pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment
Abstract
1. Introduction
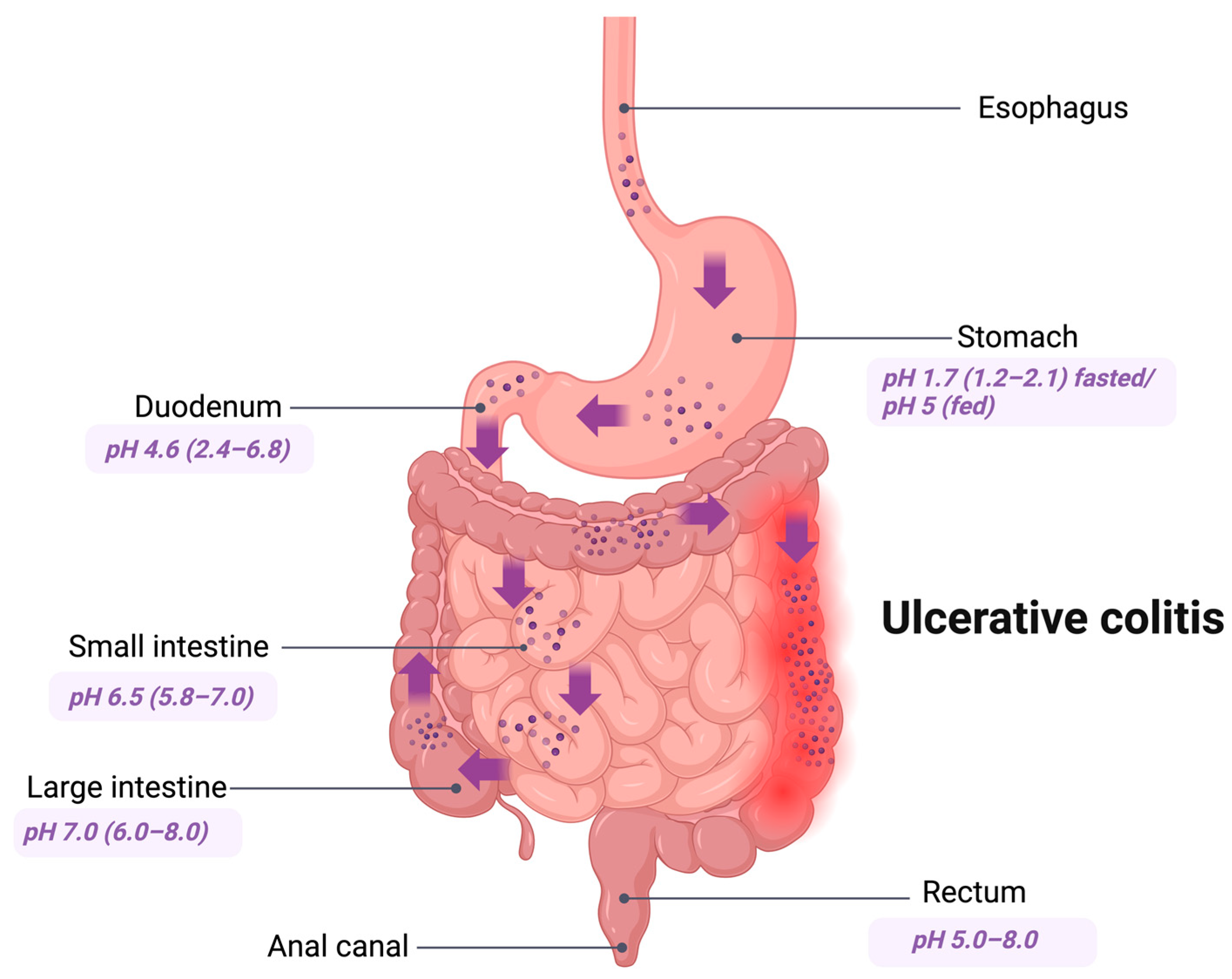
2. GIT Conditions
3. Ulcerative Colitis
4. Colon-Specific Drug Delivery Systems
Dosage Forms Suitable for Targeted Delivery
5. pH-Dependent Drug Delivery Systems
5.1. Advantages of pH-Dependent Drug Delivery Systems
5.2. Disadvantages of pH-Dependent Drug Delivery Systems
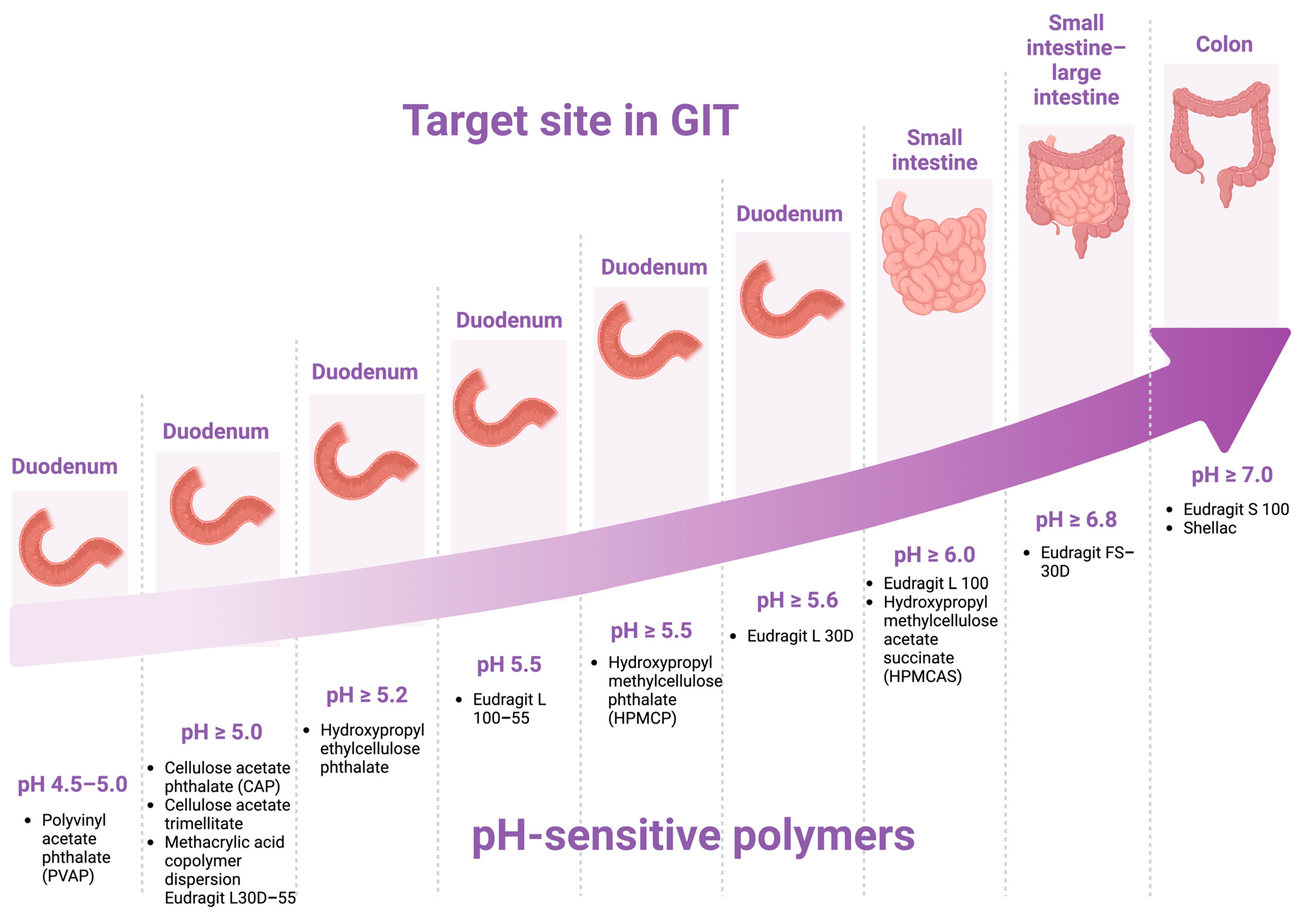
6. pH-Dependent Polymers
7. Historical Overview of pH-Dependent Strategies Utilizing Various Substances for UC Treatment over the Past 15 Years
7.1. Metronidazole
7.2. Budesonide
7.3. Prednisolone
7.4. Budesonide and Prednisolone
7.5. Hydrocortisone Sodium Succinate (HSS)
7.6. Dexamethasone
7.7. Coumarin
7.8. Notoginsenoside
7.9. Cyclosporine
7.10. Iridoid Glycosides
7.11. Flurbiprofen
7.12. Baicalin
7.13. Methotrexate
7.14. Curcumin and Combinations
7.15. Mesalamine (Mesalazine)
7.16. Mesalamine and Prednisolone
8. Marketed pH-Dependent Drug Delivery Systems
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| GIT | Gastrointestinal tract |
| 5-ASA | 5-aminosalicylic acid |
| HSS | Hydrocortisone sodium succinate |
| CsA | Cyclosporine A |
| BA | Baicalin |
| MXT | Methotrexate |
| CDDS | Colon-specific drug delivery system |
References
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-Specific Targeted Drug Delivery Systems for the Treatment of Inflammatory Bowel Disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Favaron, A.; Awad, A.; Orlu, M.; Gaisford, S.; Basit, A.W. Colonic Drug Delivery: Formulating the next Generation of Colon-Targeted Therapeutics. J. Control. Release 2023, 353, 1107–1126. [Google Scholar] [CrossRef] [PubMed]
- Vass, P.; Démuth, B.; Hirsch, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; Nagy, Z.K.; Marosi, G. Drying Technology Strategies for Colon-Targeted Oral Delivery of Biopharmaceuticals. J. Control. Release 2019, 296, 162–178. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Alshammari, N.D.; Elkanayati, R.; Vemula, S.K.; Al Shawakri, E.; Uttreja, P.; Almutairi, M.; Repka, M.A. Advancements in Colon-Targeted Drug Delivery: A Comprehensive Review on Recent Techniques with Emphasis on Hot-Melt Extrusion and 3D Printing Technologies. AAPS PharmSciTech 2024, 25, 236. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, M.; Melocchi, A.; Moutaharrik, S.; Palugan, L.; Cerea, M.; Foppoli, A.; Maroni, A.; Gazzaniga, A.; Zema, L. Administration Strategies and Smart Devices for Drug Release in Specific Sites of the Upper GI Tract. J. Control. Release 2022, 348, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Iswandana, R.; Putri, K.S.; Putri, F.A.; Gunawan, M.; Larasati, S.A. Challenge and development strategy for colon-targeted drug delivery system. Pharm. Sci. Res. 2022, 9, 17–27. [Google Scholar] [CrossRef]
- Jagtap, R.K.; Savle, M.; Sanap, G. A Review On Colon Targeted Drug Delivery System. Int. J. Pharm. Sci. 2024, 2, 152–164. [Google Scholar]
- Muhammed, R.A.; Mohammed, S.; Visht, S.; Yassen, A.O. A review on development of colon targeted drug delivery system. Int. J. Appl. Pharm. 2024, 16, 12–27. [Google Scholar] [CrossRef]
- Geetha, K.; Akshitha, S.; Rao, T.R. A Review on Colon Drug Delivery Systems. Int. J. Pharm. Sci. Rev. Res. 2023, 82, 25–35. [Google Scholar] [CrossRef]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent Advances in Colon Drug Delivery Systems. J. Control. Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Paul, S.U.; Deshmukh, V.N.; Patange, V.P. A Review on Colon Targeted Drug Delivery System and Its Approaches. World J. Pharm. Res. 2023, 12, 740–758. [Google Scholar]
- Petras, R.E.; Frankel, W.L. Large Intestine (Colon). In Modern Surgical Pathology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 755–836. ISBN 978-1-4160-3966-2. [Google Scholar]
- Dos Santos, A.M.; Carvalho, S.G.; Meneguin, A.B.; Sábio, R.M.; Gremião, M.P.D.; Chorilli, M. Oral Delivery of Micro/Nanoparticulate Systems Based on Natural Polysaccharides for Intestinal Diseases Therapy: Challenges, Advances and Future Perspectives. J. Control. Release 2021, 334, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Crnčević, N.; Hukić, M.; Deumić, S.; Selimagić, A.; Dozić, A.; Gavrankapetanović, I.; Klepo, D.; Avdić, M. Gastrointestinal Tract Microbiome Effect and Role in Disease Development. Diseases 2022, 10, 45. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Facciotti, F. Modulation of Intestinal Immune Cell Responses by Eubiotic or Dysbiotic Microbiota in Inflammatory Bowel Diseases. PharmaNutrition 2022, 21, 100303. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in Oral Nano-Delivery Systems for Colon Targeted Drug Delivery in Inflammatory Bowel Disease: Selective Targeting to Diseased versus Healthy Tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Kayal, M.; Shah, S. Ulcerative Colitis: Current and Emerging Treatment Strategies. JCM 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Łodyga, M.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the Management of Ulcerative Colitis. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev./Przegląd Gastroenterol. 2023, 18, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Teruel, A.H.; Gonzalez-Alvarez, I.; Bermejo, M.; Merino, V.; Marcos, M.D.; Sancenon, F.; Gonzalez-Alvarez, M.; Martinez-Mañez, R. New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. Int. J. Mol. Sci. 2020, 21, 6502. [Google Scholar] [CrossRef] [PubMed]
- Hmar, E.B.L.; Paul, S.; Zothantluanga, J.H.; Sharma, H.K. Ulcerative Colitis: A Review on Drug Delivery Strategies. Sci. Vis. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Evirgen, S.; İliaz, R.; Akyüz, F.; Çavuş, B.; Göktürk, S.; Örmeci, A.; Mutluay Soyer, Ö.; Baran, B.; Pınarbaşı, B.; Karaca, Ç.; et al. Cyclosporine Therapy as a Rescue Treatment in Steroid Refractory Acute Severe Ulcerative Colitis: A Real Life Data From a Tertiary Center. Turk. J. Gastroenterol. 2022, 33, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Cannatelli, R.; Monico, M.C.; Maconi, G.; Ardizzone, S. An Update on Current Pharmacotherapeutic Options for the Treatment of Ulcerative Colitis. JCM 2022, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Sands, B.E. New Therapeutics for Ulcerative Colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef]
- Danese, S.; Neurath, M.F.; Kopoń, A.; Zakko, S.F.; Simmons, T.C.; Fogel, R.; Siegel, C.A.; Panaccione, R.; Zhan, X.; Usiskin, K.; et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients with Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2526–2534. [Google Scholar] [CrossRef]
- Ghosh, S.; Majumdar, S.; Ganguly, D. A Brief Review on Colon-Specific Drug Delivery System for Targeting to Colonic Region. J. Appl. Pharm. Res. 2021, 9, 9–15. [Google Scholar] [CrossRef]
- Choudhary, L.; Jain, A.; Agarwal, D. Colon-Targeted Oral Drug Delivery Systems: A Review. Asian J. Pharm. Res. Dev. 2020, 8, 186–193. [Google Scholar]
- Awad, A.; Madla, C.M.; McCoubrey, L.E.; Ferraro, F.; Gavins, F.K.H.; Buanz, A.; Gaisford, S.; Orlu, M.; Siepmann, F.; Siepmann, J.; et al. Clinical Translation of Advanced Colonic Drug Delivery Technologies. Adv. Drug Deliv. Rev. 2022, 181, 114076. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, M.; Liu, K. Colon-Targeted Drug Delivery of Polysaccharide-Based Nanocarriers for Synergistic Treatment of Inflammatory Bowel Disease: A Review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Shukla, S.; Singhai, A.K.; Mishra, S.S. Colon drug delivery system and their benefits and the carriers for delivery of drugs to colon: A review. Asian J. Pharm. Educ. Res. 2020, 9, 15. [Google Scholar] [CrossRef]
- Sumanth, G.; Atpadkar, P.P.; Reddy, P.K.; Nihar, G. A Generous Review: Novel Approaches for Colon Targeted Drug Delivery System. Asian J. Adv. Res. Rep. 2021, 15, 92–99. [Google Scholar] [CrossRef]
- Kumari, B.; Upadhyay, P.K.; Kumar, M.; Narwal, S.; Pandurangan, A.; Malik, A. An update overview of recent advances on formulation development for colon targeting. Int. J. Pharm. Sci. Res. 2020, 11, 1571–1580. [Google Scholar] [CrossRef]
- Ghodrati, A.; Comoglu, T. An Overview on Recent Approaches for Colonic Drug Delivery Systems. Pharm. Dev. Technol. 2024, 29, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F. Making the Ideal Dosage Form: End-user considerations are becoming increasingly important as they can provide a lot of value and help to ensure commercial success of a drug. Pharm. Technol. Eur. 2020, 32, s15. [Google Scholar]
- Lin, H.; Leng, J.; Peng, F.; Xu, Z.; Ruan, G. Scalable Production of Microscopic Particles for Biological Delivery. Mater. Adv. 2023, 4, 2885–2908. [Google Scholar] [CrossRef]
- Ferraro, F.; Sonnleitner, L.M.; Neut, C.; Mahieux, S.; Verin, J.; Siepmann, J.; Siepmann, F. Colon Targeting in Rats, Dogs and IBD Patients with Species-Independent Film Coatings. Int. J. Pharm.X 2024, 7, 100233. [Google Scholar] [CrossRef] [PubMed]
- Bami, M.S.; Raeisi Estabragh, M.A.; Khazaeli, P.; Ohadi, M.; Dehghannoudeh, G. PH-Responsive Drug Delivery Systems as Intelligent Carriers for Targeted Drug Therapy: Brief History, Properties, Synthesis, Mechanism and Application. J. Drug Deliv. Sci. Technol. 2022, 70, 102987. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, L.; Yi, Y.; Yin, J.; Liang, A. Design Strategies, Advances and Future Perspectives of Colon-Targeted Delivery Systems for the Treatment of Inflammatory Bowel Disease. Asian J. Pharm. Sci. 2024, 19, 100943. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S. Novel Approaches for Colon Site-Specific Drug Delivery: An Overview of Recent Advancements. J. Pharm. Negat. Results 2022, 13, 4479–4495. [Google Scholar] [CrossRef]
- Feng, K.; Wei, Y.; Hu, T.-G.; Linhardt, R.J.; Zong, M.-H.; Wu, H. Colon-Targeted Delivery Systems for Nutraceuticals: A Review of Current Vehicles, Evaluation Methods and Future Prospects. Trends Food Sci. Technol. 2020, 102, 203–222. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Varum, F.; Al-Gousous, J.; Hofmann, M.; Page, S.; Langguth, P. In Vitro Methodologies for Evaluating Colon-Targeted Pharmaceutical Products and Industry Perspectives for Their Applications. Pharmaceutics 2022, 14, 291. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Rao, M.E.B.; Parcha, V. Smart Polymers for Colon Targeted Drug Delivery Systems: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 1130–1166. [Google Scholar] [CrossRef]
- Klaus, T.; Deshmukh, S. PH-Responsive Antibodies for Therapeutic Applications. J. Biomed. Sci. 2021, 28, 11. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Boase, N.R.B.; Gillies, E.R.; Goh, R.; Kieltyka, R.E.; Matson, J.B.; Meng, F.; Sanyal, A.; Sedláček, O. Stimuli-Responsive Polymers at the Interface with Biology. Biomacromolecules 2024, 25, 5417–5436. [Google Scholar] [CrossRef]
- Shetty, S.S.; Halagali, P.; Johnson, A.P.; Spandana, K.M.A.; Gangadharappa, H.V. Oral Insulin Delivery: Barriers, Strategies, and Formulation Approaches: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 242, 125114. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive Polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Stevens, C.A.; Kaur, K.; Klok, H.-A. Self-Assembly of Protein-Polymer Conjugates for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.-H.; Alias, S.A.; Quek, S.W.; Ng, C.Y.; Ku Marsilla, K.I. A Review of the Preparations, Properties, and Applications of Smart Biodegradable Polymers. Polym.-Plast. Technol. Mater. 2023, 62, 1273–1289. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. PH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 62, 2828–2850. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Khan, S.; Khan, S.A.; Weigmann, B. Advances in Orally-Delivered pH-Sensitive Nanocarrier Systems; an Optimistic Approach for the Treatment of Inflammatory Bowel Disease. Int. J. Pharm. 2019, 558, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH- and Ion-Sensitive Polymers for Drug Delivery. Expert. Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef]
- Seremeta, K.P.; Chiappetta, D.A.; Sosnik, A. Poly(ɛ-Caprolactone), Eudragit® RS 100 and Poly(ɛ-Caprolactone)/Eudragit® RS 100 Blend Submicron Particles for the Sustained Release of the Antiretroviral Efavirenz. Colloids Surf. B Biointerfaces 2013, 102, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Si, X.; Zhang, M.; Merlin, D. Oral Administration of pH-Sensitive Curcumin-Loaded Microparticles for Ulcerative Colitis Therapy. Colloids Surf. B Biointerfaces 2015, 135, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.K.; Jain, S.K. Metronidazole Loaded Pectin Microspheres for Colon Targeting. J. Pharm. Sci. 2009, 98, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. pH-Sensitive Nanospheres for Colon-Specific Drug Delivery in Experimentally Induced Colitis Rat Model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Naeem, M.; Choi, M.; Cao, J.; Yoon, S.; Kim, M.-S.; Jung, Y.; Lee, J.; Moon, H.R.; Ikram, M.; et al. Colon-Targeted Delivery of Budesonide Using Dual pH- and Time-Dependent Polymeric Nanoparticles for Colitis Therapy. DDDT 2015, 9, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Lee, J.; Oshi, M.A.; Cao, J.; Hlaing, S.P.; Im, E.; Jung, Y.; Yoo, J.-W. Colitis-Targeted Hybrid Nanoparticles-in-Microparticles System for the Treatment of Ulcerative Colitis. Acta Biomater. 2020, 116, 368–382. [Google Scholar] [CrossRef]
- Turanlı, Y.; Acartürk, F. Preparation and Characterization of Colon-Targeted pH/Time-Dependent Nanoparticles Using Anionic and Cationic Polymethacrylate Polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef]
- Alkazzaz, S.Z.M.; Ali, W.K. Design and In-Vitro Evaluation of Colon Targeted Prednisolone Solid Dispersion Tablets. PBJ 2015, 3, 30–41. [Google Scholar] [CrossRef]
- Liu, F.; Moreno, P.; Basit, A.W. A Novel Double-Coating Approach for Improved pH-Triggered Delivery to the Ileo-Colonic Region of the Gastrointestinal Tract. Eur. J. Pharm. Biopharm. 2010, 74, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F. In Vitro and In Vivo Evaluation of Combined Time and pH- Dependent Oral Colonic Targeted Prednisolone Microspheres. BJPR 2013, 3, 420–434. [Google Scholar] [CrossRef]
- Qu, Z.; Wong, K.Y.; Moniruzzaman, M.; Begun, J.; Santos, H.A.; Hasnain, S.Z.; Kumeria, T.; McGuckin, M.A.; Popat, A. One-Pot Synthesis of pH-Responsive Eudragit-Mesoporous Silica Nanocomposites Enable Colonic Delivery of Glucocorticoids for the Treatment of Inflammatory Bowel Disease. Adv. Ther. 2021, 4, 2000165. [Google Scholar] [CrossRef]
- Shi, X.; Yan, Y.; Wang, P.; Sun, Y.; Zhang, D.; Zou, Y.; Hu, S.; Zhang, L.; Xing, J.; Dong, Y. In Vitro and in Vivo Study of pH-Sensitive and Colon-Targeting P(LE-IA-MEG) Hydrogel Microspheres Used for Ulcerative Colitis Therapy. Eur. J. Pharm. Biopharm. 2018, 122, 70–77. [Google Scholar] [CrossRef]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J.-W. Colon-Targeted Dexamethasone Microcrystals with pH-Sensitive Chitosan/Alginate/Eudragit S Multilayers for the Treatment of Inflammatory Bowel Disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Kim, W.; Cao, J.; Jung, Y.; Yoo, J.-W. Enzyme/pH Dual Sensitive Polymeric Nanoparticles for Targeted Drug Delivery to the Inflamed Colon. Colloids Surf. B Biointerfaces 2014, 123, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tie, H.; Wang, Y.; Shang, Y.; Li, M.; Wei, X.; Wang, Z. Fabrication of pH-Dependent Solid Dispersion for Oral Colon-Targeted Delivery of Notoginsenoside R1 and Its Protective Effects on Ulcerative Colitis Mice. Heliyon 2023, 9, e20280. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.; Lee, J.; Kim, J.; Hasan, N.; Im, E.; Jung, Y.; Yoo, J.-W. pH-Responsive Alginate-Based Microparticles for Colon-Targeted Delivery of Pure Cyclosporine A Crystals to Treat Ulcerative Colitis. Pharmaceutics 2021, 13, 1412. [Google Scholar] [CrossRef]
- Gao, C.; Yu, S.; Zhang, X.; Dang, Y.; Han, D.; Liu, X.; Han, J.; Hui, M.M. Dual Functional Eudragit® S100/L30D-55 and PLGA Colon-Targeted Nanoparticles of Iridoid Glycoside for Improved Treatment of Induced Ulcerative Colitis. Int. J. Nanomed. 2021, 16, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Vashi, J.; Solanki, A. Formulation and Characterization of Ileo-Colonic Targeted Mucoadhesive Microspheres Containing Flurbiprofen for Treatment of Ulcerative Colitis. Res. J. Pharm. Technol. 2020, 13, 3377. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Shen, Y.; Wang, J.; Zhou, L.; Chen, Z. Preparation of Baicalin Colon-Targeted Granules and Its Intervention Effect on Ulcerative Colitis in Rats. Particuology 2023, 85, 13–21. [Google Scholar] [CrossRef]
- Lv, Y.; Ren, M.; Yao, M.; Zou, J.; Fang, S.; Wang, Y.; Lan, M.; Zhao, Y.; Gao, F. Colon-Specific Delivery of Methotrexate Using Hyaluronic Acid Modified PH-Responsive Nanocarrier for the Therapy of Colitis in Mice. Int. J. Pharm. 2023, 635, 122741. [Google Scholar] [CrossRef] [PubMed]
- Gugulothu, D.; Kulkarni, A.; Patravale, V.; Dandekar, P. pH-Sensitive Nanoparticles of Curcumin–Celecoxib Combination: Evaluating Drug Synergy in Ulcerative Colitis Model. J. Pharm. Sci. 2014, 103, 687–696. [Google Scholar] [CrossRef]
- Sareen, R.; Jain, N.; Rajkumari, A.; Dhar, K.L. pH Triggered Delivery of Curcumin from Eudragit-Coated Chitosan Microspheres for Inflammatory Bowel Disease: Characterization and Pharmacodynamic Evaluation. Drug Deliv. 2016, 23, 55–62. [Google Scholar] [CrossRef]
- Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Alattar, A.; Alshaman, R.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels 2023, 9, 264. [Google Scholar] [CrossRef]
- Cao, Q.; Jin, L.; Ding, Y.; Zhang, Y.; Xu, X. A Novel pH–Enzyme-Dependent Mesalamine Colon-Specific Delivery System. DDDT 2016, 10, 2021–2028. [Google Scholar] [CrossRef][Green Version]
- Patil, A.; Pawar, P.; Gharge, V.; Doltade, U.; Doijad, R. Mesalamine-Loaded Mucoadhesive Microsphere for Colon Drug Delivery System: Effect of Process Variables and in Vitro Characterization. Int. J. Pharma Investig. 2018, 8, 74. [Google Scholar] [CrossRef]
- Pawar, P.; Varsha, G. Formulation and Evaluation of Mesalamine Loaded pH Dependent Colon Specific Pulsatile Drug Delivery System. Curr. Res. Pharm. Sci. 2018, 8, 244–253. [Google Scholar] [CrossRef]
- Saraogi, G.K.; Bagri, R.; Singh, S.; Parashar, A.K. Formulation Development and Characterization of Surface Modified Pectin Microspheres for Colon Targeting. Bull. Env. Pharmacol. Life Sci. 2023, 12, 16–23. [Google Scholar]
- Ye, B. Mesalazine Preparations for the Treatment of Ulcerative Colitis: Are All Created Equal? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as Controlled Drug Delivery Carrier for the Treatment of Ulcerative Colitis: A Brief Review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef]
- Hoy, S.M. Budesonide MMX®: A Review of Its Use in Patients with Mild to Moderate Ulcerative Colitis. Drugs 2015, 75, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, A.; Roze, S.; Kuijvenhoven, J.; Ghatnekar, O.; Yip Sonderegger, Y.L. Budesonide with Multi-Matrix Technology as Second-Line Treatment for Ulcerative Colitis: Evaluation of Long-Term Cost-Effectiveness in the Netherlands. J. Med. Econ. 2018, 21, 869–877. [Google Scholar] [CrossRef]
- Prenzler, A.; Yen, L.; Mittendorf, T.; Von Der Schulenburg, J.-M. Cost Effectiveness of Ulcerative Colitis Treatment in Germany: A Comparison of Two Oral Formulations of Mesalazine. BMC Health Serv. Res. 2011, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Almadiyeva, A.; Almatova, V.; Balabayev, T.; Sultanov, M.; Absattarova, K. Clinical and Economic Efficiency of Multi-Matrix Mesalazine in Ulcerative Colitis Treatment in the Healthcare System of the Republic of Kazakhstan. J. Clin. Med. Kaz. 2019, 2, 30–36. [Google Scholar] [CrossRef]
- Brereton, N.; Bodger, K.; Kamm, M.A.; Hodgkins, P.; Yan, S.; Akehurst, R. A Cost-Effectiveness Analysis of MMX Mesalazine Compared with Mesalazine in the Treatment of Mild-to-Moderate Ulcerative Colitis from a UK Perspective. J. Med. Econ. 2010, 13, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Gomollón, F.; Méndez, I. Cost-Effectiveness Assessment through Theoretical Cost-Minimization Analysis of the Use of Two Gastro-Resistant Modified-Release Mesalazine Formulations in the Management of Ulcerative Colitis in Spain. Gastroenterol. Y Hepatol. 2016, 39, 199–212. [Google Scholar] [CrossRef] [PubMed]
- EMA. List of the Nationally Authorised Medicinal Products. Active Ingredient: Budesonide. Available online: https://www.ema.europa.eu/en/documents/psusa/budesonide-list-nationally-authorised-medicinal-products-psusa00000449202104_en.pdf (accessed on 20 January 2025).
- EMA. List of the Nationally Authorised Medicinal Products. Active Substance: Mesalazine. Available online: https://www.ema.europa.eu/en/documents/psusa/mesalazine-list-nationally-authorised-medicinal-products-psusa-00001990-202402_en.pdf (accessed on 20 January 2025).
| Author/Ref. | Year | Drug Candidates for Treatment of UC | pH-Dependent Polymer |
|---|---|---|---|
| Vaidya et al. [61] | 2009 | Metronidazole | Eudragit® S100 |
| Makhlof et al. [62] | 2009 | Budesonide | Eudragit® S100 |
| Yoo et al. [63] | 2015 | Budesonide | Eudragit® FS30D |
| Naeem et al. [64] | 2020 | Budesonide | Eudragit® FS30D |
| Turanlı and Acartürk [65] | 2022 | Budesonide | Eudragit® S100 |
| Alkazzaz et al. [66] | 2015 | Prednisolone | Eudragit® S100 |
| Liu et al. [67] | 2010 | Prednisolone | Eudragit® S100 |
| Hashem et al. [68] | 2013 | Prednisolone | Eudragit® S100 |
| Qu et al. [69] | 2021 | Budenoside and prednisolone | Eudragit® S100 |
| Shi et al. [70] | 2018 | Hydrocortisone sodium succinate | poly(ethylene glycol) methyl ether methacrylate |
| Oshi et al. [71] | 2018 | Dexamethasone | poly(ethylene glycol) methyl ether methacrylate |
| Naeem et al. [72] | 2014 | Coumarin | Eudragit® S100 |
| Tie et al. [73] | 2023 | Notoginsenoside | Eudragit® S100 |
| Oshi et al. [74] | 2021 | Cyclosporine | Eudragit® S100 |
| Gao et al. [75] | 2021 | Iridoid glycosides | Eudragit® S100 and Eudragit® L30D-55 |
| Pande et al. [76] | 2020 | Flurbiprofen | Eudragit® L 100 and Eudragit® S 100 |
| Huang et al. [77] | 2024 | Baicalin | Eudragit® S100 |
| Lv et al. [78] | 2023 | Methotrexate | Eudragit® S100 |
| Gugulothu et al. [79] | 2014 | Curcumin | Eudragit® S100 |
| Xiao et al. [60] | 2015 | Curcumin | Eudragit® S100 |
| Sareen et al. [80] | 2016 | Curcumin | Eudragit® S100 |
| Heikal et al. [81] | 2023 | Curcumin and mesalamine | Eudragit® S100 |
| Cao et al. [82] | 2016 | Mesalamine | Eudragit® S100 |
| Patil et al. [83] | 2018 | Mesalamine | Eudragit® S100 |
| Pawar et al. [84] | 2018 | Mesalamine | Eudragit® S100 and Eudragit® L100 |
| Saraogi et al. [85] | 2023 | Mesalamine and prednisolone | Eudragit® S100 |
| INN | Brand Name/Marketing Authorisation Holder | Dosage | Dosage Form | pH-Dependent Polymer |
|---|---|---|---|---|
| Mesalazine | Colitofalk® (Dr. Falk Pharma GmbH, Freiburg, Germany) | 1000 mg 1500 mg | Gastro-resistant granules | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) |
| Asacol® (Tillotts Pharma GmbH, Rheinfelden, Germany) | 800 mg 1600 mg | Modified colon-release tablets | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Asacolon® (Tillotts Pharma GmbH, Rheinfelden, Germany) | 400 mg 800 mg | Gastro-resistant tablets | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Azzavix® (Faes Farma, S.A., Leioa, Spain) | 500 mg 1000 mg | Gastro-resistant tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Asamax® Astellas Pharma Sp. z o.o., Warsaw, Poland | 400 mg 800 mg | Gastro-resistant tablets | Methacrylic acid–methacrylate–methyl methacrylate copolymer (Eudragit® FS30 D) | |
| Asamovon® (Tillotts Pharma GmbH, Rheinfelden, Germany) | 1600 mg | Modified release tablets | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Claversal® (Recordati Pharma GmbH, Ulm, Germany) | 500 mg 1000 mg | Modified colon-release tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) | |
| Salofalk® (Dr Falk GmBH, Freiburg im Breisgau, Germany) | 250 mg, 500 mg 1000 mg tablets/granules | Delayed-release granules have matrix core. Released in mid ileum to colon | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Octasa® Tillotts Pharma UK Ltd, Wellingore Lincolnshire United Kingdom | 400 mg, 800 mg 1600 mg | Modified colon-release tablets | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Mezavant® Mezavant XL® (Takeda Pharmaceuticals international AG, Dublin, Ireland) | 1200 mg tablets gastro-resistant, prolonged release tablets | MMX Multi Matrix system technology. Released in terminal ileum and entire colon | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Galtasa® (Faes Farma, S.A., Leioa, Spain) | 1000 mg | Gastro-resistant tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Delzicol® Allergan Pharmaceuticals International Limited, Madison, NJ, USA | 400 mg capsusles | Delayed-release in terminal ileum and colon | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Lialda® (Nogra Pharma Limited, Dublin, Ireland) | 1200 mg | MMX Multi Matrix system technology. Release in terminal ileum and entire colon | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Mecolvix® (Faes Farma, S.A., Leioa, Spain) | 500 mg 1000 mg | Gastro-resistant tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Mecolzine® (Faes Farma, S.A., Leioa, Spain) | 500 mg 1000 mg | Gastro-resistant tablets | Methacrylic acid polymer–ethyl acrylate copolymer (1:1) Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Mesagran® (Dr. Falk Pharma GmbH, Freiburg, Germany) | 500 mg, 1000 mg, 1500 mg, 3000 mg | Gastro-resistant sustained-release granules | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) | |
| Melazine Orion® (Faes Farma, S.A., Leioa, Spain) | 500 mg, 1000 mg | Enteric tablet | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Mesavancol® Giuliani S.p.A, Milan, Italy | 1200 mg | Modified release tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Osperzo® (Dr. Falk Pharma GmbH, Freiburg, Germany) | 1500 mg 3000 mg | Prolonged-release granules | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) | |
| Yaldigo® (Tillotts Pharma GmbH, Rheinfelden, Germany) | 1600 mg | Modified release tablets | Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) | |
| Budesonide | Budenofalk® Budenofalk Uno (Dr Falk Pharma GmbH, Freiburg, Germany) | 3 mg capsules 9 mg granules | Garstro-resistant | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) |
| Entocort CR® (Tillotts Pharma AG, Rheinfelden, Germany) | 3 mg | Garstro-resistant capsules and microgranules | Methacrylic acid–ethyl acrylate copolymer (1:1) | |
| Cortiment® Cortiment®MMX® Ferring Arzneimittel GmbH, Kiel, Germany | 9 mg | Prolonged-release tablets | Methacrylic acid–methyl methacrylate copolymer (1:1) (Eudragit® L 100) Methacrylic acid–methyl methacrylate copolymer (1:2) (Eudragit® S 100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdeva, Y.; Staynova, R. pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics 2025, 17, 226. https://doi.org/10.3390/pharmaceutics17020226
Gvozdeva Y, Staynova R. pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics. 2025; 17(2):226. https://doi.org/10.3390/pharmaceutics17020226
Chicago/Turabian StyleGvozdeva, Yana, and Radiana Staynova. 2025. "pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment" Pharmaceutics 17, no. 2: 226. https://doi.org/10.3390/pharmaceutics17020226
APA StyleGvozdeva, Y., & Staynova, R. (2025). pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics, 17(2), 226. https://doi.org/10.3390/pharmaceutics17020226








