3D-Printed Contact Lenses to Release Polyvinyl Alcohol as a Therapeutic Agent for the Treatment of Dry Eyes
Abstract
:1. Introduction
2. Materials and Methods
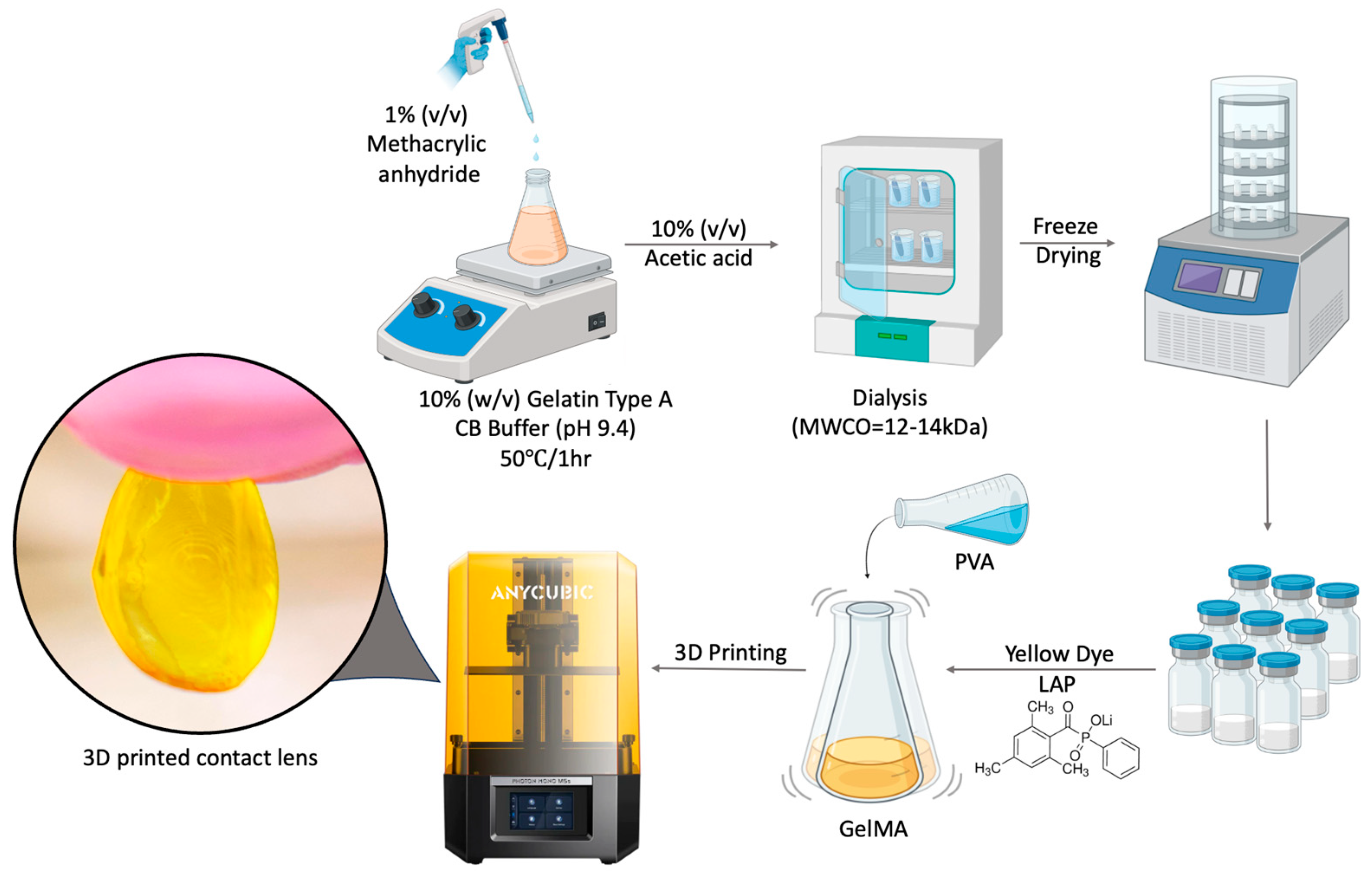
2.1. Synthesis of GelMA
2.2. Preparation of GelMA and P-Gel Inks
2.3. Three-Dimensional Printing of Contact Lenses
2.4. Print Quantification
2.5. Light Transmittance
2.6. PVA Release from 3D-Printed CLs
2.7. Drug Release Kinetics via Mathematical Modeling
2.8. Viscosity Measurements
2.9. Simulating Dry Eye Conditions via Desiccation Stress on Human Corneal Epithelial Cells
2.10. Statistical Analysis
3. Results
3.1. Three-Dimensional Printing of Contact Lenses
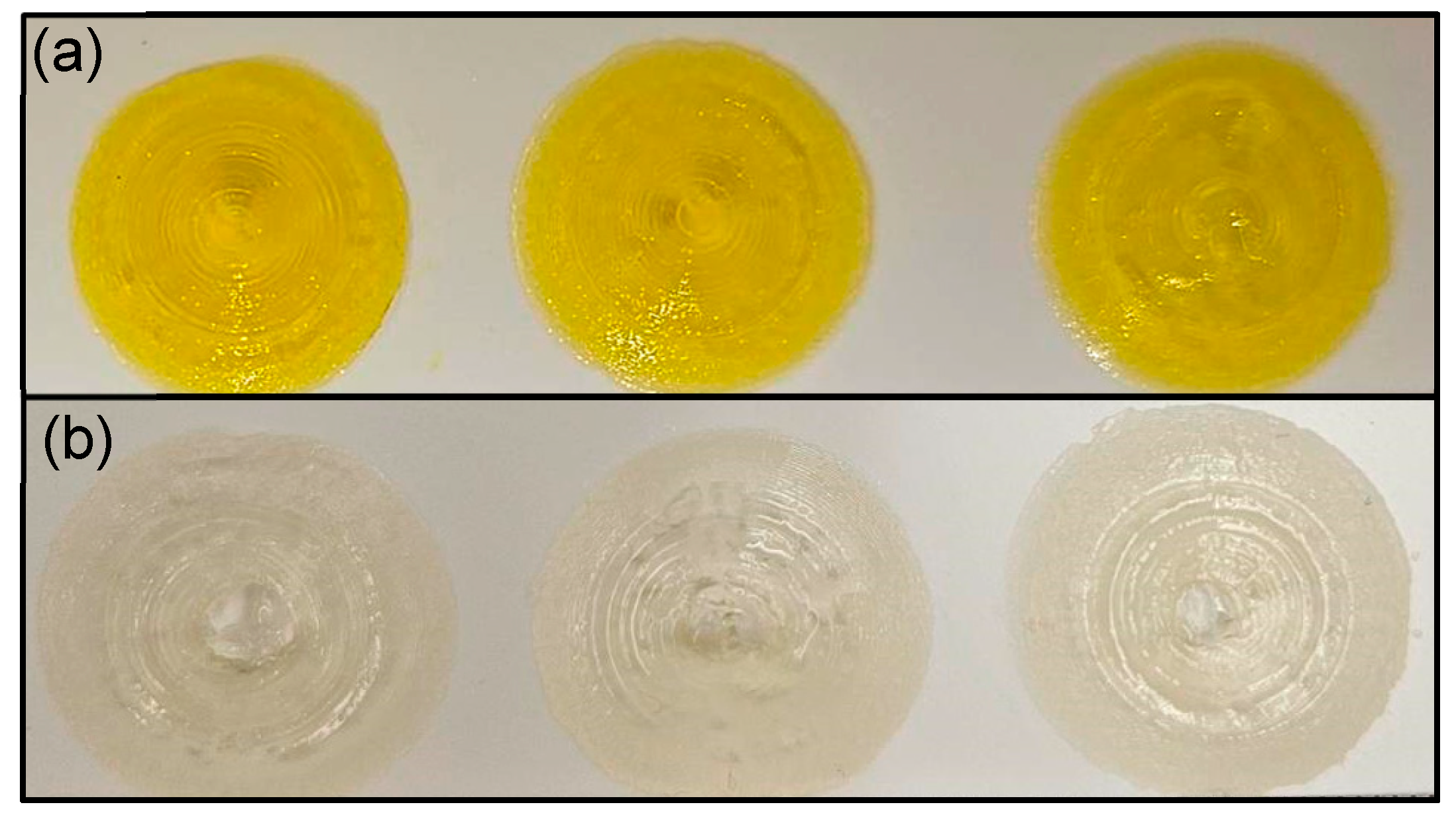
3.2. Print Quantification Analysis
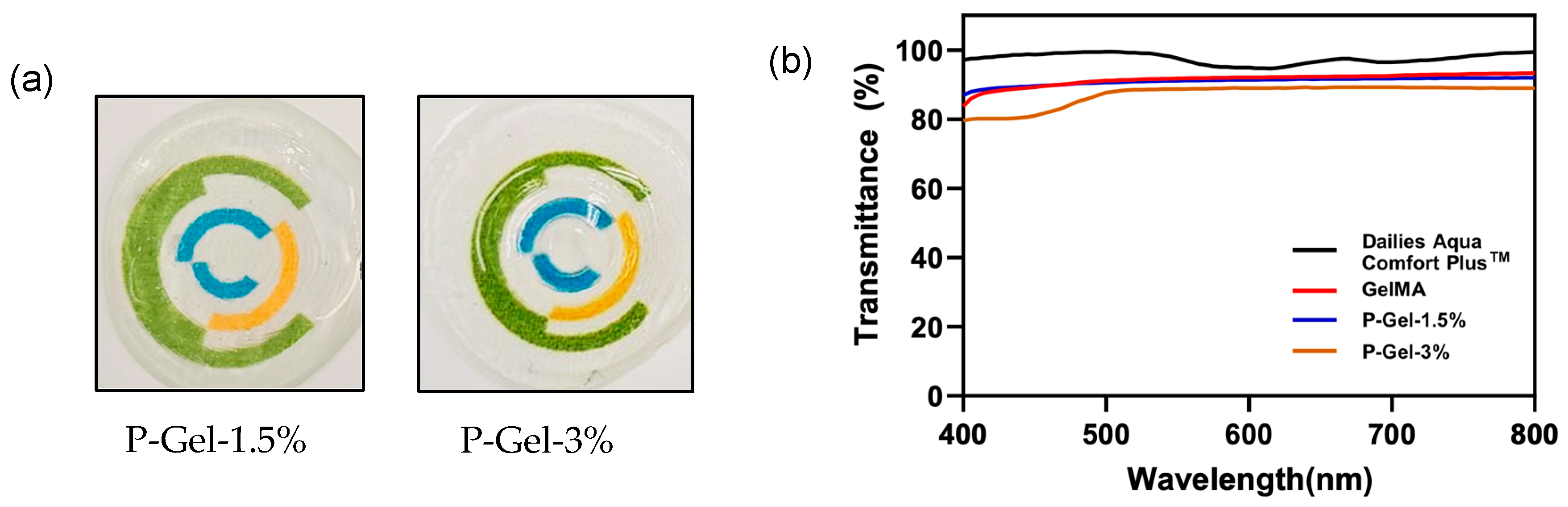
3.3. Light Transmittance and Optical Transparency
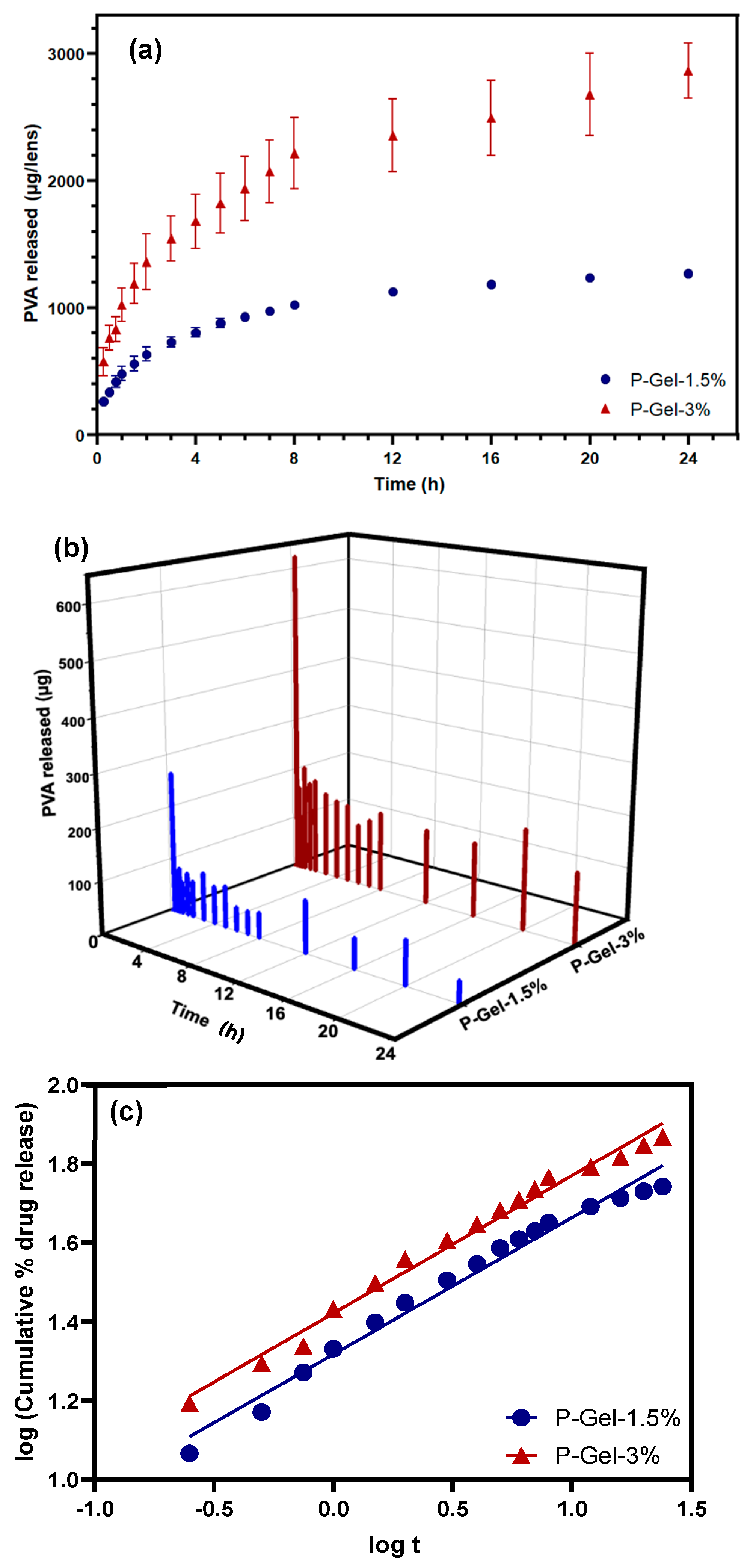
3.4. PVA Release
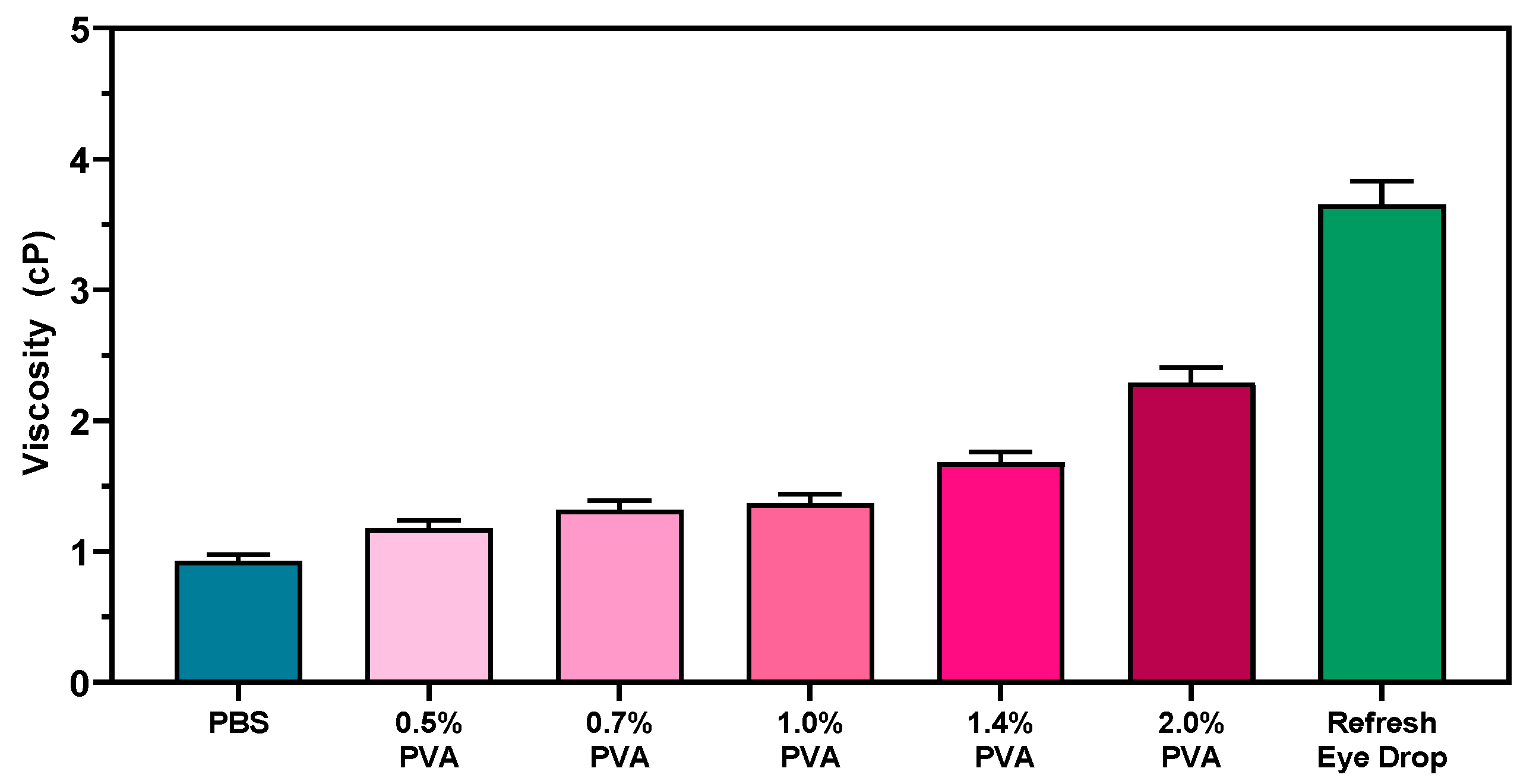
3.5. Viscosity Measurements
3.6. Ability of PVA to Protect HCECs Against Desiccation Stress
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U. TFOS DEWS II tear film report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Hui, A.; Phan, C.M.; Read, M.L.; Azar, D.; Buch, J.; Ciolino, J.B.; Naroo, S.A.; Pall, B.; Romond, K.; et al. CLEAR—Contact lens technologies of the future. Contact Lens Anterior Eye 2021, 44, 398–430. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Walther, H.; Riederer, D.; Lau, C.; Lorenz, K.O.; Subbaraman, L.N.; Jones, L. Analysis of polyvinyl alcohol release from commercially available daily disposable contact lenses using an in vitro eye model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.-M.; Subbaraman, L.N.; Jones, L.W. Uptake and release of polyvinyl alcohol from hydrogel daily disposable contact lenses. Optom. Vis. Sci. 2019, 96, 180–186. [Google Scholar] [CrossRef]
- Winterton, L.C.; Lally, J.M.; Sentell, K.B.; Chapoy, L.L. The elution of poly (vinyl alcohol) from a contact lens: The realization of a time release moisturizing agent/artificial tear. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 424–432. [Google Scholar] [CrossRef]
- Shokrollahi, P.; Garg, P.; Wulff, D.; Hui, A.; Phan, C.M.; Jones, L. Vat photopolymerization 3D printing optimization: Analysis of print conditions and print quality for complex geometries and ocular applications. Int. J. Pharm. 2025, 668, 124999. [Google Scholar] [CrossRef]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef]
- Hisham, M.; Butt, H. Vat photopolymerization printing of functionalized hydrogels on commercial contact lenses. Sci. Rep. 2024, 14, 13860. [Google Scholar] [CrossRef]
- Goto, E.; Tagami, T.; Ogawa, K.; Ozeki, T. Fabrication of 3D-printed contact lens composed of polyethylene glycol diacrylate for controlled release of Azithromycin. Biol. Pharm. Bull. 2023, 46, 1461–1467. [Google Scholar] [CrossRef]
- Hisham, M.; Salih, A.E.; Butt, H. 3D printing of multimaterial contact lenses. ACS Biomater. Sci. Eng. 2023, 9, 4381–4391. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Zou, M.; Zhang, L.; Song, Y. Suppressing the Step Effect of 3D Printing for Constructing Contact Lenses. Adv. Mater. 2022, 34, e2107249. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Aravind, M.; Chidangil, S.; George, S.D. Self-moisturizing contact lens employing capillary flow. Addit. Manuf. 2022, 55, 102842. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Hu, H.; Wang, W.; Li, Q.; Ma, L.; Ma, J.; Ye, C.; Yang, H.; Zhang, B. Liquid-Phase Integrated 3D Printed Biological Lenses for Lamellar Corneal Substitute. Adv. Healthc. Mater. 2023, 12, 2300600. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Modified low molecular weight poly-vinyl alcohol as viscosity enhancer. Mater. Today Commun. 2019, 21, 100634. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Luensmann, D.; Yu, M.; Yang, J.; Srinivasan, S.; Jones, L. Impact of cosmetics on the physical dimension and optical performance of silicone hydrogel contact lenses. Eye Contact Lens 2015, 41, 218–227. [Google Scholar] [CrossRef]
- Procházková, L.; Rodríguez-Muñoz, Y.; Procházka, J.; Wanner, J. Simple spectrophotometric method for determination of polyvinylalcohol in different types of wastewater. Int. J. Environ. Anal. Chem. 2014, 94, 399–410. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a mesoporous polydopamine@ GO/cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Shokrollahi, P.; Darge, H.F.; Phan, C.-M.; Jones, L. Controlled PVA Release from Chemical-Physical Interpenetrating Networks to Treat Dry Eyes. ACS Omega 2024, 10, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Rosencranz, R.; Bogen, S.A. Clinical laboratory measurement of serum, plasma, and blood viscosity. Pathol. Patterns Rev. 2006, 125 (Suppl. S1), S78–S86. [Google Scholar] [CrossRef] [PubMed]
- Molladavoodi, S.; Robichaud, M.; Wulff, D.; Gorbet, M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS ONE 2017, 12, e0178981. [Google Scholar] [CrossRef]
- Griffith, M.; Osborne, R.; Munger, R.; Xiong, X.; Doillon, C.J.; Laycock, N.L.; Hakim, M.; Song, Y.; Watsky, M.A. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- Rangarajan, R.; Ketelson, H.A.; Do, R.; McCanna, D.J.; Suko, A.; Enstone, D.; Subbaraman, L.N.; Dantam, J.; Jones, L.W. Effect of artificial tear formulations on the metabolic activity of human corneal epithelial cells after exposure to desiccation. JoVE J. Vis. Exp. 2020, 159, e60812. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, X.J.; Sun, Y.; Gao, Q.; Fu, J.Z.; Cai, X.J.; He, Y. Printability during projection-based 3D bioprinting. Bioact. Mater. 2022, 11, 254–267. [Google Scholar] [CrossRef]
- Bose, S.; Phan, C.M.; Rizwan, M.; Tse, J.W.; Yim, E.; Jones, L.; Pignatello, R.; Almeida, H.; Santonocito, D.; Puglia, C. Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material. Pharmaceutics 2024, 16, 26. [Google Scholar] [CrossRef]
- Zhu, M.X.; Wang, Y.Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Guo, A.; Zhang, S.; Yang, R.; Sui, C. Enhancing the mechanical strength of 3D printed GelMA for soft tissue engineering applications. Mater. Today Bio 2023, 24, 100939. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing chemical and physical stability of pharmaceuticals using freeze-thaw method: Challenges and opportunities for process optimization through quality by design approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef]
- Mandal, P.; Stokes, K.; Hernández, G.; Brandell, D.; Mindemark, J. Influence of binder crystallinity on the performance of si electrodes with poly (vinyl alcohol) binders. ACS Appl. Energy Mater. 2021, 4, 3008–3016. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, R.; Liu, S.; Liu, Y.; Gu, Y.; Zhang, H. 3D printing GelMA/PVA interpenetrating polymer networks scaffolds mediated with CuO nanoparticles for angiogenesis. Macromol. Biosci. 2022, 22, 2200208. [Google Scholar] [CrossRef]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K.; Letourneur, D.; Le Visage, C. Evaluation of hemocompatibility and endothelialization of hybrid poly (vinyl alcohol)(PVA)/gelatin polymer films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1549–1559. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Rao, J.; Zhou, Q.; Chen, J.; Gu, J.; Wang, Y.; Liu, Y. Carbodiimide crosslinked decellularized lenticules as a drug carrier for sustained antibacterial eye treatments. Biomed. Mater. 2023, 18, 025009. [Google Scholar] [CrossRef]
- Gupta, R.K.; Alzayed, M.A.; Alkhayl, A.A.A.; Bedaiwi, T.S. Effect of Light Sources on Transmittance of Commercially Available Contact Lenses. Cureus 2024, 16, e62093. [Google Scholar] [CrossRef]
- Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Marcos Luciano, B., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Onogi, S.; Masuda, T.; Miyanaga, N.; Kimura, Y. Dependence of viscosity of concentrated polymer solutions upon molecular weight and concentration. J. Polym. Sci. Part A-2 Polym. Phys. 1967, 5, 899–913. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [PubMed]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-349/subpart-B/section-349.12 (accessed on 3 June 2024).
- Labetoulle, M.; Benitez-del-Castillo, J.M.; Barabino, S.; Herrero Vanrell, R.; Daull, P.; Garrigue, J.-S.; Rolando, M. Artificial tears: Biological role of their ingredients in the management of dry eye disease. Int. J. Mol. Sci. 2022, 23, 2434. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Greco, G.; Bonechi, C.; Lamponi, S.; Rossi, C.; Magnani, A. A PVA/PVP hydrogel for human lens substitution: Synthesis, rheological characterization, and in vitro biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 278–288. [Google Scholar] [CrossRef] [PubMed]

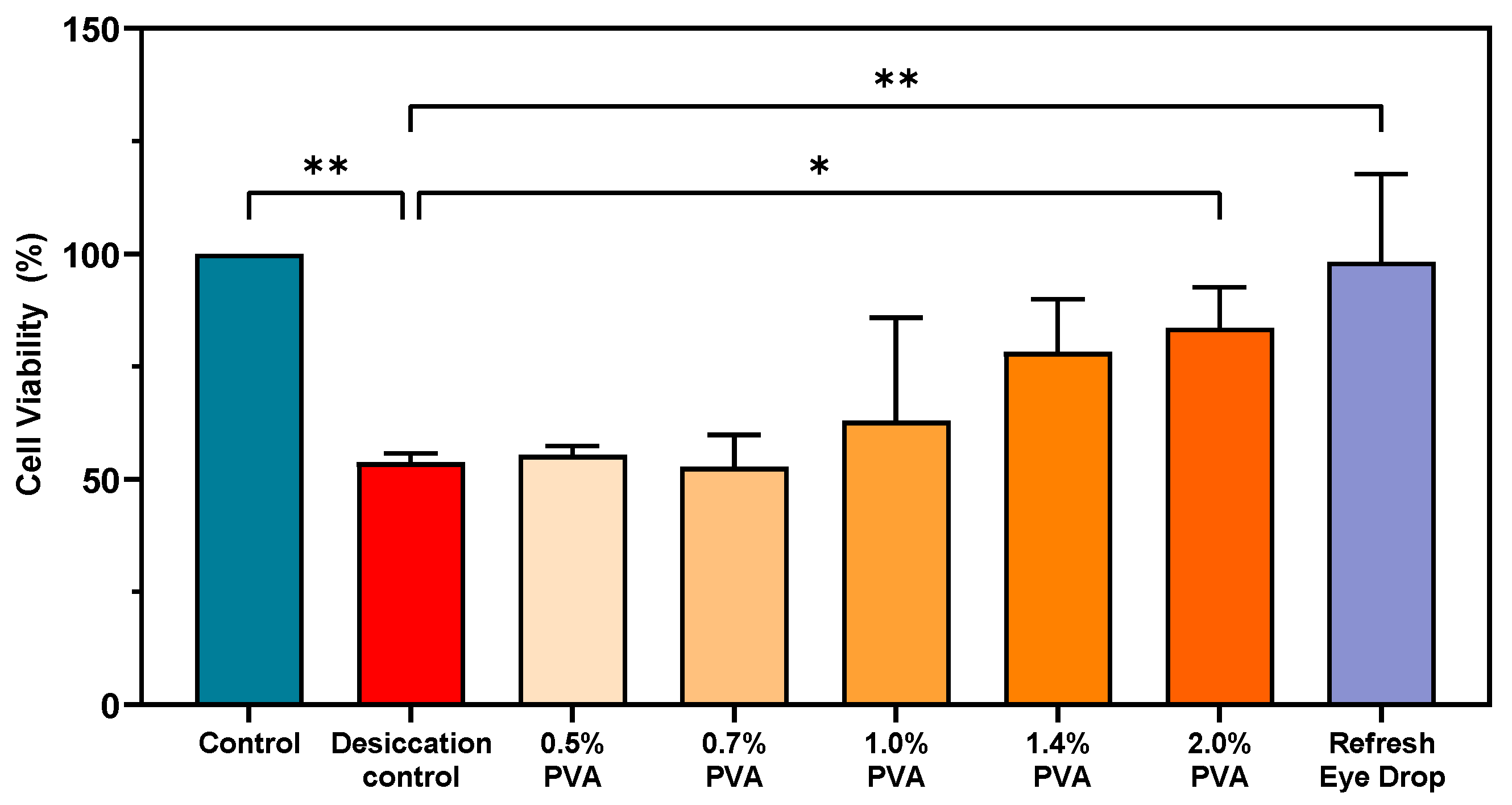
| Shape Fidelity | ||
|---|---|---|
| Zero Order | First Order | Higuichi Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|
| Equation | ||||
| Graph | cumulative drug release vs. time | log cumulative % drug remaining vs. time | cumulative % drug release vs. | log (cumulative % drug release vs. log t |
| Kinetic Model | P-Gel-1.5% | P-Gel-3% |
|---|---|---|
| Zero-order | 0.7824 | 0.8131 |
| First order | 0.8487 | 0.9181 |
| Higuchi | 0.9364 | 0.9532 |
| Korsmeyer–Peppas | 0.9806 | 0.9864 |
| Diffusion exponent (n-value) * | 0.3464 | 0.3476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, P.; Shokrollahi, P.; Darge, H.F.; Phan, C.-M.; Jones, L. 3D-Printed Contact Lenses to Release Polyvinyl Alcohol as a Therapeutic Agent for the Treatment of Dry Eyes. Pharmaceutics 2025, 17, 219. https://doi.org/10.3390/pharmaceutics17020219
Garg P, Shokrollahi P, Darge HF, Phan C-M, Jones L. 3D-Printed Contact Lenses to Release Polyvinyl Alcohol as a Therapeutic Agent for the Treatment of Dry Eyes. Pharmaceutics. 2025; 17(2):219. https://doi.org/10.3390/pharmaceutics17020219
Chicago/Turabian StyleGarg, Piyush, Parvin Shokrollahi, Haile Fentahun Darge, Chau-Minh Phan, and Lyndon Jones. 2025. "3D-Printed Contact Lenses to Release Polyvinyl Alcohol as a Therapeutic Agent for the Treatment of Dry Eyes" Pharmaceutics 17, no. 2: 219. https://doi.org/10.3390/pharmaceutics17020219
APA StyleGarg, P., Shokrollahi, P., Darge, H. F., Phan, C.-M., & Jones, L. (2025). 3D-Printed Contact Lenses to Release Polyvinyl Alcohol as a Therapeutic Agent for the Treatment of Dry Eyes. Pharmaceutics, 17(2), 219. https://doi.org/10.3390/pharmaceutics17020219







