Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgery and Delivery of Maternal MitoQ and Dexamethasone
- Saline (5 mL IV daily 105–137 ± 2 dGA, n = 17);
- Dexamethasone (Dex; 12 mg in 2 mL saline IM at 115 and 116 dGA; n = 25);
- MitoQ (6 mg/kg MS010 (20% w/w mixture of mitoquinol and mitoquinone, the reduced and oxidised forms of the same molecule, respectively, and β-cyclodextrin to improve solubility; MRC Mitochondrial Biology Unit, Cambridge, UK) in 5 mL saline IV, daily bolus 105–137 ± 2 dGA; n = 17; [28]);
- Co-treatment with Dex and MitoQ (Dex+MitoQ (as above); n = 14).
2.2. Post-Mortem
2.3. Microsome Extraction
2.4. In Vitro Quantification of Hepatic Cytochrome P450 Activity
2.5. Hepatic Protein Extraction
2.6. Quantification of Hepatic Proteins
2.7. Statistical Analysis
3. Results
3.1. Antenatal MitoQ Increases Liver Weight in the Fetus and Increases Bodyweight in 9 mo Lambs
3.2. Metabolism of Substrates in the CYP3A Family Is Altered in Fetuses and Young Adult Offspring According to Antenatal Treatment
3.3. Decreased Hepatic CYP Activity in Young Adult Lambs as a Result of Maternal Dexamethasone Treatment in Pregnancy Can Be Ameliorated by MitoQ Co-Treatment
3.4. Antenatal MitoQ Increases Endogenous Antioxidant Catalase Expression in 9 mo Lambs
3.5. Mitochondrial Abundance Is Decreased in 9 mo Lambs, but Not Fetuses, Co-Treated with Dex+MitoQ
3.6. CYP Transcription Regulators Were Not Affected by Antenatal Treatments
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 11β-HSD2 | 11-beta-hydroxysteroid dehydrogenase 2 |
| 4HNE | 4-hydroxynonenal |
| ACS | Antenatal corticosteroids |
| CAT | Catalase |
| CYP | Cytochrome P450 |
| Dex | Dexamethasone |
| dGA | Days gestational age |
| DRP1 | Dynamin-related protein 1 |
| ER | Endoplasmic reticulum |
| GR | Glucocorticoid receptor |
| HNF-4α | Hepatocyte nuclear factor 4 alpha |
| IM | Intramuscular |
| IV | Intravenous |
| mo | Months of age |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OPA1 | Mitochondrial dynamin-like GTPase 1 |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| RDS | Respiratory Distress Syndrome |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| SOD | Superoxide dismutase |
References
- Keramat, S.A.; Alam, K.; Al-Hanawi, M.K.; Gow, J.; Biddle, S.J.H.; Hashmi, R. Trends in the prevalence of adult overweight and obesity in Australia, and its association with geographic remoteness. Sci. Rep. 2021, 11, 11320. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- McBride, G.M.; Wiese, M.D.; Soo, J.Y.; Darby, J.R.T.; Berry, M.J.; Varcoe, T.J.; Morrison, J.L. The impact of intrauterine growth restriction on cytochrome P450 enzyme expression and activity. Placenta 2020, 99, 50–62. [Google Scholar] [CrossRef]
- Zhou, S.F.; Liu, J.P.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef]
- Mitchell, A.A.; Gilboa, S.M.; Werler, M.M.; Kelley, K.E.; Louik, C.; Hernandez-Diaz, S.; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obs. Gynecol. 2011, 205, 51.e51–51.e58. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020, 12, CD004454. [Google Scholar] [CrossRef]
- Fowden, A.L.; Li, J.; Forhead, A.J. Glucocorticoids and the preparation for life after birth: Are there long-term consequences of the life insurance? Proc. Nutr. Soc. 1998, 57, 113–122. [Google Scholar] [CrossRef]
- Jellyman, J.K.; Fletcher, A.J.W.; Fowden, A.L.; Giussani, D.A. Glucocorticoid Maturation of Fetal Cardiovascular Function. Trends Mol. Med. 2020, 26, 170–184. [Google Scholar] [CrossRef]
- Blankenship, S.A.; Brown, K.E.; Simon, L.E.; Stout, M.J.; Tuuli, M.G. Antenatal corticosteroids in preterm small-for-gestational age infants: A systematic review and meta-analysis. Am. J. Obs. Gynecol. MFM 2020, 2, 100215. [Google Scholar] [CrossRef] [PubMed]
- McGillick, E.V.; Orgeig, S.; Williams, M.T.; Morrison, J.L. Risk of Respiratory Distress Syndrome and Efficacy of Glucocorticoids: Are They the Same in the Normally Grown and Growth-Restricted Infant? Reprod. Sci. 2016, 23, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Ford, G.W.; Davis, N.M.; Callanan, C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin. Sci. 2000, 98, 137–142. [Google Scholar] [CrossRef]
- Raikkonen, K.; Gissler, M.; Kajantie, E. Associations Between Maternal Antenatal Corticosteroid Treatment and Mental and Behavioral Disorders in Children. J. Am. Med. Assoc. 2020, 323, 1924–1933. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Soo, P.S.; McGillick, E.V.; Hiscock, J.; Zhang, S.; McMillen, I.C.; Orgeig, S. Antenatal steroids and the IUGR fetus: Are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? J. Pregnancy 2012, 2012, 839656. [Google Scholar] [CrossRef]
- Verhaeghe, J.; van Bree, R.; Van Herck, E. Oxidative stress after antenatal betamethasone: Acute downregulation of glutathione peroxidase-3. Early Hum. Dev. 2009, 85, 767–771. [Google Scholar] [CrossRef]
- Adler, A.; Camm, E.J.; Hansell, J.A.; Richter, H.G.; Giussani, D.A. Investigation of the use of antioxidants to diminish the adverse effects of postnatal glucocorticoid treatment on mortality and cardiac development. Neonatology 2010, 98, 73–83. [Google Scholar] [CrossRef]
- Camm, E.J.; Tijsseling, D.; Richter, H.G.; Adler, A.; Hansell, J.A.; Derks, J.B.; Cross, C.M.; Giussani, D.A. Oxidative stress in the developing brain: Effects of postnatal glucocorticoid therapy and antioxidants in the rat. PLoS ONE 2011, 6, e21142. [Google Scholar] [CrossRef]
- Tijsseling, D.; Camm, E.J.; Richter, H.G.; Herrera, E.A.; Kane, A.D.; Niu, Y.; Cross, C.M.; de Vries, W.B.; Derks, J.B.; Giussani, D.A. Statins prevent adverse effects of postnatal glucocorticoid therapy on the developing brain in rats. Pediatr. Res. 2013, 74, 639–645. [Google Scholar] [CrossRef]
- Garrud, T.A.C.; Giussani, D.A. Combined Antioxidant and Glucocorticoid Therapy for Safer Treatment of Preterm Birth. Trends Endocrinol. Metab. 2019, 30, 258–269. [Google Scholar] [CrossRef]
- Niu, Y.; Herrera, E.A.; Evans, R.D.; Giussani, D.A. Antioxidant treatment improves neonatal survival and prevents impaired cardiac function at adulthood following neonatal glucocorticoid therapy. J. Physiol. 2013, 591, 5083–5093. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.L.; Allison, B.J.; Niu, Y.; Cross, C.M.; Itani, N.; Kane, A.D.; Herrera, E.A.; Skeffington, K.L.; Botting, K.J.; Giussani, D.A. Intervention against hypertension in the next generation programmed by developmental hypoxia. PLoS Biol. 2019, 17, e2006552. [Google Scholar] [CrossRef] [PubMed]
- Itani, N.; Skeffington, K.L.; Beck, C.; Niu, Y.; Giussani, D.A. Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J. Pineal. Res. 2016, 60, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Ota, E.; Nagata, C.; Shahrook, S.; Crowther, C.A. Vitamin C supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD004072. [Google Scholar] [CrossRef]
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD004069. [Google Scholar] [CrossRef]
- Verteramo, R.; Pierdomenico, M.; Greco, P.; Milano, C. The Role of Melatonin in Pregnancy and the Health Benefits for the Newborn. Biomedicines 2022, 10, 3252. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Botting, K.J.; Skeffington, K.L.; Niu, Y.; Allison, B.J.; Brain, K.L.; Itani, N.; Beck, C.; Logan, A.; Murray, A.J.; Murphy, M.P.; et al. Translatable mitochondria-targeted protection against programmed cardiovascular dysfunction. Sci. Adv. 2020, 6, eabb1929. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef]
- Spiroski, A.M.; Niu, Y.; Nicholas, L.M.; Austin-Williams, S.; Camm, E.J.; Sutherland, M.R.; Ashmore, T.J.; Skeffington, K.L.; Logan, A.; Ozanne, S.E.; et al. Mitochondria antioxidant protection against cardiovascular dysfunction programmed by early-onset gestational hypoxia. FASEB J. 2021, 35, e21446. [Google Scholar] [CrossRef]
- Lakshman, R.; Spiroski, A.M.; McIver, L.B.; Murphy, M.P.; Giussani, D.A. Noninvasive Biomarkers for Cardiovascular Dysfunction Programmed in Male Offspring of Adverse Pregnancy. Hypertension 2021, 78, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Lock, M.C.; Botting, K.J.; Allison, B.J.; Niu, Y.; Ford, S.G.; Murphy, M.P.; Orgeig, S.; Giussani, D.A.; Morrison, J.L. MitoQ as an antenatal antioxidant treatment improves markers of lung maturation in healthy and hypoxic pregnancy. J. Physiol. 2023, 601, 3647–3665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Camm, E.J.; Nuzzo, A.M.; Spiroski, A.M.; Skeffington, K.L.; Ashmore, T.J.; Rolfo, A.; Todros, T.; Logan, A.; Ma, J.; et al. In vivo mitochondria-targeted protection against uterine artery vascular dysfunction and remodelling in rodent hypoxic pregnancy. J. Physiol. 2024, 602, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.; Murphy, M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study, G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cocheme, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- Rodriguez-Cuenca, S.; Cocheme, H.M.; Logan, A.; Abakumova, I.; Prime, T.A.; Rose, C.; Vidal-Puig, A.; Smith, A.C.; Rubinsztein, D.C.; Fearnley, I.M.; et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic. Biol. Med. 2010, 48, 161–172. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs then and now: The need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar]
- Soo, J.Y.; Wiese, M.D.; Berry, M.J.; McMillen, I.C.; Morrison, J.L. Intrauterine growth restriction may reduce hepatic drug metabolism in the early neonatal period. Pharmacol. Res. 2018, 134, 68–78. [Google Scholar] [CrossRef]
- McBride, G.M.; Soo, J.Y.; Varcoe, T.; Morrison, J.L.; Wiese, M.D. Development of a method to determine cytochrome P450 1A2, 2C9, 2D6 and 3A4 activity sheep hepatic microsomes. J. Pharmacol. Toxicol. Methods 2020, 106, 106934. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.D.; Meakin, A.S.; Varcoe, T.J.; Darby, J.R.T.; Sarr, O.; Kiser, P.; Bradshaw, E.L.; Regnault, T.R.H.; Morrison, J.L. Hepatic cytochrome P450 function is reduced by life-long Western diet consumption in guinea pig independent of birth weight. Life Sci. 2021, 287, 120133. [Google Scholar] [CrossRef] [PubMed]
- Meakin, A.S.; Amirmostofian, M.; Darby, J.R.; Holman, S.L.; Morrison, J.L.; Wiese, M.D. Characterisation of cytochrome P450 isoenzyme activity in sheep liver and placental microsomes. Placenta 2023, 131, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lie, S.; Morrison, J.L.; Williams-Wyss, O.; Suter, C.M.; Humphreys, D.T.; Ozanne, S.E.; Zhang, S.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K.; et al. Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1013–E1024. [Google Scholar] [CrossRef]
- Bertossa, M.R.; Darby, J.R.T.; Holman, S.L.; Meakin, A.S.; Li, C.; Huber, H.F.; Wiese, M.D.; Nathanielsz, P.W.; Morrison, J.L. Maternal high fat-high energy diet alters metabolic factors in the non-human primate fetal heart. J. Physiol. 2024, 602, 4251–4269. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Zhang, S.; Holman, S.L.; Muhlhausler, B.S.; McMillen, I.C.; Morrison, J.L. Cardiac growth and metabolism of the fetal sheep are not vulnerable to a 10 day increase in fetal glucose and insulin concentrations during late gestation. Heliyon 2023, 9, e18292. [Google Scholar] [CrossRef]
- Soo, P.S.; Hiscock, J.; Botting, K.J.; Roberts, C.T.; Davey, A.K.; Morrison, J.L. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod. Toxicol. 2012, 33, 374–381. [Google Scholar] [CrossRef]
- Darby, J.R.T.; McMillen, I.C.; Morrison, J.L. Maternal undernutrition in late gestation increases IGF2 signalling molecules and collagen deposition in the right ventricle of the fetal sheep heart. J. Physiol. 2018, 596, 2345–2358. [Google Scholar] [CrossRef]
- Brosen, K. Some aspects of genetic polymorphism in the biotransformation of antidepressants. Therapie 2004, 59, 5–12. [Google Scholar] [CrossRef]
- Faber, M.S.; Jetter, A.; Fuhr, U. Assessment of CYP1A2 activity in clinical practice: Why, how, and when? Basic Clin. Pharmacol. Toxicol. 2005, 97, 125–134. [Google Scholar] [CrossRef]
- Ariyoshi, N.; Ohara, M.; Kaneko, M.; Afuso, S.; Kumamoto, T.; Nakamura, H.; Ishii, I.; Ishikawa, T.; Kitada, M. Q172H replacement overcomes effects on the metabolism of cyclophosphamide and efavirenz caused by CYP2B6 variant with Arg262. Drug Metab. Dispos. 2011, 39, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Klein, K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): Advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Dacasto, M.; Eeckhoutte, C.; Capolongoa, F.; Dupuy, J.; Carletti, M.; Calleja, C.; Nebbia, C.; Alvinerie, M.; Galtier, P. Effect of breed and gender on bovine liver cytochrome P450 3A (CYP3A) expression and inter-species comparison with other domestic ruminants. Vet. Res. 2005, 36, 179–190. [Google Scholar] [CrossRef] [PubMed]
- International Sheep Genomics Consortium; Archibald, A.L.; Cockett, N.E.; Dalrymple, B.P.; Faraut, T.; Kijas, J.W.; Maddox, J.F.; McEwan, J.C.; Hutton Oddy, V.; Raadsma, H.W.; et al. The sheep genome reference sequence: A work in progress. Anim. Genet. 2010, 41, 449–453. [Google Scholar] [CrossRef]
- Jacobs, T.G.; Marzolini, C.; Back, D.J.; Burger, D.M. Dexamethasone is a dose-dependent perpetrator of drug-drug interactions: Implications for use in people living with HIV. J. Antimicrob. Chemother. 2022, 77, 568–573. [Google Scholar] [CrossRef]
- Slaviero, K.A.; Clarke, S.J.; Rivory, L.P. Inflammatory response: An unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003, 4, 224–232. [Google Scholar] [CrossRef]
- Abramson, J.L.; Hooper, W.C.; Jones, D.P.; Ashfaq, S.; Rhodes, S.D.; Weintraub, W.S.; Harrison, D.G.; Quyyumi, A.A.; Vaccarino, V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis 2005, 178, 115–121. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fletcher, A.J.; Goodfellow, M.R.; Forhead, A.J.; Gardner, D.S.; McGarrigle, H.H.; Fowden, A.L.; Giussani, D.A. Low doses of dexamethasone suppress pituitary-adrenal function but augment the glycemic response to acute hypoxemia in fetal sheep during late gestation. Pediatr. Res. 2000, 47, 684–691. [Google Scholar] [CrossRef]
- Jellyman, J.K.; Gardner, D.S.; McGarrigle, H.H.; Fowden, A.L.; Giussani, D.A. Antenatal glucocorticoid therapy increases glucose delivery to cerebral circulations during acute hypoxemia in fetal sheep during late gestation. Am. J. Obs. Gynecol. 2009, 201, 82.e8. [Google Scholar] [CrossRef]
- Ridderstrom, M.; Zamora, I.; Fjellstrom, O.; Andersson, T.B. Analysis of selective regions in the active sites of human cytochromes P450, 2C8, 2C9, 2C18, and 2C19 homology models using GRID/CPCA. J. Med. Chem. 2001, 44, 4072–4081. [Google Scholar] [CrossRef]
- Clifton, V.L. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef]
- Bhaumik, S.; Lockett, J.; Cuffe, J.; Clifton, V.L. Glucocorticoids and Their Receptor Isoforms: Roles in Female Reproduction, Pregnancy, and Foetal Development. Biology 2023, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Meakin, A.S.; Cuffe, J.S.M.; Darby, J.R.T.; Morrison, J.L.; Clifton, V.L. Let’s Talk about Placental Sex, Baby: Understanding Mechanisms That Drive Female- and Male-Specific Fetal Growth and Developmental Outcomes. Int. J. Mol. Sci. 2021, 22, 6386. [Google Scholar] [CrossRef]
- Vatten, L.J.; Skjaerven, R. Offspring sex and pregnancy outcome by length of gestation. Early Hum. Dev. 2004, 76, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Wright, I.M.; Clifton, V.L. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R510–R514. [Google Scholar] [CrossRef] [PubMed]
- Meakin, A.S.; Nathanielsz, P.W.; Li, C.; Clifton, V.L.; Wiese, M.D.; Morrison, J.L. Maternal obesity impacts fetal liver androgen signalling in a sex-specific manner. Life Sci. 2024, 337, 122344. [Google Scholar] [CrossRef]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015, 116, 264–278. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Hanada, Y.; Hasuzawa, N.; Moriyama, Y.; Nomura, M.; Yamamoto, K. Dynamin-related protein 1 deficiency accelerates lipopolysaccharide-induced acute liver injury and inflammation in mice. Commun. Biol. 2021, 4, 894. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Zhang, M.; Li, Z.; Liu, B.; Liu, H.; Hao, J.; Li, X. Synergistic mechanism between the endoplasmic reticulum and mitochondria and their crosstalk with other organelles. Cell Death Discov. 2023, 9, 51. [Google Scholar] [CrossRef]
- Volgyi, K.; Juhasz, G.; Kovacs, Z.; Penke, B. Dysfunction of Endoplasmic Reticulum (ER) and Mitochondria (MT) in Alzheimer’s Disease: The Role of the ER-MT Cross-Talk. Curr. Alzheimer Res. 2015, 12, 655–672. [Google Scholar] [CrossRef]
- Escribano-Lopez, I.; Banuls, C.; Diaz-Morales, N.; Iannantuoni, F.; Rovira-Llopis, S.; Gomis, R.; Rocha, M.; Hernandez-Mijares, A.; Murphy, M.P.; Victor, V.M. The Mitochondria-Targeted Antioxidant MitoQ Modulates Mitochondrial Function and Endoplasmic Reticulum Stress in Pancreatic beta Cells Exposed to Hyperglycaemia. Cell Physiol. Biochem. 2019, 52, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, F.V.; Islas, F.; Jimenez-Gonzalez, S.; Luaces, M.; Ramchandani, B.; Romero-Miranda, A.; Delgado-Valero, B.; Roldan-Molina, E.; Saiz-Pardo, M.; Ceron-Nieto, M.A.; et al. Mitochondrial Oxidative Stress Promotes Cardiac Remodeling in Myocardial Infarction through the Activation of Endoplasmic Reticulum Stress. Antioxidants 2022, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, F.V.; Jimenez-Gonzalez, S.; Delgado-Valero, B.; Jurado-Lopez, R.; Genty, M.; Romero-Miranda, A.; Rodriguez, C.; Nieto, M.L.; Martinez-Martinez, E.; Cachofeiro, V. The Interplay of Mitochondrial Oxidative Stress and Endoplasmic Reticulum Stress in Cardiovascular Fibrosis in Obese Rats. Antioxidants 2021, 10, 1274. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Allison, B.J.; Brain, K.L.; Patey, O.V.; Niu, Y.; Botting, K.J.; Ford, S.G.; Garrud, T.A.; Wooding, P.F.B.; Shaw, C.J.; et al. Chronic Hypoxia in Ovine Pregnancy Recapitulates Physiological and Molecular Markers of Preeclampsia in the Mother, Placenta, and Offspring. Hypertension 2022, 79, 1525–1535. [Google Scholar] [CrossRef]
- Tong, W.; Giussani, D.A. Preeclampsia link to gestational hypoxia. J. Dev. Orig. Health Dis. 2019, 10, 322–333. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: Implications in drug development. Drug Discov. Today 2021, 26, 2754–2773. [Google Scholar] [CrossRef]
- Razaz, N.; Skoll, A.; Fahey, J.; Allen, V.M.; Joseph, K.S. Trends in optimal, suboptimal, and questionably appropriate receipt of antenatal corticosteroid prophylaxis. Obs. Gynecol. 2015, 125, 288–296. [Google Scholar] [CrossRef]
- Baghlaf, H.; Snelgrove, J.W.; Li, Q.; Huszti, E.; McDonald, S.D.; Asztalos, E.; Palermo, M.S.F.; Murphy, K.E. One vs 2 courses of antenatal corticosteroids in pregnancies at risk of preterm birth: A secondary analysis of the MACS trial. Am. J. Obs. Gynecol. MFM 2023, 5, 101002. [Google Scholar] [CrossRef]
- Humbeck, C.; Jonassen, S.; Bringewatt, A.; Pervan, M.; Rody, A.; Bossung, V. Timing of antenatal steroid administration for imminent preterm birth: Results of a prospective observational study in Germany. Arch. Gynecol. Obs. 2023, 308, 839–847. [Google Scholar] [CrossRef]
- Familiari, A.; Napolitano, R.; Visser, G.H.A.; Lees, C.; Wolf, H.; Prefumo, F.; on behalf of the TRUFFLE-2 feasibility study investigators. Antenatal corticosteroids and perinatal outcome in late fetal growth restriction: Analysis of prospective cohort. Ultrasound Obs. Gynecol. 2023, 61, 191–197. [Google Scholar] [CrossRef]
- Rood, K.M.; Ugwu, L.G.; Grobman, W.A.; Bailit, J.L.; Wapner, R.J.; Varner, M.W.; Thorp, J.M., Jr.; Caritis, S.N.; Tita, A.T.N.; Saade, G.R.; et al. Obstacles to Optimal Antenatal Corticosteroid Administration to Eligible Patients. Am. J. Perinatol. 2024, 41, e594–e600. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.A.; Lewandowski, A.J.; Worton, S.A.; Davis, E.F.; Lazdam, M.; Francis, J.; Neubauer, S.; Lucas, A.; Singhal, A.; Leeson, P. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 2012, 129, e1282–e1290. [Google Scholar] [CrossRef] [PubMed]
- McBride, G.M.; Meakin, A.S.; Soo, J.Y.; Darby, J.R.T.; Varcoe, T.J.; Bradshaw, E.L.; Lock, M.C.; Holman, S.L.; Saini, B.S.; Macgowan, C.K.; et al. Intrauterine growth restriction alters the activity of drug metabolising enzymes in the maternal-placental-fetal unit. Life Sci. 2021, 285, 120016. [Google Scholar] [CrossRef] [PubMed]

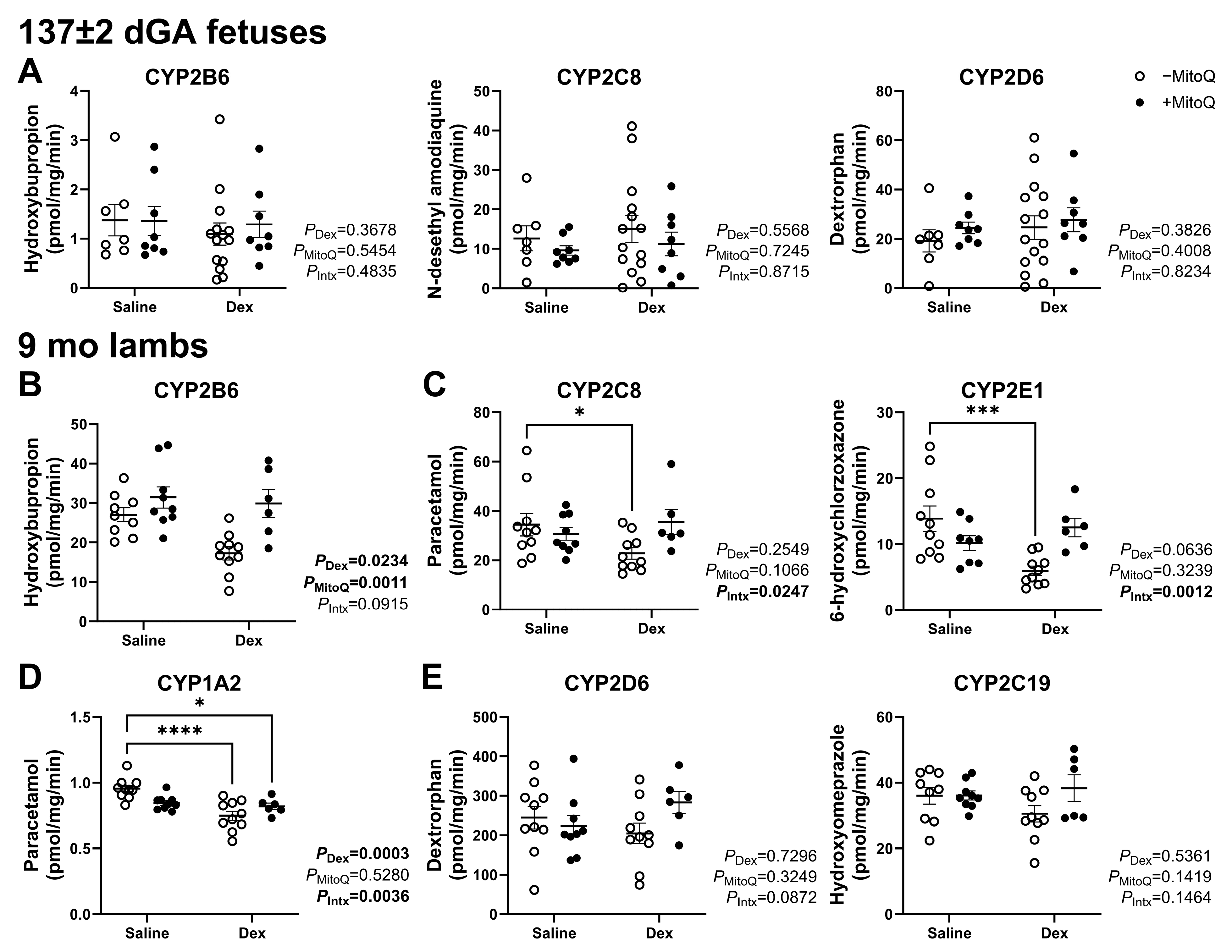
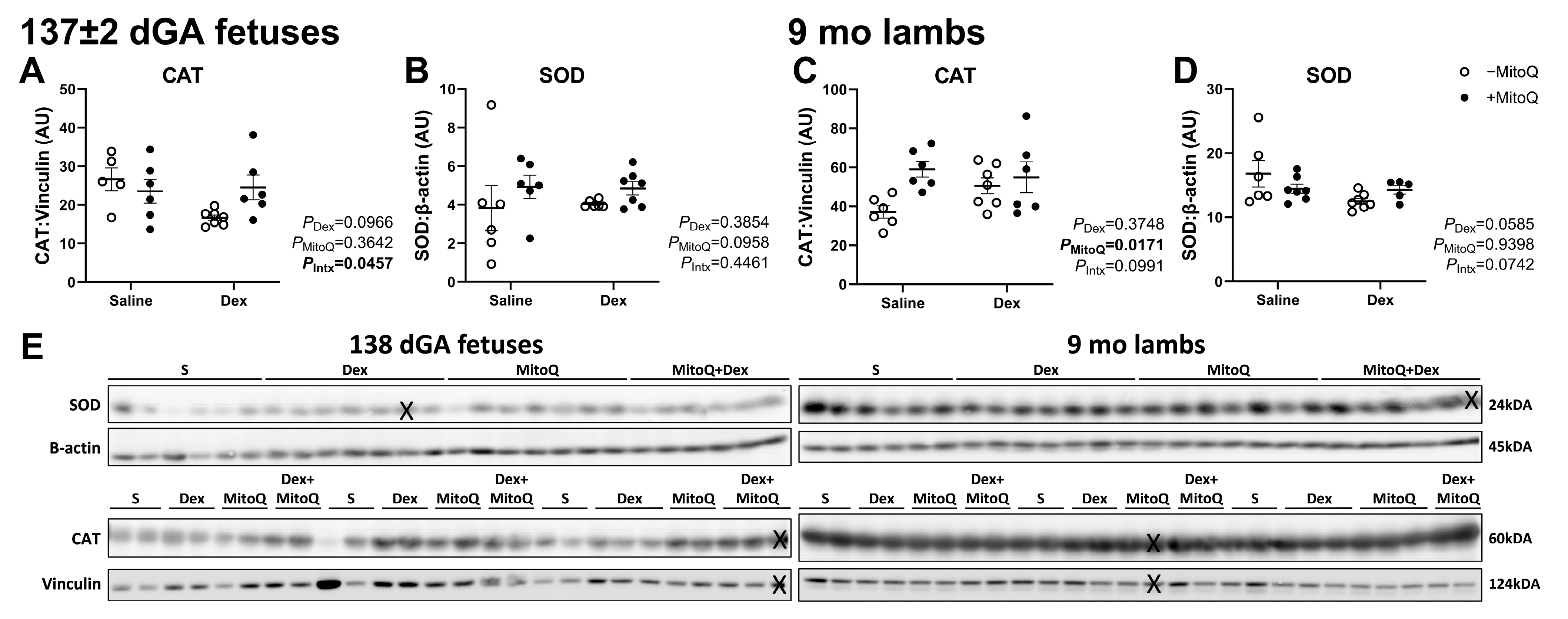


| Saline | Dex | |||||||
|---|---|---|---|---|---|---|---|---|
| −MitoQ | +MitoQ | −MitoQ | +MitoQ | PDex | PMitoQ | PIntx | ||
| Fetal (137 ± 2 dGA) | n = 7 | n = 8 | n = 10 | n = 8 | ||||
| Post-mortem bodyweight (kg) | 3.34 ± 0.20 | 4.11 ± 0.26 | 3.73 ± 0.16 | 3.82 ± 0.15 | 0.7989 | 0.0395 | 0.1013 | |
| Liver weight (g) | 68.26 ± 4.82 | 97.36 ± 5.20 | 75.38 ± 4.75 | 75.28 ± 2.80 | 0.1762 | 0.0115 | 0.0110 | |
| Liver to bodyweight ratio (g/kg) | 20.44 ± 0.79 | 24.01 ± 1.32 | 20.22 ± 1.09 | 19.12 ± 0.62 | 0.0461 | 0.3232 | 0.0672 | |
| Young adults (9 mo) | n = 10 | n = 9 | n = 10 | n = 6 | ||||
| Gestational age at birth (dGA) | 147.20 ± 0.63 | 146.89 ± 0.84 | 146.90 ± 1.09 | 148.00 ± 1.10 | 0.6692 | 0.6777 | 0.4586 | |
| Birthweight (kg) | 3.36 ± 0.21 | 3.34 ± 0.21 | 3.58 ± 0.29 | 3.63 ± 0.30 | 0.3291 | 0.9435 | 0.8788 | |
| Post-mortem bodyweight (kg) | 25.70 ± 1.51 | 28.44 ± 1.83 | 26.55 ± 1.86 | 34.35 ± 2.08 | 0.0770 | 0.0076 | 0.1808 | |
| Liver weight (g) | 387.39 ± 19.61 | 374.74 ± 21.56 | 374.76 ± 18.48 | 452.59 ± 23.60 | 0.1324 | 0.1326 | 0.0400 | |
| Liver to bodyweight ratio (g/kg) | 14.51 ± 0.63 | 13.30 ± 0.51 | 14.73 ± 1.20 | 13.25 ± 0.51 | 0.9247 | 0.1318 | 0.8816 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennett, M.G.A.; Meakin, A.S.; Botting-Lawford, K.J.; Niu, Y.; Ford, S.G.; Murphy, M.P.; Wiese, M.D.; Giussani, D.A.; Morrison, J.L. Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone. Pharmaceutics 2025, 17, 285. https://doi.org/10.3390/pharmaceutics17030285
Bennett MGA, Meakin AS, Botting-Lawford KJ, Niu Y, Ford SG, Murphy MP, Wiese MD, Giussani DA, Morrison JL. Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone. Pharmaceutics. 2025; 17(3):285. https://doi.org/10.3390/pharmaceutics17030285
Chicago/Turabian StyleBennett, Millicent G. A., Ashley S. Meakin, Kimberley J. Botting-Lawford, Youguo Niu, Sage G. Ford, Michael P. Murphy, Michael D. Wiese, Dino A. Giussani, and Janna L. Morrison. 2025. "Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone" Pharmaceutics 17, no. 3: 285. https://doi.org/10.3390/pharmaceutics17030285
APA StyleBennett, M. G. A., Meakin, A. S., Botting-Lawford, K. J., Niu, Y., Ford, S. G., Murphy, M. P., Wiese, M. D., Giussani, D. A., & Morrison, J. L. (2025). Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone. Pharmaceutics, 17(3), 285. https://doi.org/10.3390/pharmaceutics17030285








