Novel Peptide-Modified Zeolitic Imidazolate Framework-8 Nanoparticles with pH-Sensitive Release of Doxorubicin for Targeted Treatment of Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
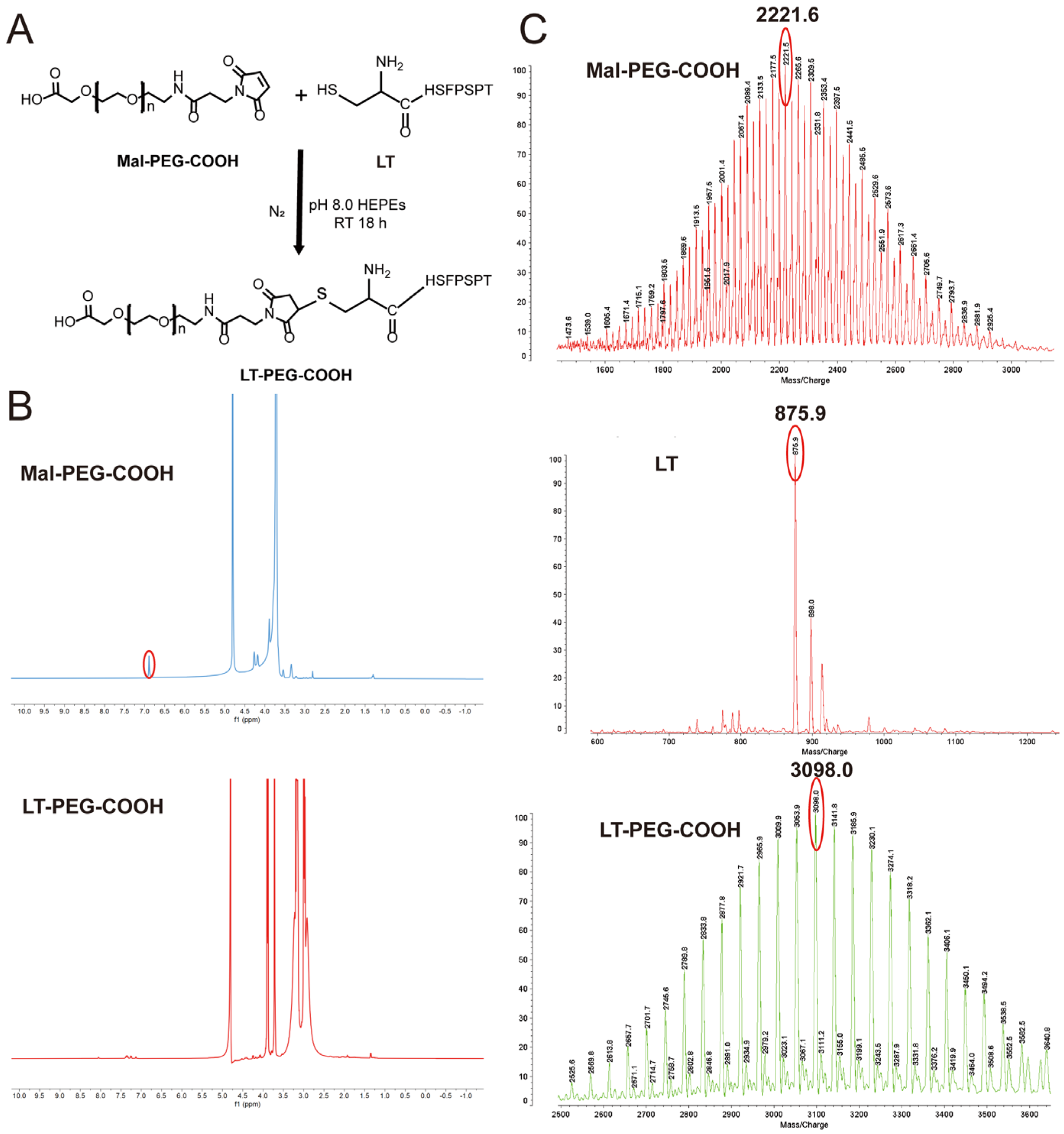
2.2. Synthesis and Characterization of LT-PEG-COOH
2.3. Preparation of LT-PEG@DOX@ZIF-8
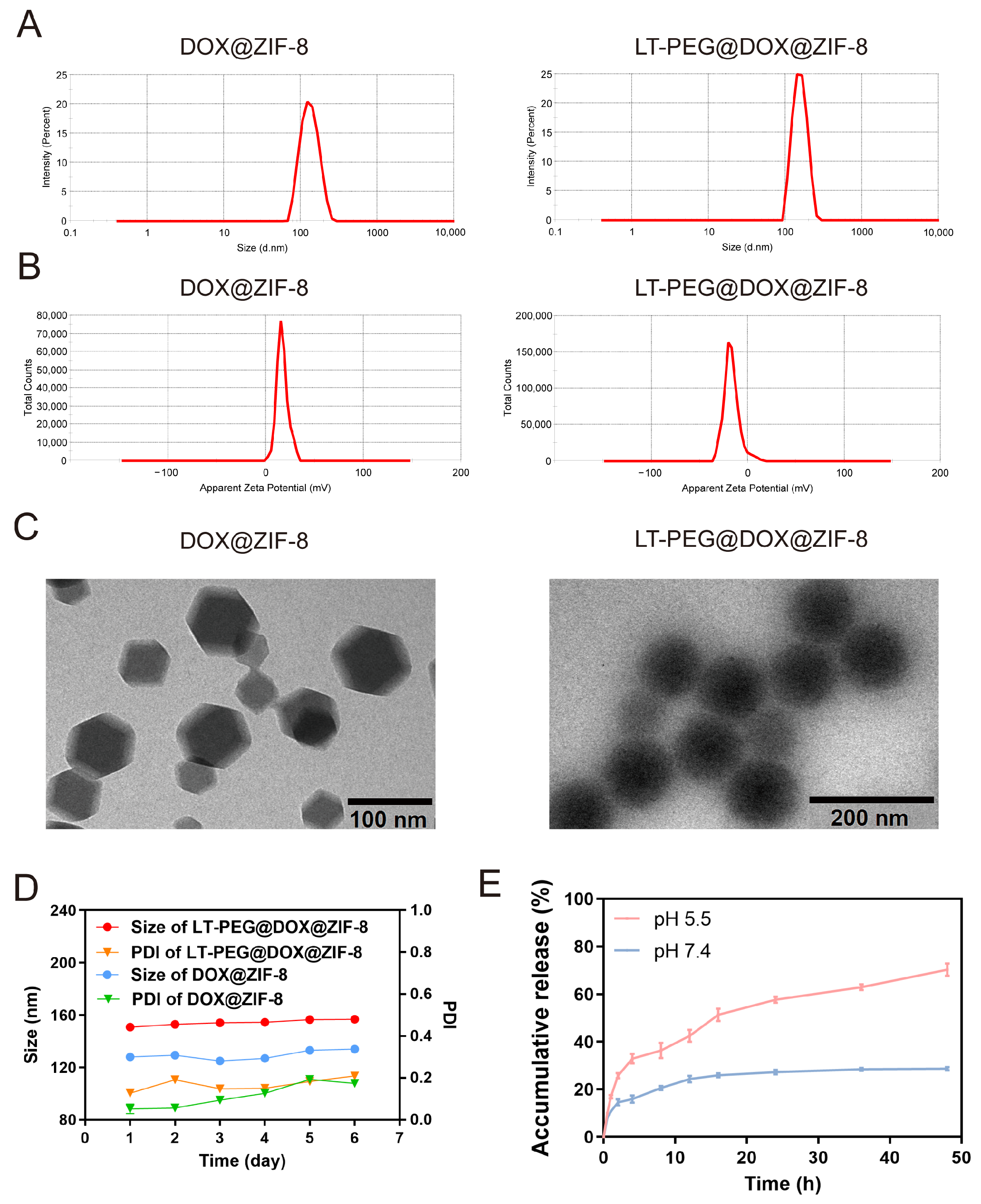
2.4. Characterization of LT-PEG@DOX@ZIF-8
2.5. Stability of LT-PEG@DOX@ZIF-8
2.6. In Vitro Release Assay
2.7. Cell Culture
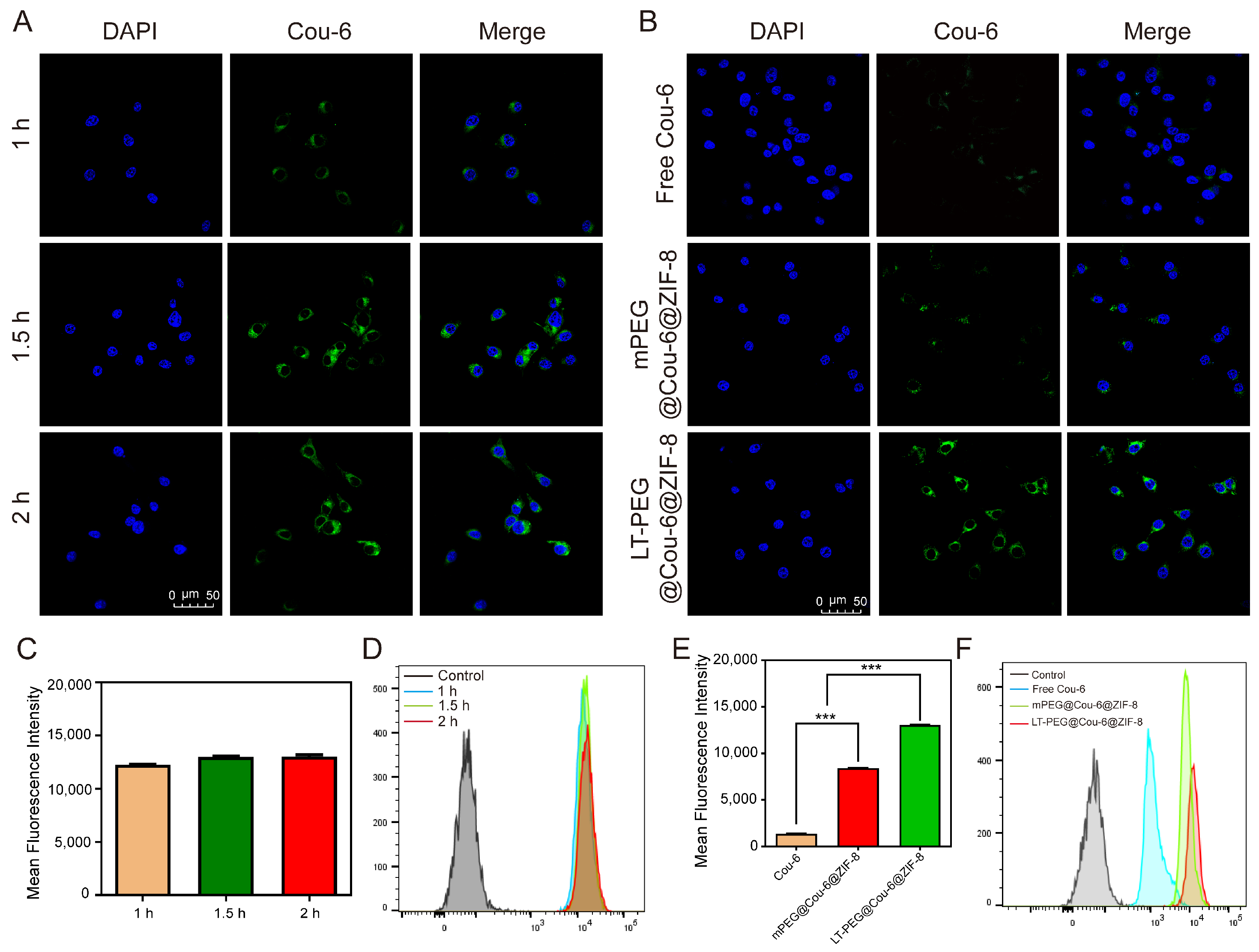
2.8. Cellular Uptake Studies In Vitro
2.9. Cell Viability Assay
2.10. Cell Apoptosis Assay
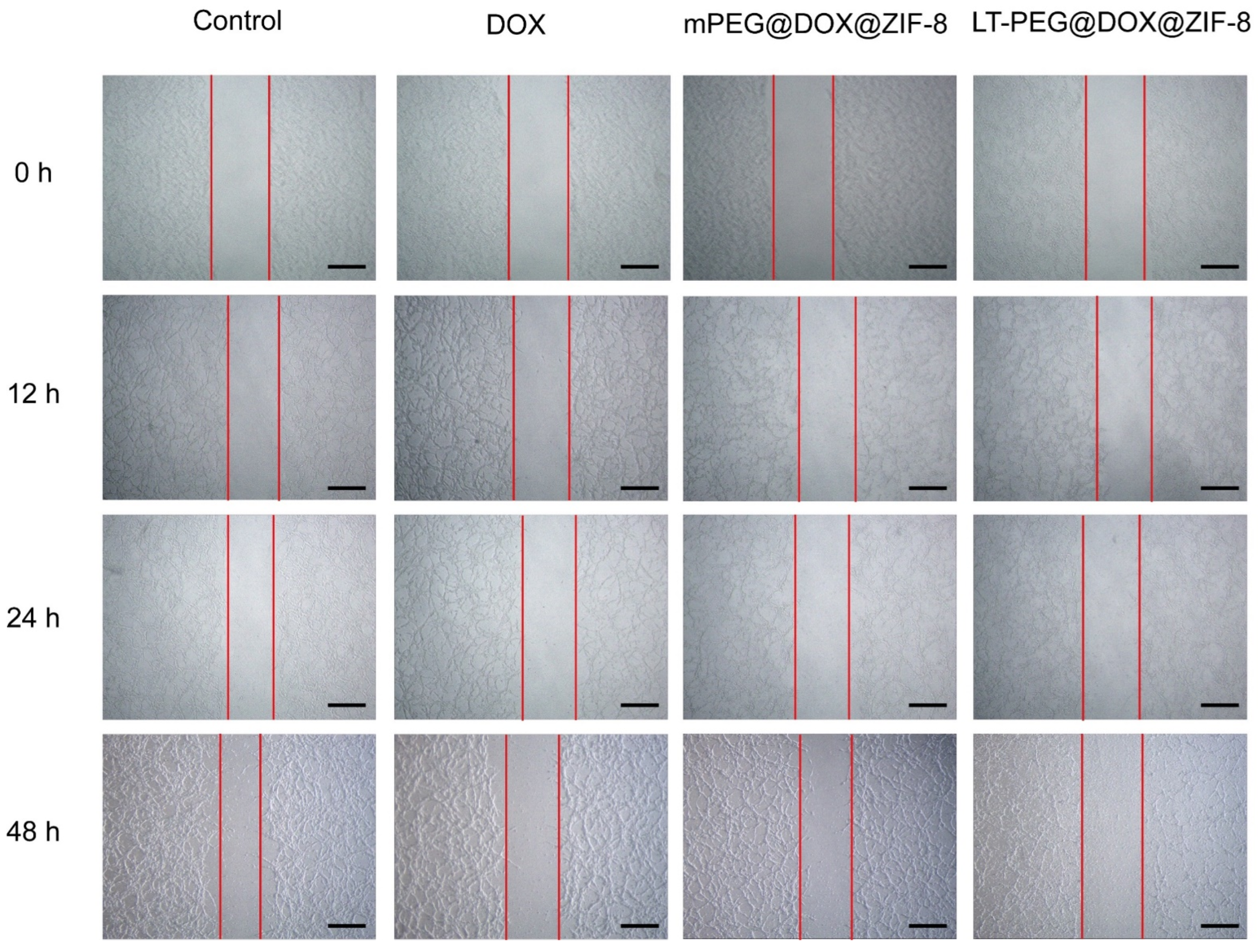
2.11. In Vitro Wound-Healing Assay
2.12. Establishment of CRC Model
2.13. Biodistribution and In Vivo Imaging
2.14. In Vivo Colorectal Tumor Cell Distribution
2.15. In Vivo Anti-Tumor Efficiency and Biosafety Evaluation
2.16. Statistical Analysis
3. Results
3.1. Synthesis of LT-PEG-COOH
3.2. Characterization of LT-PEG@DOX@ZIF-8
3.3. Cellular Uptake Study
3.4. Cell Viability Assay
3.5. In Vitro Cell Apoptosis Study
3.6. Wound-Healing Assay
3.7. In Vivo Biodistribution
3.8. Biodistribution in Tumors
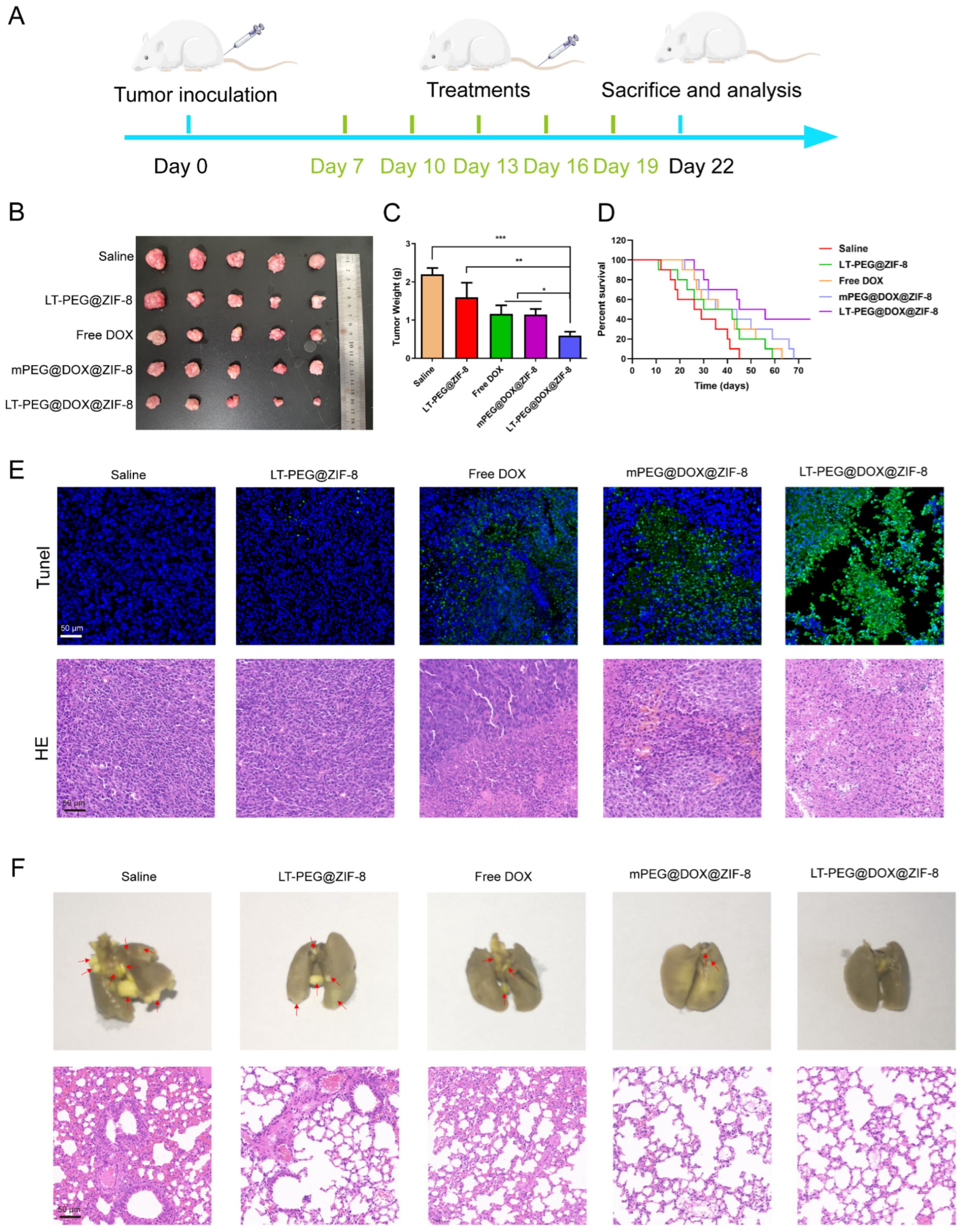
3.9. In Vivo Anti-Tumor and Anti-Metastasis Efficacy
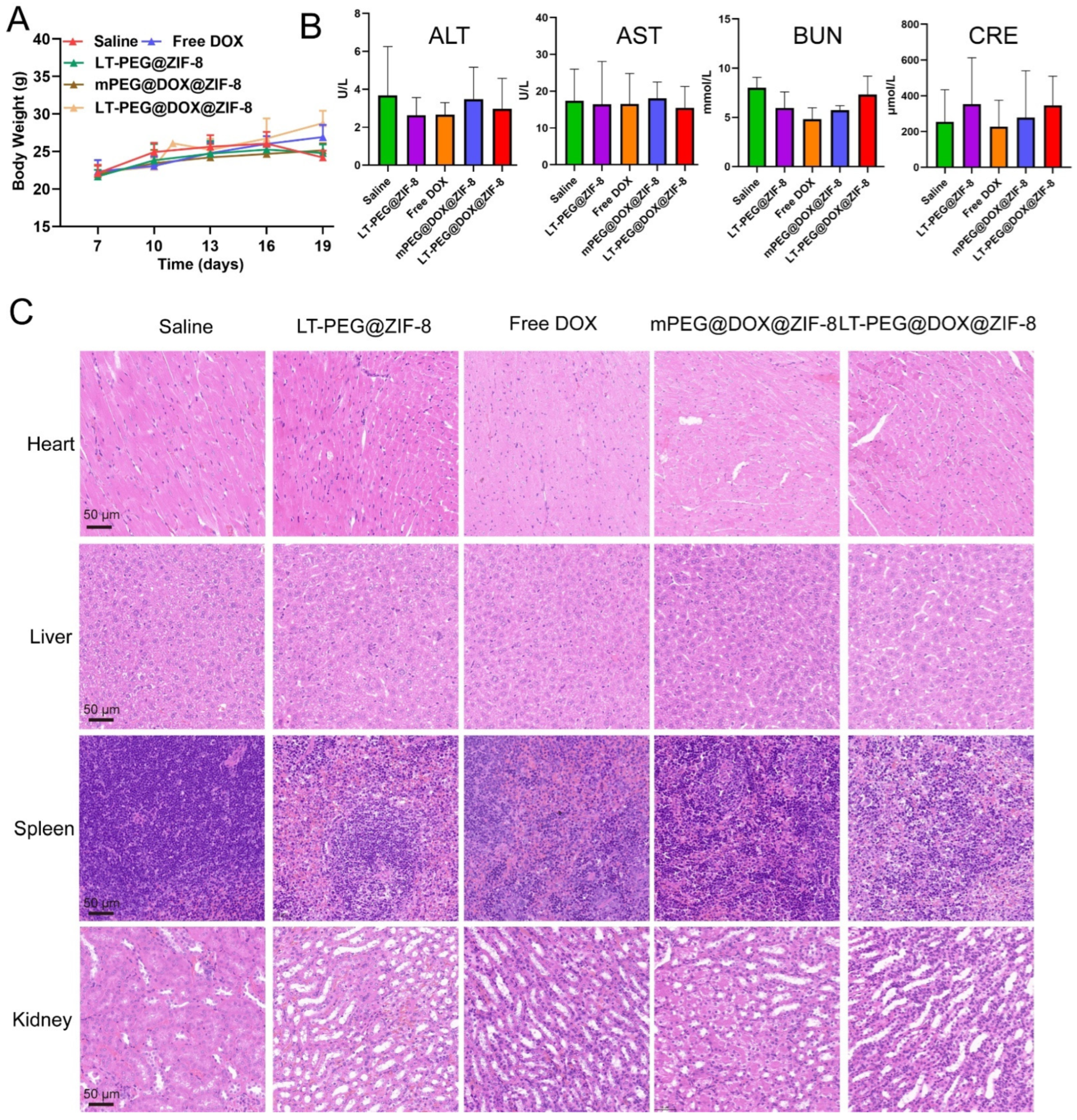
3.10. In Vivo Biocompatibility and Biosafety Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Y.; Zhang, H.; Shao, Q.; Hu, Q.; Ma, J.; Wan, Y.; Guo, L.; Wan, X.; Sun, H.; et al. A Tumor Environment-Activated Photosensitized Biomimetic Nanoplatform for Precise Photodynamic Immunotherapy of Colon Cancer. Adv. Sci. 2024, 11, e2402465. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhang, W.; Wang, Y.; Zhai, Y.; Li, Y.; Li, W.; Chang, J.; Zhao, X.; Huang, M.; et al. A liquid biopsy signature of circulating extracellular vesicles-derived RNAs predicts response to first line chemotherapy in patients with metastatic colorectal cancer. Mol. Cancer 2023, 22, 199. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C.; Wang, H.; Yang, K.; Huang, M.; Zhang, W.; Yu, Q.; Wang, H.; Zhang, L.; Zhao, Y.; et al. Multifunctional Nanoparticle-Loaded Injectable Alginate Hydrogels with Deep Tumor Penetration for Enhanced Chemo-Immunotherapy of Cancer. ACS Nano 2024, 18, 18604–18621. [Google Scholar] [CrossRef]
- Go, G.; Lee, C.S.; Yoon, Y.M.; Lim, J.H.; Kim, T.H.; Lee, S.H. PrP(C) Aptamer Conjugated-Gold Nanoparticles for Targeted Delivery of Doxorubicin to Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1976. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef]
- Lin, D.; Lv, W.; Qian, M.; Jiang, G.; Lin, X.; Gantulga, D.; Wang, Y. Engineering cell membrane-camouflaged COF-based nanosatellite for enhanced tumor-targeted photothermal chemoimmunotherapy. Biomaterials 2025, 314, 122869. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, S.; Zhang, Z.; Fu, S.; Liu, S.; Liu, J.; Ma, Q.; Xia, Z.; Gu, P.; Gao, S.; et al. Regulating tumor cells to awaken T cell antitumor function and enhance melanoma immunotherapy. Biomaterials 2025, 316, 123034. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Mei, T.; Liu, Y.; Wu, K.; Le, W.; Hu, Y. Nanoscale MOFs in nanomedicine applications: From drug delivery to therapeutic agents. J. Mater. Chem. B 2023, 11, 3273–3294. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Kong, S.; Zhang, J.; Xia, H.; Chen, R.; Guo, Z.; Li, Z.; Ahmed, R.; Rehman, A.; Kazemian, H.; et al. Hydrophilic Metal-Organic Frameworks Regulated by Biomineralized Protein for Enhanced Stability and Drug Delivery. Nano Lett. 2024, 24, 15652–15661. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef]
- He, L.; Huang, G.; Liu, H.; Sang, C.; Liu, X.; Chen, T. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci. Adv. 2020, 6, eaay9751. [Google Scholar] [CrossRef]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O′Keeffe, M.; Yaghi, O.M. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Duren, T. Opening the gate: Framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, X.; Liu, Z.; Xie, Z.; Wang, Y.; Wang, H.; Ni, D. Bimetallic Nanoplatforms for Prostate Cancer Treatment by Interfering Cellular Communication. J. Am. Chem. Soc. 2024, 146, 22530–22540. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic Imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Wang, H.; Fang, T.; Wang, J.; Zhang, M.; Mu, X.; Gao, T.; Wei, T.; Dai, Z. Adaptive Size Evolution of an MOFs-in-MOF Nanovehicle for Enhanced Nucleus-Targeted Tumor Chemotherapy. Nano Lett. 2024, 24, 10605–10613. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Q.; Mo, D.; Feng, J.; Wang, Y.; Li, T.; Zhang, Y.; Wei, H. A Self-Cascade Oxygen-Generating Nanomedicine for Multimodal Tumor Therapy. Small 2024, 20, e2403523. [Google Scholar] [CrossRef]
- Li, Z.J.; Wu, W.K.; Ng, S.S.; Yu, L.; Li, H.T.; Wong, C.C.; Wu, Y.C.; Zhang, L.; Ren, S.X.; Sun, X.G.; et al. A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J. Control. Release 2010, 148, 292–302. [Google Scholar] [CrossRef]
- Liu, Z.; Gray, B.D.; Barber, C.; Bernas, M.; Cai, M.; Furenlid, L.R.; Rouse, A.; Patel, C.; Banerjee, B.; Liang, R.; et al. Characterization of TCP-1 probes for molecular imaging of colon cancer. J. Control. Release 2016, 239, 223–230. [Google Scholar] [CrossRef]
- Liu, Z.; Gray, B.D.; Barber, C.; Wan, L.; Furenlid, L.R.; Liang, R.; Li, Z.; Woolfenden, J.M.; Pak, K.Y.; Martin, D.R. PEGylated and Non-PEGylated TCP-1 Probes for Imaging of Colorectal Cancer. Mol. Imaging Biol. 2023, 25, 133–143. [Google Scholar] [CrossRef]
- Chu, X.Y.; Huang, W.; Wang, Y.L.; Meng, L.W.; Chen, L.Q.; Jin, M.J.; Chen, L.; Gao, C.H.; Ge, C.; Gao, Z.G.; et al. Improving antitumor outcomes for palliative intratumoral injection therapy through lecithin- chitosan nanoparticles loading paclitaxel- cholesterol complex. Int. J. Nanomed. 2019, 14, 689–705. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.Y.; Pei, X.; Li, Y.H.; Feng, H.; He, Z.H.; Xie, W.J.; Pei, X.B.; Zhu, Z.; Wan, Q.B.; et al. One-Pot Facile Encapsulation of Dimethyloxallyl Glycine by Nanoscale Zeolitic Imidazolate Frameworks-8 for Enhancing Vascularized Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2202317. [Google Scholar] [CrossRef]
- Rajabi, A.; Nejati, M.; Homayoonfal, M.; Arj, A.; Razavi, Z.S.; Ostadian, A.; Mohammadzadeh, B.; Vosough, M.; Karimi, M.; Rahimian, N.; et al. Doxorubicin-loaded zymosan nanoparticles: Synergistic cytotoxicity and modulation of apoptosis and Wnt/β-catenin signaling pathway in C26 colorectal cancer cells. Int. J. Biol. Macromol. 2024, 260, 128949. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ding, Y.; Fang, S.; Yang, W.; Chen, J.; Ma, J.; Wang, M.; Wang, J.; Zhang, F.; Guo, X.; et al. Potentiated Calcium Carbonate with Enhanced Calcium Overload Induction and Acid Neutralization Capabilities to Boost Chemoimmunotherapy against Liver Cancer. ACS Nano 2024, 18, 27597–27616. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, C.; Li, Z.; Yao, Z.; Tian, J.; Shan, Y.; Chen, S.; Song, W.; Pan, W.; Ping, Y.; et al. Tumor Microenvironment-Activated In Situ Synthesis of Peroxynitrite for Enhanced Chemodynamic Therapy. ACS Nano 2024, 18, 27042–27054. [Google Scholar] [CrossRef]
- Chen, C.; Yu, M.; Li, Q.; Zhou, Y.; Zhang, M.; Cai, S.; Yu, J.; Huang, Z.; Liu, J.; Kuang, Y.; et al. Programmable Tetrahedral DNA-RNA Nanocages Woven with Stimuli-Responsive siRNA for Enhancing Therapeutic Efficacy of Multidrug-Resistant Tumors. Adv. Sci. 2024, 11, e2404112. [Google Scholar] [CrossRef]
- Fang, S.; Zheng, L.; Shu, G.-F.; Xiaoxiao, C.; Guo, X.; Ding, Y.; Yang, W.; Chen, J.; Zhao, Z.; Tu, J.; et al. Multiple Immunomodulatory Strategies Based on Targeted Regulation of Proprotein Convertase Subtilisin/Kexin Type 9 and Immune Homeostasis against Hepatocellular Carcinoma. ACS Nano 2024, 18, 8811–8826. [Google Scholar] [CrossRef]
- Yan, H.; Xu, P.; Ma, H.; Li, Y.; Zhang, R.; Cong, H.; Yu, B.; Shen, Y. Enzyme-triggered transcytosis of drug carrier system for deep penetration into hepatoma tumors. Biomaterials 2023, 301, 122213. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, J.; Liang, N.; Zhang, X.; Shabiti, S.; Wang, Z.; Yu, S.; Pan, Z.-Y.; Li, W.; Cai, L. Bioorthogonal/Ultrasound Activated Oncolytic Pyroptosis Amplifies In Situ Tumor Vaccination for Boosting Antitumor Immunity. ACS Nano 2024, 18, 9413–9430. [Google Scholar] [CrossRef]
- Xu, C.; Yang, S.; Jiang, Z.; Zhou, J.; Yao, J. Self-Propelled Gemini-like LMWH-Scaffold Nanodrugs for Overall Tumor Microenvironment Manipulation via Macrophage Reprogramming and Vessel Normalization. Nano Lett. 2019, 20, 372–383. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiong, H.; Yang, S.; Lu, Y.; Deng, Y.; Yao, J.; Yao, J. Jet-Lagged Nanoparticles Enhanced Immunotherapy Efficiency through Synergistic Reconstruction of Tumor Microenvironment and Normalized Tumor Vasculature. Adv. Healthc. Mater. 2020, 9, e2000075. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, H.; Hou, X.; Wang, D.; Wang, Z.; Shang, Y.; Zhang, S.; Liu, J.; Ren, C.; Liu, J. Cancer Stem-Like Cells-Oriented Surface Self-Assembly to Conquer Radioresistance. Adv. Mater. 2023, 35, 2302916. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tao, W.; Li, S.; Chen, Y.; Sun, Y.; He, Z.; Sun, B.; Sun, J. Apoptotic body–mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci. Adv. 2021, 7, eabg0880. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Chen, L.; Wang, S.; Liu, C.; Zhao, H.; Jin, M.; Chang, S.; Quan, X.; Cui, M.; et al. Combined Biomimetic MOF-RVG15 Nanoformulation Efficient Over BBB for Effective Anti-Glioblastoma in Mice Model. Int. J. Nanomed. 2022, 17, 6377–6398. [Google Scholar] [CrossRef] [PubMed]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Mokhtarzadeh, A.; Ramezani, M. In Vitro and In Vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur. J. Pharm. Biopharm. 2017, 113, 60–74. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Zhao, H.; Chen, L.; Liu, Y.; Wu, H.; Liu, C.; Feng, J.; Liu, C.; Xiao, C.; Wang, Q.; et al. Novel Peptide-Modified Zeolitic Imidazolate Framework-8 Nanoparticles with pH-Sensitive Release of Doxorubicin for Targeted Treatment of Colorectal Cancer. Pharmaceutics 2025, 17, 246. https://doi.org/10.3390/pharmaceutics17020246
Gong L, Zhao H, Chen L, Liu Y, Wu H, Liu C, Feng J, Liu C, Xiao C, Wang Q, et al. Novel Peptide-Modified Zeolitic Imidazolate Framework-8 Nanoparticles with pH-Sensitive Release of Doxorubicin for Targeted Treatment of Colorectal Cancer. Pharmaceutics. 2025; 17(2):246. https://doi.org/10.3390/pharmaceutics17020246
Chicago/Turabian StyleGong, Liming, Heming Zhao, Liqing Chen, Yanhong Liu, Hao Wu, Chao Liu, Jing Feng, Chenfei Liu, Congcong Xiao, Qiming Wang, and et al. 2025. "Novel Peptide-Modified Zeolitic Imidazolate Framework-8 Nanoparticles with pH-Sensitive Release of Doxorubicin for Targeted Treatment of Colorectal Cancer" Pharmaceutics 17, no. 2: 246. https://doi.org/10.3390/pharmaceutics17020246
APA StyleGong, L., Zhao, H., Chen, L., Liu, Y., Wu, H., Liu, C., Feng, J., Liu, C., Xiao, C., Wang, Q., Jin, M., Gao, Z., Huang, W., & Guan, Y. (2025). Novel Peptide-Modified Zeolitic Imidazolate Framework-8 Nanoparticles with pH-Sensitive Release of Doxorubicin for Targeted Treatment of Colorectal Cancer. Pharmaceutics, 17(2), 246. https://doi.org/10.3390/pharmaceutics17020246






