as Dry Powder Inhalation for the Treatment of Respiratory Infections. Pharmaceutics 2018, 10, 252. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; Kluitman, M.P.G.; Kloezen, M.; Frijlink, H.W.; Grasmeijer, F. Dispersibility and Storage Stability Optimization of High Dose Isoniazid Dry Powder Inhalation Formulations with L-Leucine or Trileucine. Pharmaceutics 2019, 12, 24. [Google Scholar] [CrossRef]
- Lechuga-Ballesteros, D.; Charan, C.; Stults, C.L.M.; Stevenson, C.L.; Miller, D.P.; Vehring, R.; Tep, V.; Kuo, M.C. Trileucine Improves Aerosol Performance and Stability of Spray-Dried Powders for Inhalation. J. Pharm. Sci. 2008, 97, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A.V. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Harinck, L.; Lokras, A.G.; Gerde, P.; Selg, E.; Sjöberg, C.-O.; Franzyk, H.; Thakur, A.; Foged, C. Leucine improves the aerosol performance of dry powder inhaler formulations of siRNA-loaded nanoparticles. Int. J. Pharm. 2022, 621, 121758. [Google Scholar] [CrossRef] [PubMed]
- Seville, P.C.; Learoyd, T.P.; Li, H.Y.; Williamson, I.J.; Birchall, J.C. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Shur, J.; Nevell, T.G.; Ewen, R.J.; Price, R.; Smith, A.; Barbu, E.; Conway, J.H.; Carroll, M.P.; Shute, J.K.; Smith, J.R. Cospray-dried unfractionated heparin with L-leucine as a dry powder inhaler mucolytic for cystic fibrosis therapy. J. Pharm. Sci. 2008, 97, 4857–4868. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Gregson, F.K.A.; Wang, H.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. On the particle formation of leucine in spray drying of inhalable microparticles. Int. J. Pharm. 2021, 592, 120102. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an excipient in spray dried powder for inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef]
- Raula, J.; Seppälä, J.; Malm, J.; Karppinen, M.; Kauppinen, E.I. Structure and Dissolution of l-Leucine-Coated Salbutamol Sulphate Aerosol Particles. AAPS PharmSciTech 2012, 13, 707–712. [Google Scholar] [CrossRef]
- Vartiainen, V.; Bimbo, L.M.; Hirvonen, J.; Kauppinen, E.I.; Raula, J. Drug permeation and cellular interaction of amino acid-coated drug combination powders for pulmonary delivery. Int. J. Pharm. 2016, 504, 89–97. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.T.; Sun, S.-P.; Denman, J.A.; Gengenbach, T.R.; Barraud, N.; Rice, S.A.; Li, J.; Yang, M.; Chan, H.-K. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. AAPS J. 2016, 18, 372–384. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Ordoubadi, M.; Gregson, F.K.A.; Wang, H.; Carrigy, N.B.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. Trileucine as a dispersibility enhancer of spray-dried inhalable microparticles. J. Control. Release 2021, 336, 522–536. [Google Scholar] [CrossRef]
- Shetty, N.; Ahn, P.; Park, H.; Bhujbal, S.; Zemlyanov, D.; Cavallaro, A.; Mangal, S.; Li, J.; Zhou, Q.T. Improved Physical Stability and Aerosolization of Inhalable Amorphous Ciprofloxacin Powder Formulations by Incorporating Synergistic Colistin. Mol. Pharm. 2018, 15, 4004–4020. [Google Scholar] [CrossRef]
- Zheng, J.; Lyu, Y.; Wu, B.; Wang, S. Defect engineering of the protection layer for photoelectrochemical devices. EnergyChem 2020, 2, 100039. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, S.; Ahmed, M.U.; Zhou, Q.T. Liposomal Formulation Reduces Transport and Cell Uptake of Colistin in Human Lung Epithelial Calu-3 Cell and 3D Human Lung Primary Tissue Models. AAPS PharmSciTech 2024, 25, 40. [Google Scholar] [CrossRef]
- May, S.; Jensen, B.; Wolkenhauer, M.; Schneider, M.; Lehr, C.M. Dissolution Techniques for In Vitro Testing of Dry Powders for Inhalation. Pharm. Res. 2012, 29, 2157–2166. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef]
- Mangal, S.; Park, H.; Nour, R.; Shetty, N.; Cavallaro, A.; Zemlyanov, D.; Thalberg, K.; Puri, V.; Nicholas, M.; Narang, A.S.; et al. Correlations between surface composition and aerosolization of jet-milled dry powder inhaler formulations with pharmaceutical lubricants. Int. J. Pharm. 2019, 568, 118504. [Google Scholar] [CrossRef]
- Munis, A.M.; Hyde, S.C.; Gill, D.R. A human surfactant B deficiency air-liquid interface cell culture model suitable for gene therapy applications. Mol. Ther. Methods Clin. Dev. 2021, 20, 237–246. [Google Scholar] [CrossRef]
- Chai, G.; Park, H.; Yu, S.; Zhou, F.; Li, J.; Xu, Q.; Zhou, Q. Evaluation of co-delivery of colistin and ciprofloxacin in liposomes using an in vitro human lung epithelial cell model. Int. J. Pharm. 2019, 569, 118616. [Google Scholar] [CrossRef]
- Pathak, V.; Park, H.; Zemlyanov, D.; Bhujbal, S.V.; Ahmed, M.U.; Azad, M.A.K.; Li, J.; Zhou, Q.T. Improved Aerosolization Stability of Inhalable Tobramycin Powder Formulation by Co-Spray Drying with Colistin. Pharm. Res. 2022, 39, 2781–2799. [Google Scholar] [CrossRef]
- Yu, J.; Chan, H.-K.; Gengenbach, T.; Denman, J.A. Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2017, 119, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, E.; Vlasenko, S.; Martin, S.; Koop, T.; Pöschl, U. Amorphous and crystalline aerosol particles interacting with water vapor: Conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations. Atmos. Chem. Phys. 2009, 9, 9491–9522. [Google Scholar] [CrossRef]
- Andronis, V.; Yoshioka, M.; Zografi, G. Effects of Sorbed Water on the Crystallization of Indomethacin from the Amorphous State. J. Pharm. Sci. 1997, 86, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.-H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A.V. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef]
- Wang, H.; Nobes, D.S.; Vehring, R. Particle Surface Roughness Improves Colloidal Stability of Pressurized Pharmaceutical Suspensions. Pharm. Res. 2019, 36, 43. [Google Scholar] [CrossRef]
- Berruyer, P.; Lindkvist, M.; Gracin, S.; Starciuc, T.; Bertarello, A.; Busi, B.; Schantz, S.; Emsley, L. Hierarchy of the Components in Spray-Dried, Protein-Excipient Particles Using DNP-Enhanced NMR Spectroscopy. Mol. Pharm. 2023, 20, 5682–5689. [Google Scholar] [CrossRef]
- Mohan, M.; Lee, S.; Guo, C.; Peri, S.P.; Doub, W.H. Evaluation of Abbreviated Impactor Measurements (AIM) and Efficient Data Analysis (EDA) for Dry Powder Inhalers (DPIs) Against the Full-Resolution Next Generation Impactor (NGI). AAPS PharmSciTech 2017, 18, 1585–1594. [Google Scholar] [CrossRef]
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.-M.; Ehrhardt, C. The Cell Line NCl-H441 Is a Useful In Vitro Model for Transport Studies of Human Distal Lung Epithelial Barrier. Mol. Pharm. 2014, 11, 995–1006. [Google Scholar] [CrossRef]
- Ren, H.; Birch, N.P.; Suresh, V. An Optimised Human Cell Culture Model for Alveolar Epithelial Transport. PLoS ONE 2016, 11, e0165225. [Google Scholar] [CrossRef]
- Martinez, M.N.; Amidon, G.L. A Mechanistic Approach to Understanding the Factors Affecting Drug Absorption: A Review of Fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643. [Google Scholar] [CrossRef]






| Formulation | Total Solid Content | Colistin (%, w/w) | Leucine (%, w/w) | Trileucine (%, w/w) |
|---|---|---|---|---|
| Colistin | 20 mg/mL | 100 | - | - |
| 95Col-5Leu | 95 | 5 | - | |
| 90Col-10Leu | 90 | 10 | - | |
| 80Col-20Leu | 80 | 20 | - | |
| 98Col-2Trileu | 98 | - | 2 | |
| 95Col-5Trileu | 95 | - | 5 | |
| 90Col-10Trileu | 90 | - | 10 |
| Formulation | D10 (µm) | D50 (µm) | D90 (µm) | Span |
|---|---|---|---|---|
| Colistin | 1.13 ± 0.01 | 2.41 ± 0.07 | 5.34 ± 0.21 | 1.74 ± 0.04 |
| 95Col-5Leu | 1.08 ± 0.02 | 2.22 ± 0.05 | 4.81 ± 0.22 | 1.68 ± 0.06 |
| 90Col-10Leu | 1.05 ± 0.01 | 2.21 ± 0.02 | 4.85 ± 0.10 | 1.72 ± 0.04 |
| 80Col-20Leu | 1.05 ± 0.01 | 2.10 ± 0.02 | 4.22 ± 0.03 | 1.51 ± 0.00 |
| 98Col-2Trileu | 1.09 ± 0.03 | 2.34 ± 0.11 | 5.19 ± 0.45 | 1.75 ± 0.11 |
| 95Col-5Trileu | 1.05 ± 0.01 | 2.25 ± 0.03 | 5.06 ± 0.14 | 1.78 ± 0.03 |
| 90Col-10Trileu | 1.08 ± 0.02 | 2.28 ± 0.05 | 5.04 ± 0.19 | 1.73 ± 0.04 |
| SD Powders | Atomic Percentage of Sulfur (%) | Percentage of Colistin Reduction (C) = 100% − B/A × 100% | |
|---|---|---|---|
| Theoretical Value (A) | Normalized Experimental Value (B) | ||
| Colistin | 2.67 | 2.67 ± 0.04 | 0.00 ± 1.46 |
| 95Col-5Leu | 2.54 | 2.43 ± 0.03 | 4.25 ± 1.17 |
| 90Col-10Leu | 2.40 | 2.29 ± 0.08 | 4.39 ± 3.19 |
| 80Col-20Leu | 2.13 | 2.02 ± 0.01 | 5.14 ± 0.49 |
| 98Col-2Trileu | 2.62 | 2.38 ± 0.09 | 9.13 ± 3.26 |
| 95Col-5Trileu | 2.53 | 2.07 ± 0.03 | 18.29 ± 1.31 |
| 90Col-10Trileu | 2.40 | 1.75 ± 0.08 | 26.81 ± 3.37 |
| SD Powders | FPF Reduction (%) = (FPF0_week − FPF1_week)/ FPF0_week × 100% |
|---|---|
| Colistin | 76.16 ± 2.24 |
| 80Col-20Leu | 6.23 ± 2.36 |
| 90Col-10Leu | 22.00 ± 0.43 |
| 95Col-5Leu | 52.63 ± 2.94 |
| 90Col-10Trileu | 7.87 ± 1.16 |
| 95Col-5Trileu | 18.41 ± 1.99 |
| 98Col-2Trileu | 19.56 ± 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Arte, K.S.; Patil, C.D.; Zhou, Q.; Qu, L. Impact of Co-Spray Drying with Leucine or Trileucine on Aerosol Performance, In Vitro Dissolution, and Cellular Uptake of Colistin Powder Formulations for Inhalation. Pharmaceutics 2025, 17, 199. https://doi.org/10.3390/pharmaceutics17020199
Huang Y, Arte KS, Patil CD, Zhou Q, Qu L. Impact of Co-Spray Drying with Leucine or Trileucine on Aerosol Performance, In Vitro Dissolution, and Cellular Uptake of Colistin Powder Formulations for Inhalation. Pharmaceutics. 2025; 17(2):199. https://doi.org/10.3390/pharmaceutics17020199
Chicago/Turabian StyleHuang, Yijing, Kinnari Santosh Arte, Chanakya D. Patil, Qi Zhou, and Li Qu. 2025. "Impact of Co-Spray Drying with Leucine or Trileucine on Aerosol Performance, In Vitro Dissolution, and Cellular Uptake of Colistin Powder Formulations for Inhalation" Pharmaceutics 17, no. 2: 199. https://doi.org/10.3390/pharmaceutics17020199
APA StyleHuang, Y., Arte, K. S., Patil, C. D., Zhou, Q., & Qu, L. (2025). Impact of Co-Spray Drying with Leucine or Trileucine on Aerosol Performance, In Vitro Dissolution, and Cellular Uptake of Colistin Powder Formulations for Inhalation. Pharmaceutics, 17(2), 199. https://doi.org/10.3390/pharmaceutics17020199







