Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview
Abstract
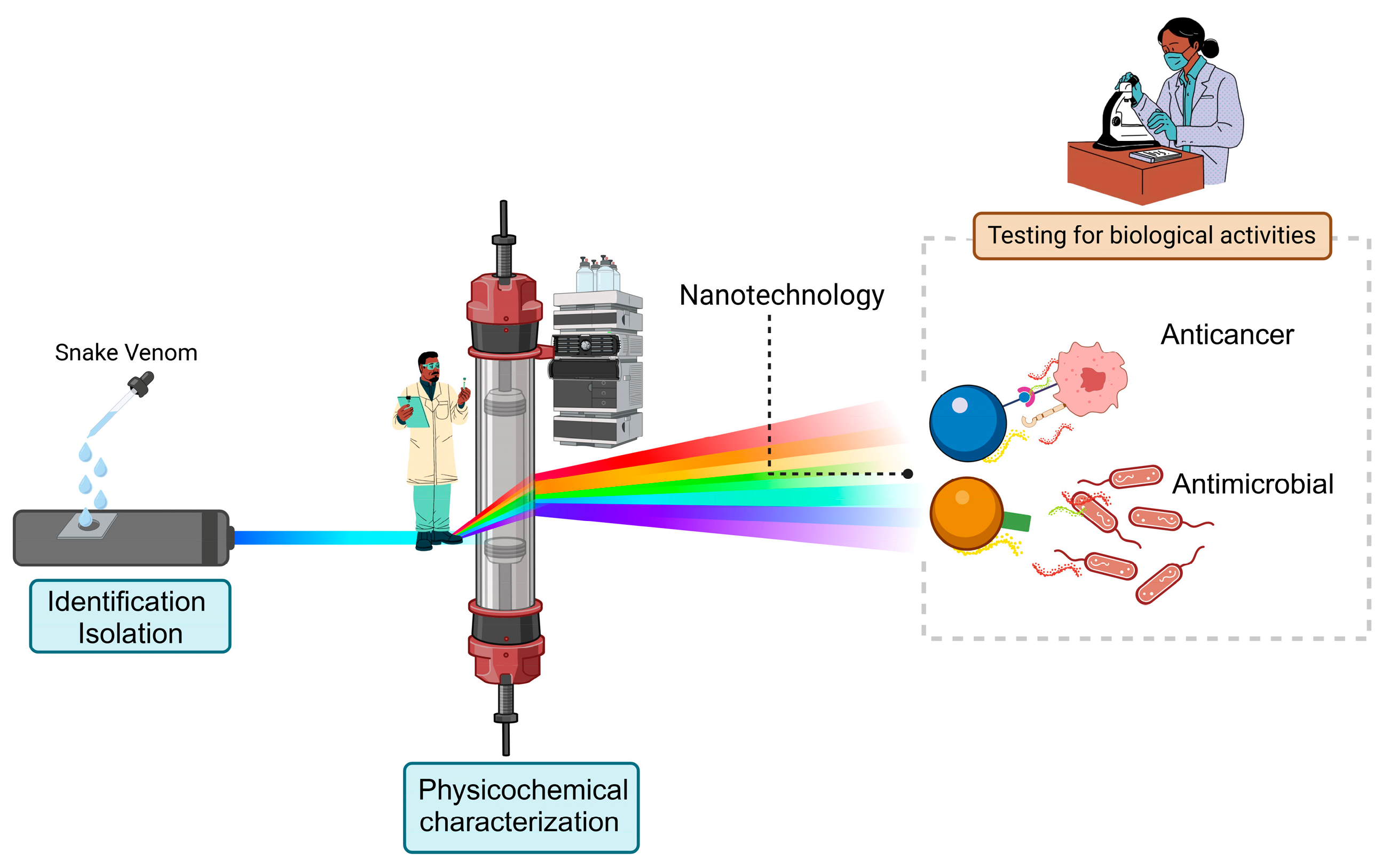
1. Introduction
2. Snake Venom Composition
3. Snake Venom as Treatment
4. Challenges in Venom Therapy: Addressing Formulation Needs and the Role of Nanotechnology
5. Commonly Used Nanosystems to Encapsulate Snake Venom
6. Encapsulation of Venom Fractions with Bioactivity and Its Applications
6.1. Nanosystems Containing Bothrops Snake Venom
6.2. Nanosystems Containing Crotalus Snake Venom
6.3. Nanosystems Containing Lachesis Snake Venom
7. Theragnostic Applications of Polymeric Nanosystems
8. Clinical Trial, Patents, and Market
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mirtschin, P.J.; Dunstan, N.; Hough, B.; Hamilton, E.; Klein, S.; Lucas, J.; Millar, D.; Madaras, F.; Nias, T. Venom yields from australian and some other species of snakes. Ecotoxicology 2006, 15, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Knudsen, C.; Oliveira, I.S.; Rimbault, C.; Cerni, F.A.; Wen, F.H.; Sachett, J.; Sartim, M.A.; Laustsen, A.H.; Monteiro, W.M. Current knowledge on snake dry bites. Toxins 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; World Health Organization. Snakebite Envenoming. Available online: https://www.who.int/health-topics/snakebite (accessed on 6 September 2023).
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Aron, M.B.; Mulwafu, M.; Mailosi, B.; Kreuels, B.; Dullie, L.; Kachimanga, C.; Blessmann, J.; Ndarama, E.; Sambani, C.; Munyaneza, F.; et al. Experiences and practices of traditional healers on snakebite treatment and prevention in rural Malawi. PLoS Negl. Trop. Dis. 2023, 17, e0011653. [Google Scholar] [CrossRef] [PubMed]
- Strand, E.; Murta, F.; Tupetz, A.; Barcenas, L.; Phillips, A.J.; Farias, A.S.; Santos, A.C.; Rocha, G.S.; Staton, C.A.; Ramos, F.R.; et al. Perspectives on snakebite envenoming care needs across different sociocultural contexts and health systems: A comparative qualitative analysis among us and brazilian health providers. Toxicon X 2023, 17, 100143. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Paterson, C. What future for policing? Some reflections on the concept and purpose of policing and their implications for police reform in England and Wales. Int. J. Law Public Adm. 2019, 2, 12–22. [Google Scholar] [CrossRef]
- Schneider, M.C.; Min, K.; Hamrick, P.N.; Montebello, L.R.; Ranieri, T.M.; Mardini, L.; Camara, V.M.; Raggio Luiz, R.; Liese, B.; Vuckovic, M.; et al. Overview of snakebite in Brazil: Possible drivers and a tool for risk mapping. PLoS Negl. Trop. Dis. 2021, 15, e0009044. [Google Scholar] [CrossRef]
- Araújo, S.C.M.; Câmara, J.T.; Guedes, T.B. Snakebites in northeastern Brazil: Accessing clinical-epidemiological profile as a strategy to deal with neglected tropical diseases. Rev. Soc. Bras. Med. Trop. 2023, 56, e02242023. [Google Scholar] [CrossRef] [PubMed]
- Duque, B.R.; Bruno, S.F.; Ferreira, V.; Guedes, T.B.; Machado, C.; Hamdan, B. Venomous snakes of medical importance in the brazilian state of Rio de Janeiro: Habitat and taxonomy against ophidism. Braz. J. Biol. 2023, 83, e272811. [Google Scholar] [CrossRef]
- Bordon, K.D.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Werner, R.M.; Soffa, A.N. Considerations for the development of a field-based medical device for the administration of adjunctive therapies for snakebite envenoming. Toxicon X 2023, 20, 100169. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, A.; Laraba-Djebari, F. Development and evaluation of polymeric nanoparticles as a delivery system for snake envenoming prevention. Biologicals 2021, 70, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; van-der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef]
- Jenner, R.; Undheim, E. Venom: The Secrets of Nature’s Deadliest Weapon; Smithsonian Books: Washington, DC, USA, 2017. [Google Scholar]
- Schendel, R.; Jenner, R.; Undheim, E. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.C.; Casewell, N.R.; Elliott, C.T.; Harvey, A.L.; Jamieson, A.G.; Strong, P.N.; Turner, A.D. Friends or foes? Emerging impacts of biological toxins. Trends Biochem. Sci. 2019, 44, 365–379. [Google Scholar] [CrossRef]
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal protein toxins: Origins and therapeutic applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef]
- Singh, S.B.; Pelaez, F. Biodiversity, chemical diversity and drug discovery. In Natural Compounds as Drugs, Volume I; Birkhäuser: Basel, Switzerland, 2008; pp. 141–174. [Google Scholar]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The three-finger toxin fold: A multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017, 142 (Suppl. S2), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Song, J.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef] [PubMed]
- McCleary, R.J.R.; Kini, R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon 2013, 62, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA2, a promising target for broad-spectrum antivenom drug development. Biomed. Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A current perspective on snake venom composition and constituent protein families. Arch. Toxicol. 2023, 97, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Viegas, M.F.; Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef]
- Slagboom, J.; Mladić, M.; Xie, C.; Kazandjian, T.D.; Vonk, F.; Somsen, G.W.; Casewell, N.R.; Kool, J. high throughput screening and identification of coagulopathic snake venom proteins and peptides using nanofractionation and proteomics approaches. PLoS Negl. Trop. Dis. 2020, 14, e0007802. [Google Scholar] [CrossRef]
- Koh, C.Y.; Kini, R.M. from snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Wenzel, U.; Ziyadeh, F.N.; Stahl, R.A.K. Angiotensin converting-enzyme inhibitor treatment reduces glomerular P16 INK4 and P27 Kip1 expression in diabetic BBdp rats. Diabetologia 1999, 42, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.W.; Harris, J.B. Myotoxic activity of the toxic phospholipase, notexin, from the venom of the australian tiger snake. J. Neuropathol. Exp. Neurol. 1996, 55, 1230–1237. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Ponce-Soto, L.A.; Marangoni, S.; Lomonte, B. Systemic and local myotoxicity induced by snake venom group II Phospholipases A2: Comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon 2008, 51, 80–92. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Samuel, S.P.; Bickler, P.E.; Lewin, M.R. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J. Trop. Med. 2018, 2018, 4320175. [Google Scholar] [CrossRef] [PubMed]
- Greener, M. The next generation of venom-based drugs. Prescriber 2020, 31, 28–32. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Soares, A.M.; Silva, S.L. Why to study peptides from venomous and poisonous animals? Int. J. Pept. Res. Ther. 2023, 29, 76. [Google Scholar] [CrossRef]
- Trim, C.M.; Byrne, L.J.; Trim, S.A. Utilisation of compounds from venoms in drug discovery. Prog. Med. Chem. 2021, 60, 1–66. [Google Scholar] [PubMed]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. From snake venom’s disintegrins and C-type lectins to anti-platelet drugs. Toxins 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic potential of peptides derived from animal venoms: Current views and emerging drugs for diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 117955142110060. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Song, S.; Liu, Y.; Jiao, B.; Meng, R. Use of Batroxobin in central and peripheral ischemic vascular diseases: A systematic review. Front. Neurol. 2021, 12, 716778. [Google Scholar] [CrossRef]
- Faioli, C.N.; Domingos, T.F.S.; Oliveira, E.C.; Sanchez, E.F.; Ribeiro, S.; Muricy, G.; Fuly, A.L. appraisal of antiophidic potential of marine sponges against Bothrops jararaca and Lachesis muta venom. Toxins 2013, 5, 1799–1813. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm activity of acidic phospholipase isoform isolated from Bothrops erythromelas snake venom. Toxins 2020, 12, 606. [Google Scholar] [CrossRef]
- Uetz, P. The Reptile Database. Available online: http://www.reptile-database.org/ (accessed on 2 February 2024).
- Pompeia, C.; Frare, E.O.; Peigneur, S.; Tytgat, J.; Silva, Á.P.; Oliveira, E.B.; Pereira, A.; Kerkis, I.; Kolonin, M.G. Synthetic polypeptide crotamine: Characterization as a myotoxin and as a target of combinatorial peptides. J. Mol. Med. 2022, 100, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Yin, C.; Wang, W.; Davies, P.; Sanchez, E.; Suntravat, M.; Zawieja, D.; Cromer, W. Effect of the snake venom component crotamine on lymphatic endothelial cell responses and lymph transport. Microcirculation 2023, 30, e12775. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.F.; Campeiro, J.D.; Yonamine, C.M. Toxicon revisiting the potential of south american rattlesnake Crotalus durissus terrificus toxins as therapeutic, theranostic and/or biotechnological agents. Toxicon 2022, 206, 1–13. [Google Scholar] [CrossRef]
- Giardini, A.C.; Evangelista, B.G.; Sant’Anna, M.B.; Martins, B.B.; Lancellotti, C.L.P.; Ciena, A.P.; Chacur, M.; Pagano, R.L.; Ribeiro, O.G.; Zambelli, V.O.; et al. Crotalphine attenuates pain and neuroinflammation induced by experimental autoimmune encephalomyelitis in mice. Toxins 2021, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a novel potent analgesic peptide from the venom of the south american rattlesnake Crotalus durissus terrificus. Peptides 2008, 29, 1293–1304. [Google Scholar] [CrossRef]
- Silva, A.; Isbister, G.K. Current research into snake antivenoms, their mechanisms of action and applications. Biochem. Soc. Trans. 2020, 48, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.L.; Rowan, E.G.; Albericio, F.; Stábeli, R.G.; Calderon, L.A.; Soares, A.M. Animal toxins and their advantages in biotechnology and pharmacology. Biomed. Res. Int. 2014, 2014, 951561. [Google Scholar] [CrossRef]
- Peigneur, S.; Tytgat, J. Toxins in drug discovery and pharmacology. Toxins 2018, 10, 126. [Google Scholar] [CrossRef]
- Slagboom, J.; Otvos, R.A.; Cardoso, F.C.; Iyer, J.; Visser, J.C.; van Doodewaerd, B.R.; McCleary, R.J.R.; Niessen, W.M.A.; Somsen, G.W.; Lewis, R.J.; et al. Neurotoxicity fingerprinting of venoms using on-line microfluidic AChBP profiling. Toxicon 2018, 148, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Undheim, E.A.B.; Pineda, S.S.; Jin, A.-H.; Lavergne, V.; Fry, B.G.; Lewis, R.J.; Alewood, P.F.; King, G.F. Venoms-based drug discovery: Proteomic and transcriptomic approaches. In Venom to Drugs; King, G.F., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 80–96. [Google Scholar]
- Souza, G.H.M.F.; Catharino, R.R.; Ifa, D.R.; Eberlin, M.N.; Hyslop, S. Peptide fingerprinting of snake venoms by direct infusion nanoelectrospray ionization mass spectrometry: Potential use in venom identification and taxonomy. J. Mass Spec. 2008, 43, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Sciani, J.M.; Vigerelli, H.; Costa, A.S.; Câmara, D.A.D.; Junior, P.L.-S.; Pimenta, D.C. An unexpected cell-penetrating peptide from Bothrops jararaca venom identified through a novel size exclusion chromatography screening. J. Pept. Sci. 2017, 23, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Gomes, A.; Sengupta, J.; Datta, P.; Singha, S.; Dasgupta, A.K.; Gomes, A. Nanoparticle-conjugated animal venom-toxins and their possible therapeutic potential. J. Venom. Res. 2012, 3, 15–21. [Google Scholar]
- Munawar, A.; Ali, S.; Akrem, A.; Betzel, C. Snake venom peptides: Tools of biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef]
- Hamimed, S.; Abdeljelil, N.; Landoulsi, A.; Chatti, A.; Aljabali, A.A.A.; Barhoum, A. Bacterial cellulose nanofibers. In Handbook of Nanocelluloses; Springer: Cham, Switzerland, 2022; pp. 1–38. [Google Scholar]
- Langer, R.; Weissleder, R. Nanotechnology. JAMA 2015, 313, 135. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotech. 2018, 16, 71. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Modern trends in animal venom research—Omics and nanomaterials. World J. Biol. Chem. 2017, 8, 4–12. [Google Scholar] [CrossRef]
- Gomes, A.; Ghosh, S.; Sengupta, J.; Saha, K.; Gomes, A. Nanotechnology in snake venom research—An overview. Indian J. Exp. Biol. 2018, 56, 707–715. [Google Scholar]
- Soares, K.; Gláucia-Silva, F.; Silva, A.D.; Torres-Rêgo, M.; Araújo, N.; Menezes, Y.; Damasceno, I.; Tambourgi, D.; Silva-Júnior, A.; Fernandes-Pedrosa, M. Antivenom production against Bothrops jararaca and Bothrops erythromelas snake venoms using cross-linked chitosan nanoparticles as an immunoadjuvant. Toxins 2018, 10, 158. [Google Scholar] [CrossRef]
- Santos-Silva, E.d.; Torres-Rêgo, M.; Gláucia-Silva, F.; Feitosa, R.C.; Lacerda, A.F.; Rocha, H.A.d.O.; Fernandes-Pedrosa, M.d.F.; Silva-Júnior, A.A. Cationic PLGA nanoparticle formulations as biocompatible immunoadjuvant for serum production and immune response against Bothrops jararaca venom. Toxins 2022, 14, 888. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.C.F.; Paula, M.O.; Lastra, H.C.B.; Alves, B.B.; Moreno, D.A.N.; Yoshida, E.H.; Amaral Filho, J.; Cogo, J.C.; Varanda, E.A.; Rai, M.; et al. Activity of silver nanoparticles on prokaryotic cells and Bothrops jararacussu snake venom. Drug Chem. Toxicol. 2019, 42, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Gomes, A.; Dasgupta, S.C.; Gomes, A. Snake venom as therapeutic agents: From toxin to drug development. Indian J. Exp. Biol. 2002, 40, 1353–1358. [Google Scholar]
- Calmette, A.; Saenz, A.; Costil, L. Effects du venin de cobra sur les greffes cancereuses et sur le cancer spontane adenocarcinome de la souris. Comptes Rendus Acad. Sci. 1933, 197, 205–210. [Google Scholar]
- Tu, A.T.; Giltner, J.B. Cytotoxic effects of snake venoms on kb and yoshida sarcoma cells. Res. Commun. Chem. Pathol. Pharmacol. 1974, 9, 783–786. [Google Scholar] [PubMed]
- Iwaguchi, T.; Takechi, M.; Hayashi, K. Cytolytic activity of cytotoxin isolated from indian cobra venom against experimental tumor cells. Biochem. Int. 1985, 10, 343–349. [Google Scholar] [PubMed]
- Debnath, A.; Saha, A.; Gomes, A.; Biswas, S.; Chakrabarti, P.; Giri, B.; Biswas, A.K.; Gupta, S.D.; Gomes, A. A lethal cardiotoxic–cytotoxic protein from the indian monocellate cobra Naja kaouthia venom. Toxicon 2010, 56, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.P.; Bhowmik, T.; Dasgupta, A.K.; Gomes, A. In vivo and in vitro toxicity of nanogold conjugated snake venom protein toxin GNP-NKCT1. Toxicol. Rep. 2014, 1, 74–84. [Google Scholar] [CrossRef]
- Aubin-Tam, M.-E. Conjugation of nanoparticles to proteins. In Nanomaterial Interfaces in Biology; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 19–27. [Google Scholar]
- Aubin-Tam, M.-E.; Hamad-Schifferli, K. Structure and function of nanoparticle–protein conjugates. Biomed. Mater. 2008, 33, 034001. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly-Ɛ-caprolactone microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Rosenberg, R.T.; Siegel, S.J.; Dan, N. Release of highly hydrophilic drugs from Poly-Ɛ-caprolactone matrices. J. Appl. Polym. Sci. 2008, 107, 3149–3156. [Google Scholar] [CrossRef]
- Lin, M.; Meng, S.; Zhong, W.; Li, Z.; Du, Q.; Tomasik, P. Novel biodegradable blend matrices for controlled drug release. J. Pharm. Sci. 2008, 97, 4240–4248. [Google Scholar] [CrossRef] [PubMed]
- Nait Mohamed, F.A.; Laraba-Djebari, F. Development and characterization of a new carrier for vaccine delivery based on calcium-alginate nanoparticles: Safe immunoprotective approach against scorpion envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone PCL based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef] [PubMed]
- Lotocki, V.; Kakkar, A. Miktoarm star polymers: Branched architectures in drug delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of polyε-caprolactone microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Najafi-Hajivar, S.; Zakeri-Milani, P.; Mohammadi, H.; Niazi, M.; Soleymani-Goloujeh, M.; Baradaran, B.; Valizadeh, H. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed. Pharmacother. 2016, 83, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Han, J.; Zhang, Y.; Wei, L.; Yu, S.; Wang, X.; Jin, Z.; Wang, Y. Enhancing mucosal immune response of newcastle disease virus DNA vaccine using n-2-hydroxypropyl trimethylammonium chloride chitosan and n,o-carboxymethyl chitosan nanoparticles as delivery carrier. Mol. Pharm. 2018, 151, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, H.; Hu, Y.; Xu, D.; Yin, C.; Han, Q.; Zhang, J. chitosan nanovaccines as efficient carrier adjuvant system for il-12 with enhanced protection against hbv. Int. J. Nanomed. 2021, 16, 4913–4928. [Google Scholar] [CrossRef] [PubMed]
- De França, A.C.R.B.; Soares, K.S.R.; Silva, F.G.; Silva, A.D.; Rêgo, M.T.; Silva, D.P.; Ferreira, S.S.; Furtado, A.A.; Silva-Júnior, A.A.; Pedrosa, M.F.F. Evaluation of self-assembled Bothrops jararacussu snake venom proteins cross-linked chitosan nanoparticles activity for use as a potential antibacterial. Toxicon 2019, 168, S32–S33. [Google Scholar] [CrossRef]
- Tsuruta, L.R.; Moro, A.M.; Tambourgi, D.V.; Sant’Anna, O.A. Oral tolerance induction by Bothrops jararaca venom in a murine model and cross-reactivity with toxins of other snake venoms. Toxins 2021, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; Jeong, D. Oral tolerance development and maintenance. Immunol. Allergy Clin. N. Am. 2018, 38, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.H.B.; Sant`Anna, O.A.; Quintilio, W.; Schwendener, R.A.; Araujo, P.S. A rational design for the nanoencapsulation of poisonous animal venoms in liposomes prepared with natural phospholipids. Curr. Drug Deliv. 2012, 9, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.E.; Fisk, E.; Crowe, L.M.; Araujo, P.S.; Crowe, J.H. Evidence of phospholipase activity in phospholipid bilayers under conditions of low hydration. J. Plant Physiol. 1997, 150, 661–667. [Google Scholar] [CrossRef]
- Ferreira, T.L.; Ward, R.J. The interaction of bothropstoxin-I Lys49-PLA2 with liposome membranes. Toxicon 2009, 54, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.C.C.; Fatima, M.; Furtado, D.; Correa, T.C.; Raw, I. Characterization of the snake venoms from seven brazilian species of Bothrops by FPLC anion-exchange chromatography. Comp. Biochem. 1992, 102, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.E.; Crowe, L.M.; Araujo, P.S.; Fisk, E.; Crowe, J.H. Arbutin inhibits PLA2 in partially hydrated model systems. Biochim. Biophys. Acta 1996, 1302, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.T.; Gomes, R.T.; Viotti, A.P.; Freitas, T.V. Immunization with liposome-encapsulated Bothrops jararaca venom. Toxicon 2000, 38, 881–886. [Google Scholar] [CrossRef]
- Kirby, C.; Gregoriadis, G. Dehydration-rehydration vesicles: A simple method for high yield drug entrapment in liposomes. Nat. Biotechnol. 1984, 2, 979–984. [Google Scholar] [CrossRef]
- Barros, N.B.; Macedo, S.R.A.; Ferreira, A.S.; Tagliari, M.P.; Zanchi, F.B.; Kayano, A.M.; Soares, A.M.; Nicolete, R. Liposomes containing an ASP49-Phospholipase A2 from Bothrops jararacussu snake venom as experimental therapy against cutaneous leishmaniasis. Int. Immunopharmacol. 2016, 36, 225–231. [Google Scholar] [CrossRef]
- Barros, N.B.; Macedo, S.R.A.; Ferreira, A.S.; Tagliari, M.P.; Kayano, A.M.; Nicolete, L.D.F.; Soares, A.M.; Nicolete, R. ASP49-Phospholipase A2-loaded liposomes as experimental therapy in cutaneous leishmaniasis model. Int. Immunopharmacol. 2018, 55, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Gláucia-Silva, F.; Torres-Rêgo, M.; Rocha Soares, K.S.; Damasceno, I.Z.; Tambourgi, D.V.; Silva-Júnior, A.A.; Fernandes-Pedrosa, M.F. A biotechnological approach to immunotherapy: Antivenom against Crotalus durissus cascavella snake venom produced from biodegradable nanoparticles. Int. J. Biol. Macromol. 2018, 120, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Sartim, M.A.; Menaldo, D.L.; Sampaio, S.V. immunotherapeutic potential of crotoxin: Anti-inflammatory and immunosuppressive properties. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 39. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, M.B.; Giardini, A.C.; Ribeiro, M.A.C.; Lopes, F.S.R.; Teixeira, N.B.; Kimura, L.F.; Bufalo, M.C.; Ribeiro, O.G.; Borrego, A.; Cabrera, W.H.K.; et al. The Crotoxin:SBA-15 complex down-regulates the incidence and intensity of experimental autoimmune encephalomyelitis through peripheral and central actions. Front. Immunol. 2020, 11, 591563. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, M.B.; Lopes, F.S.R.; Kimura, L.F.; Giardini, A.C.; Sant’Anna, O.A.; Picolo, G. Crotoxin conjugated to SBA-15 nanostructured mesoporous silica induces long-last analgesic effect in the neuropathic pain model in mice. Toxins 2019, 11, 679. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- Karpel, R.L.; Silva Liberato, M.; Campeiro, J.D.; Bergeon, L.; Szychowski, B.; Butler, A.; Marino, G.; Cusic, J.F.; Oliveira, L.C.G.; Oliveira, E.B.; et al. Design and characterization of crotamine-functionalized gold nanoparticles. Colloids Surf. B Biointerfaces 2018, 163, 1–8. [Google Scholar] [CrossRef]
- Jimenez-Canale, J.; Fernandez-Quiroz, D.; Teran-Saavedra, N.G.; Diaz-Galvez, K.R.; Gallegos-Tabanico, A.; Burgara-Estrella, A.J.; Sarabia-Sainz, H.M.; Guzman-Partida, A.M.; Robles-Burgueño, M.d.R.; Vazquez-Moreno, L.; et al. Cytotoxic activity of a northern black-tailed rattlesnake Crotalus molossus molossus venom-loaded in chitosan nanoparticles as a potential antitumoral system. Acta Biochim. Pol. 2022, 69, 233–243. [Google Scholar] [CrossRef]
- Magalhães, T.; Viotti, A.P.; Gomes, R.T.; Freitas, T.V. Effect of membrane composition and of coencapsulation of immunostimulants in a liposome-entrapped crotoxin. Biotechnol. Appl. Biochem. 2001, 33, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.V.; Frézard, F. encapsulation of native crotoxin in liposomes: A safe approach for the production of antivenom and vaccination against Crotalus durissus terrificus venom. Toxicon 1997, 35, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Baudou, F.G.; Fusco, L.; Giorgi, E.; Diaz, E.; Municoy, S.; Desimone, M.F.; Leiva, L.; De Marzi, M.C. Physicochemical and biological characterization of nanovenoms, a new tool formed by silica nanoparticles and Crotalus durissus terrificus venom. Colloids Surf. B Biointerfaces 2020, 193, 111128. [Google Scholar] [CrossRef]
- Gomes, R.T.; Camargos, R.P.F.; Viotti, A.P.; Tavares, A.P.; Revelo, M.P.; Freitas, T.V. Comparison of the biodistribution of free or liposome-entrapped Crotalus durissus terrificus south american rattlesnake venom in mice. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 295–301. [Google Scholar] [CrossRef]
- Stransky, S.; Costal-Oliveira, F.; -Souza, L.L.; Duarte, C.G.; Chávez-Olórtegui, C.; Braga, V.M.M. In vitro assessment of cytotoxic activities of Lachesis muta muta snake venom. PLoS Negl. Trop. Dis. 2018, 12, e0006427. [Google Scholar] [CrossRef] [PubMed]
- Fuly, A.L.; Machado, O.L.; Alves, E.W.; Carlini, C.R. Mechanism of inhibitory action on platelet activation of a phospholipase A2 isolated from Lachesis muta bushmaster snake venom. Thromb. Haemost. 1997, 78, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.M.; Pimenta, A.M.C.; Oliveira, M.C.; Lima, M.E. Venoms, toxins and derivatives from the brazilian fauna: Valuable sources for drug discovery. Sheng Li Xue Bao 2015, 67, 261–270. [Google Scholar]
- Kerkis, A.; Kerkis, I.; Rádis-Baptista, G.; Oliveira, E.B.; Vianna-Morgante, A.M.; Pereira, L.V.; Yamane, T. Crotamine is a novel cellpenetrating protein from the venom of rattlesnake Crotalus durissus terrificus. FASEB J. 2004, 18, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, A.; Hayashi, M.A.F.; Yamane, T.; Kerkis, I. Properties of cell penetrating peptides CPPs. IUBMB Life 2006, 58, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Porta, L.C.; Campeiro, J.D.; Hayashi, M.A.F. A native CPP from rattlesnake with therapeutic and theranostic properties. In Cell Penetrating Peptides; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; pp. 91–104. [Google Scholar]
- Nascimento, F.D.; Hayashi, M.A.F.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Tersariol, I.L.S.; Kerkis, I. Crotamine mediates gene delivery into cells through the binding to heparan sulfate proteoglycans. J. Bio. Chem. 2007, 282, 21349–21360. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Nascimento, F.D.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Pereira, A.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Kerkis, I.; et al. Cytotoxic effects of crotamine are mediated through lysosomal membrane permeabilization. Toxicon 2008, 52, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, N.; Budagavi, D.P.; Chugh, A. CyLoP-1: Membrane-active peptide with cell-penetrating and antimicrobial properties. Biochim. Biophys. Acta 2017, 1859, 167–176. [Google Scholar] [CrossRef]
- Rádis-Baptista, G.; LaTorre, B.G.; Andreu, D. A Novel Cell-penetrating peptide sequence derived by structural minimization of a snake toxin exhibits preferential nucleolar localization. J. Med. Chem. 2008, 51, 7041–7044. [Google Scholar] [CrossRef]
- Rodrigues, M.; LaTorre, B.G.; Rádis-Baptista, G.; Santos, N.C.; Andreu, D. efficient cellular delivery of β-galactosidase mediated by NrTPs, a new family of cell-penetrating peptides. Bioconjug. Chem. 2011, 22, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Santos, A.; LaTorre, B.G.; Rádis-Baptista, G.; Andreu, D.; Santos, N.C. molecular characterization of the interaction of crotamine-derived nucleolar targeting peptides with lipid membranes. Biochim. Biophys. Acta 2012, 1818, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Tansi, F.L.; Filatova, M.P.; Koroev, D.O.; Volpina, O.M.; Lange, S.; Schumann, C.; Teichgräber, U.K.; Reissmann, S.; Hilger, I. New generation cpps show distinct selectivity for cancer and noncancer cells. J. Cell. Biochem. 2019, 120, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; LaTorre, B.G.; Pérez-Peinado, C.; Barron, A.E.; Andreu, D.; Rádis-Baptista, G. Vipericidins: A novel family of cathelicidin-related peptides from the venom gland of south american pit vipers. Amino Acids 2014, 46, 2561–2571. [Google Scholar] [CrossRef]
- Falcao, C.B.; Radis-Baptista, G. Crotamine and Crotalicidin, membrane active peptides from Crotalus durissus terrificus rattlesnake venom, and their structurally-minimized fragments for applications in medicine and biotechnology. Peptides 2020, 126, 170234. [Google Scholar] [CrossRef] [PubMed]
- Peinado, C.P.; Defaus, S.; Andreu, D. Hitchhiking with nature: Snake venom peptides to fight cancer and superbugs. Toxins 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- VanHoek, M.L. Antimicrobial peptides in reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef]
- Zhou, H.; Dou, J.; Wang, J.; Chen, L.; Wang, H.; Zhou, W.; Li, Y.; Zhou, C. The antibacterial activity of bf-30 in vitro and in infected burned rats is through interference with cytoplasmic membrane integrity. Peptides 2011, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Juba, M.L.; Porter, D.K.; Williams, E.H.; Rodriguez, C.A.; Barksdale, S.M.; Bishop, B.M. Helical cationic antimicrobial peptide length and its impact on membrane disruption. Biochim. Biophys. Acta 2015, 1848, 1081–1091. [Google Scholar] [CrossRef]
- Cavalcante, C.S.P.; Aguiar, F.L.L.; Fontenelle, R.O.S.; Menezes, R.R.P.P.B.; Martins, A.M.C.; Falcão, C.B.; Andreu, D.; Rádis-Baptista, G. Insights into the candidacidal mechanism of ctn[15–34]—A carboxyl-terminal, crotalicidin-derived peptide related to cathelicidins. J. Med. Microbiol. 2018, 67, 129–138. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Dias, S.A.; Domingues, M.M.; Benfield, A.H.; Freire, J.M.; Rádis-Baptista, G.; Gaspar, D.; Castanho, M.A.R.B.; Craik, D.J.; Henriques, S.T.; et al. Mechanisms of bacterial membrane permeabilization by crotalicidin ctn and its fragment ctn15–34, antimicrobial peptides from rattlesnake venom. J. Bio. Chem. 2018, 293, 1536–1549. [Google Scholar] [CrossRef]
- Aguiar, F.L.L.D.; Santos, N.C.; de Paula Cavalcante, C.S.; Andreu, D.; Baptista, G.R.; Gonçalves, S. Antibiofilm activity on candida albicans and mechanism of action on biomembrane models of the antimicrobial peptide Ctn[15–34]. Int. J. Mol. Sci. 2020, 21, 8339. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; Peinado, C.P.; La Torre, B.G.; Mayol, X.; Carreras, H.Z.; Jiménez, M.Á.; Rádis-Baptista, G.; Andreu, D. Structural dissection of crotalicidin, a rattlesnake venom cathelicidin, retrieves a fragment with antimicrobial and antitumor activity. J. Med. Chem. 2015, 58, 8553–8563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chan, J.Y.W.; Rêgo, J.V.; Chong, C.-M.; Ai, N.; Falcão, C.B.; Rádis-Baptista, G.; Lee, S.M.Y. rhodamine b-conjugated encrypted vipericidin nonapeptide is a potent toxin to zebrafish and associated with in vitro cytotoxicity. Biochim. Biophys. Acta 2015, 1850, 1253–1260. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Mendes, B.; Almeida, J.R.; Vale, N.; Gomes, P.; Gadelha, F.R.; Silva, S.L.; Miguel, D.C. Potential use of 13-mer peptides based on phospholipase and oligoarginine as leishmanicidal agents. Comp. Biochem. Physiol. 2019, 226, 108612. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Twardowski, T.; Wohlgemuth, R. Bioeconomy for sustainable development. Biotechnol. J. 2019, 14, e1800638. [Google Scholar] [CrossRef]
- Woźniak, E.; Tyczewska, A.; Twardowski, T. Bioeconomy development factors in the european union and poland. New Biotechnol. 2021, 60, 2–8. [Google Scholar] [CrossRef]
- Kuckertz, A.; Berger, E.S.C.; Brändle, L. Entrepreneurship and the sustainable bioeconomy transformation. Environ. Innov. Soc. Transit. 2020, 37, 332–344. [Google Scholar] [CrossRef]
- Bugge, M.; Hansen, T.; Klitkou, A. What is the bioeconomy? a review of the literature. Sustainability 2016, 8, 691. [Google Scholar] [CrossRef]
- Patermann, C.; Aguilar, A. The origins of the bioeconomy in the european union. New Biotechnol. 2018, 40, 20–24. [Google Scholar] [CrossRef]
- Vivien, F.D.; Nieddu, M.; Befort, N.; Debref, R.; Giampietro, M. The hijacking of the bioeconomy. Ecol. Econ. 2019, 159, 189–197. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Biotechnology, in vitro production of natural bioactive compounds, herbal preparation, and disease management (treatment and prevention). In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2; Progress in Drug Research; Springer: Cham, Switzerland, 2018; pp. 585–664. [Google Scholar]
- Ferreira, S.H. A bradykinin-potentiating factor BPF present in the venom of Bothrops jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Boldrini-França, J.; Pinheiro-Junior, E.L.; Peigneur, S.; Pucca, M.B.; Cerni, F.A.; Borges, R.J.; Costa, T.R.; Carone, S.E.I.; Fontes, M.R.D.M.; Sampaio, S.V.; et al. Beyond hemostasis: A snake venom serine protease with potassium channel blocking and potential antitumor activities. Sci. Rep. 2020, 10, 4476. [Google Scholar] [CrossRef]
- USPTO. Cooperative Patent Classification—CPC. Available online: https://www.uspto.gov/web/patents/classification/cpc/html/cpc.html (accessed on 6 March 2024).
- Oliveira, Á.S.; Fantinel, A.L.; Artuzo, F.D.; Oliveira, L.; Singer, R.B.; da Frota Júnior, M.L.C.; Dewes, H.; Talamini, E. Applications of venom biodiversity in agriculture. EFB Bioecon. J. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake Bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bolton, F.; Casewell, N.; Al-Abdulla, I. Snake antivenom trial. Vet. Rec. 2014, 174, 126. [Google Scholar] [CrossRef]
- Williams, D.J.; Habib, A.G.; Warrell, D.A. Clinical studies of the effectiveness and safety of antivenoms. Toxicon 2018, 150, 1–10. [Google Scholar] [CrossRef]
- Metkar, S.; Udayakumar, S.; Girigoswami, A.; Girigoswami, K. Natural serine proteases and their applications in combating amyloid formation: Review article. ADMET DMPK 2024, 12, 797–820. [Google Scholar]



| Molecule/Toxin | Classification/Source | Nanosystems | Characterization Size (nm); PDI; PZ (mV) | Encapsulation Efficiency (%) | Biological Activities | References |
|---|---|---|---|---|---|---|
| Whole venom | Bothrops jararaca (BJ) | Cationic PLGA nanoparticles (CNps) +1.0% BJ venom | 168.7 ± 3.8 nm, 0.09 ± 0.01 +35.6 ± 13.4 mV | - | Immunoadjuvant | [68] |
| Whole venom | Bothrops jararaca and Bothrops erythromelas (Bery) | Chitosan nanoparticles (CNPs) | BJ 10%: 174.7 ± 5.0 nm, 0.203 ± 0.07 +24.91 ± 2.91 mV Bery 10%: 160.0 ± 2.3 nm, 0.302 ± 0.01 +19.00 ± 2.76 mV | BJ 67.7 Bery 87.6 | Immunoadjuvant | [67] |
| Whole venom | Bothrops jararacussu | CNPs | 160 nm Inferior to 0.5 | More than 70% | Antimicrobial activity against Gram-positive bacteria | [91] |
| Whole venom | Bothrops jararaca | Nanostructured SBA-15 silica | - | 1 µg of venom adsorbed /encapsulated | Oral tolerance induction | [92] |
| Whole venom | Pool of venom containing Bothrops alternatus, Bothrops jararaca, Bothrops jararacussu, Bothrops Bothrops moojeni, Bothrops neuwiedi, and Crotalus durissus terrificus | Liposomes with Bothrops venom (LB), and with crotalic venom (LC) | LB (119 ± 47 nm) and LC (147 ± 56 nm) | The liposomal efficiencies of protein incorporation were 99% for the bothropic and 59% for the crotalic venoms | Development of antisera to treat snakebites, reducing the stress of the horse | [94] |
| Whole venom | Bothrops jararaca | Liposomes | - | - | Immunostimulants | [99] |
| PLA2 toxin | Bothrops jararacussu | Liposomes | Size: 241.9 and 205.2 nm Zeta: −18 mV and −25.4 mV | - | Anti-Leishmania amazonensis activity in vitro and in vivo | [101,102] |
| Whole venom | Crotalus molossus molossus | CNPs | Size: 415.9 ± 21.67 nm PdI: 0.44 ± 0.03 Zeta: +28.3 ± 1.17 mV | 48.29 ± 3.84 | Cytotoxic activity against T-47D breast carcinoma cells | [109] |
| Whole venom | Crotalus durissus cascavella | CNPs + C. d. cascavella venom 1.0% | Size: 147.7 nm PdI: 0.323 Zeta: +41.80 mV | 98.9 | Immunostimulants | [103] |
| Crotoxin | Crotalus durissus Terrificus | Nanostructured mesoporous silica SBA-15 | - | - | Immunomodulatory and anti-inflammatory activity in multiple sclerosis | [105] |
| Crotamine | Crotalus durissus Terrificus | Gold nanoparticle GNPs | Size: 43 nm Zeta: −0.6 ± 3.9 mV | - | Cell-penetrating ability | [108] |
| Crotoxin | Crotalus durissus Terrificus | Liposomes | - | - | Immunostimulants | [110,111] |
| Whole venom | Crotalus durissus terrificus | Liposomes | - | - | Biodistribution of free and encapsulated liposomes | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, Á.E.F.; Barros, A.B.C.; Silva, L.C.F.; Carvalho, L.M.M.; Pereira, G.M.A.; Uchôa, A.F.C.; Barbosa-Filho, J.M.; Silva, M.S.; Luna, K.P.O.; Soares, K.S.R.; et al. Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview. Pharmaceutics 2025, 17, 204. https://doi.org/10.3390/pharmaceutics17020204
Alves ÁEF, Barros ABC, Silva LCF, Carvalho LMM, Pereira GMA, Uchôa AFC, Barbosa-Filho JM, Silva MS, Luna KPO, Soares KSR, et al. Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview. Pharmaceutics. 2025; 17(2):204. https://doi.org/10.3390/pharmaceutics17020204
Chicago/Turabian StyleAlves, Álisson E. F., Anne B. C. Barros, Lindomara C. F. Silva, Lucas M. M. Carvalho, Graziela M. A. Pereira, Ana F. C. Uchôa, José M. Barbosa-Filho, Marcelo S. Silva, Karla P. O. Luna, Karla S. R. Soares, and et al. 2025. "Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview" Pharmaceutics 17, no. 2: 204. https://doi.org/10.3390/pharmaceutics17020204
APA StyleAlves, Á. E. F., Barros, A. B. C., Silva, L. C. F., Carvalho, L. M. M., Pereira, G. M. A., Uchôa, A. F. C., Barbosa-Filho, J. M., Silva, M. S., Luna, K. P. O., Soares, K. S. R., & Xavier-Júnior, F. H. (2025). Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview. Pharmaceutics, 17(2), 204. https://doi.org/10.3390/pharmaceutics17020204







