Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside
Abstract
1. Introduction
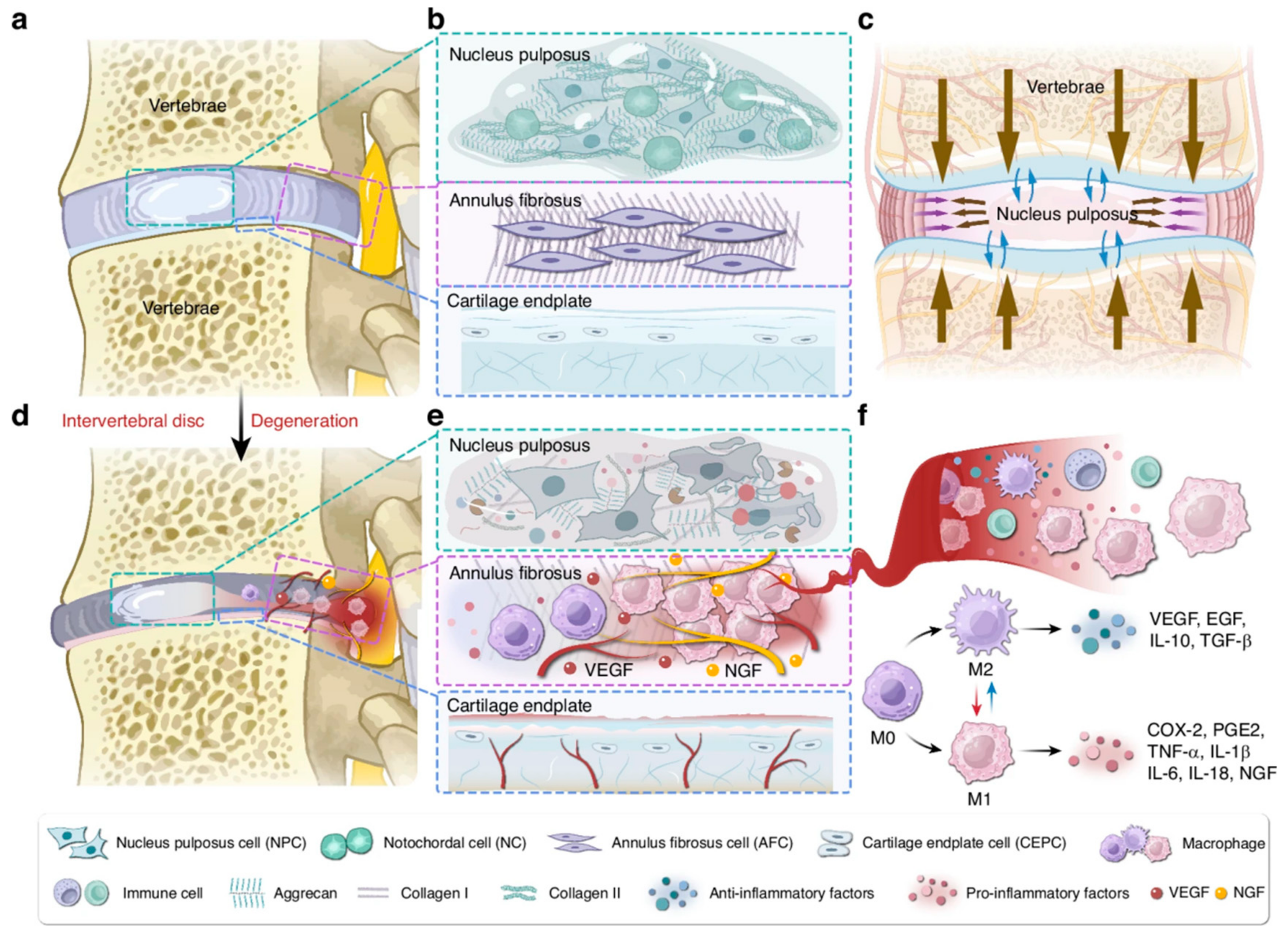
2. Mechanisms and Therapeutic Approaches for IDD
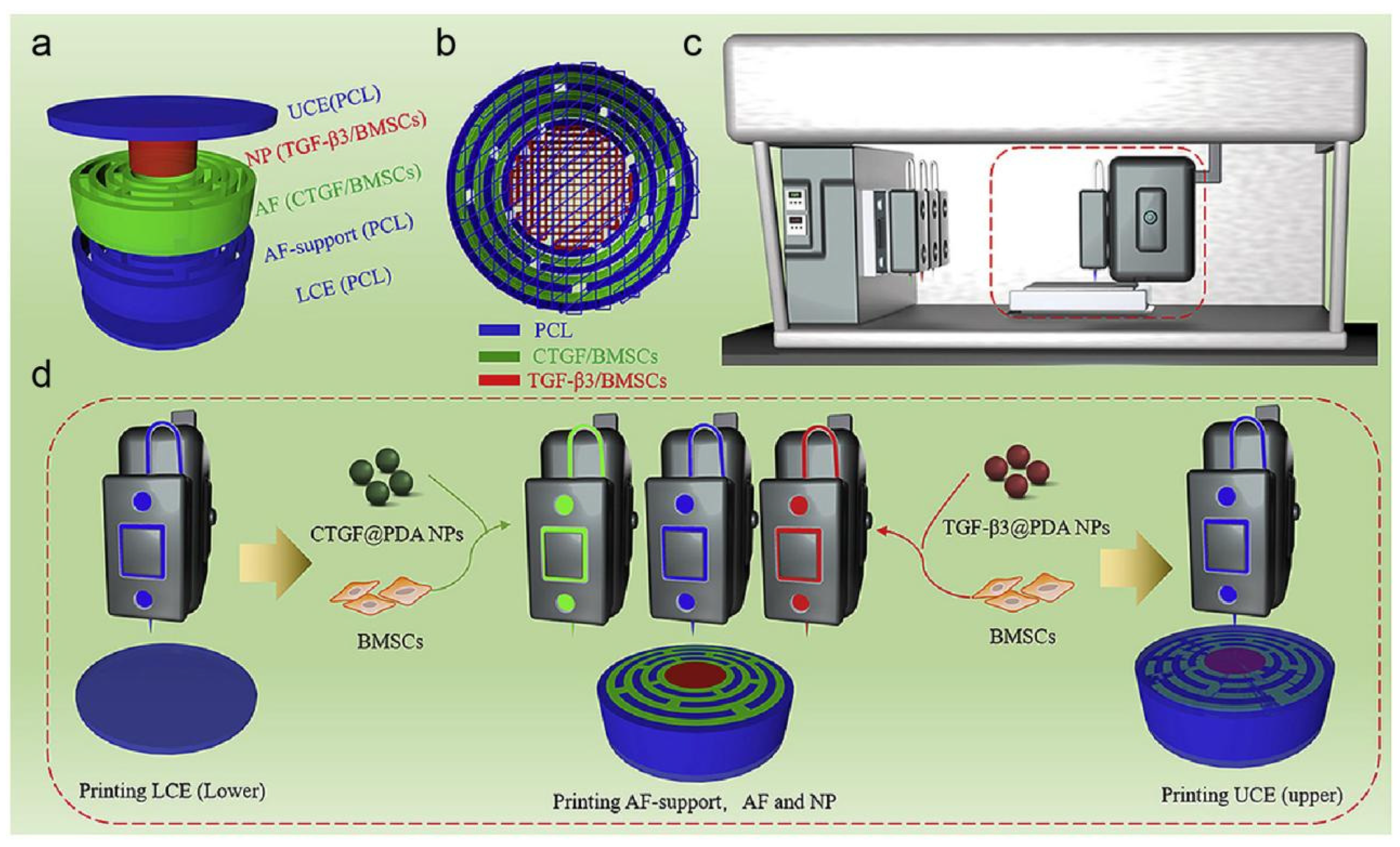
3. Nanomedicine Provides New Perspectives for the Treatment of IDD
3.1. Drug Delivery Routes for IDD Therapy
3.2. Nanoparticles
3.2.1. IVD Targeted Delivery
3.2.2. Promotion of Anabolic Metabolism
3.2.3. Promotion of Stem Cell Migration and Differentiation
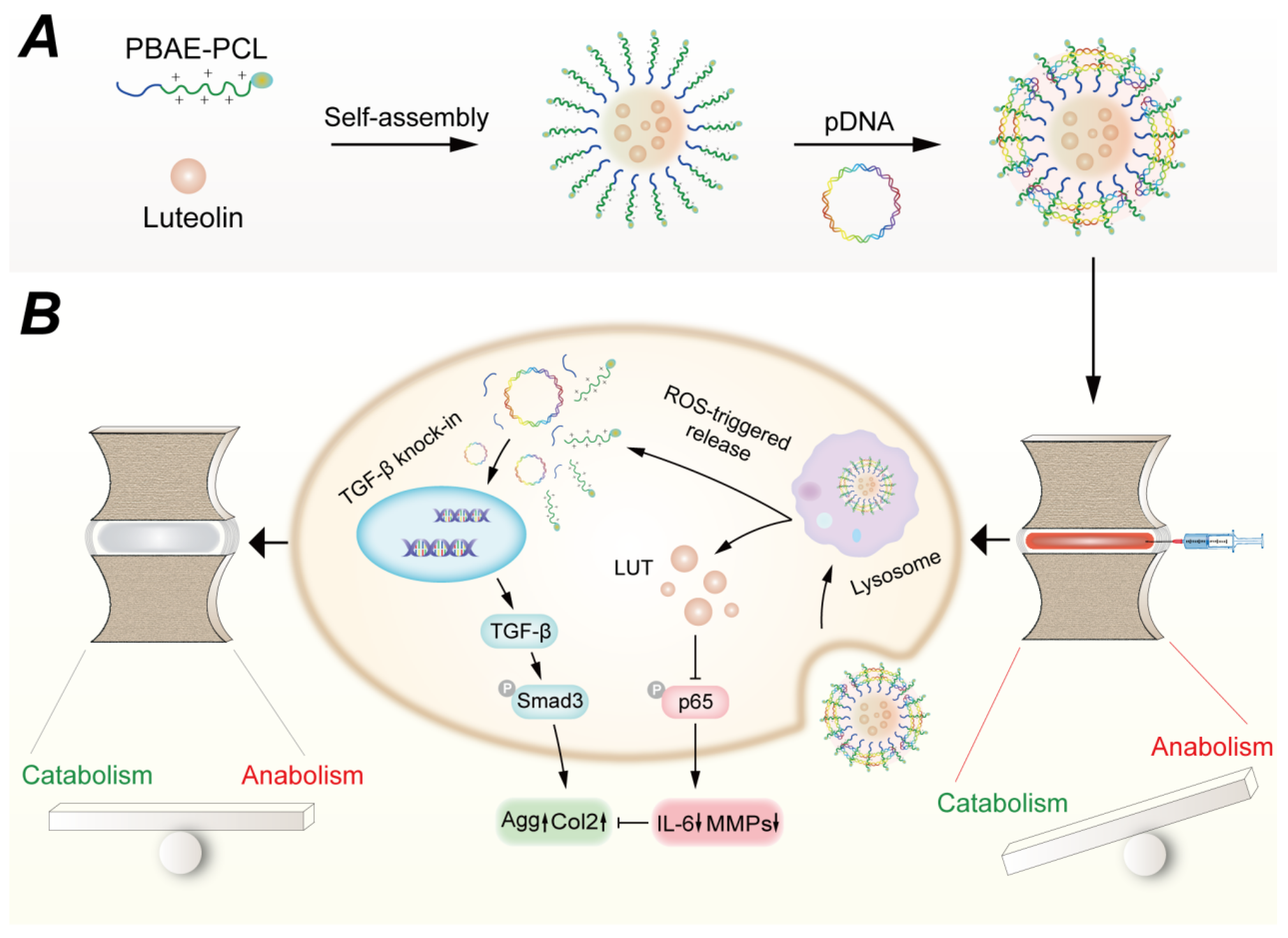
3.3. Gene-Nanoparticle Complexes
3.3.1. Anti-Inflammation
3.3.2. Anti-Fibrosis
3.3.3. Promotion of Stem Cell Migration and Differentiation
| Type | Composition | Gene | Effect | References |
|---|---|---|---|---|
| Thermo-responsive mixed polyplex micelles | Poly(ethylene glycol)-block-poly [PEG-b-PAsp(DET)] and poly(N-isopropylacrylamide)-block-PAsp(DET) [PNIPAM-b-PAsp(DET)] | Heme oxygenase-1 (HO-1) | Delivery vector: resistance to nuclease decomposition and protein adsorption Gene: anti-inflammation | [47] |
| Nanofibrous spongy microspheres (NF-SMS) loaded hyperbranched polymer (HP)/PLGA (HP/NS) | Hyperbranched polymer (HP), PLGA, nanofibrous spongy microspheres (NF-SMS) | Orphan nuclear receptor-4A1 NR4A1 | Delivery vector: sustained release over 30 days Gene: anti-fibrosis | [49] |
| Polyplex Micelle-Loaded Injectable Hydrogels | PEG114-GPLGVRG-PAsp(Det)48-Chole | miR-29 | Delivery vector: MMP2 responsiveness Gene: anti-fibrosis | [51] |
| Nanofibrous spongy microspheres (NF-SMS) loaded hyperbranched polymer (HP)/PLGA (HP/NS) | Hyperbranched polymer (HP), PLGA, nanofibrous spongy microspheres (NF-SMS); MSCs | anti-miR-199a | Delivery vector: MSCs loading ability and promote their differentiation Gene: anti-calcification | [52] |
| ROS-responsive cationic copolymer co-delivery nanoparticles | Luteolin-pTGF-β1 plasmid@PBC(PBAE-PCL) | TGF-β1 | Delivery vector: ROS-Responsiveness; Co-delivery Gene: promote ECM production | [16] |
3.4. Fullerene Derivatives—Fullerol
3.5. Microspheres
3.5.1. Anti-Inflammation
3.5.2. Promotion of Stem Cell Migration and Differentiation

3.6. Biological Source Nanocarriers—Exosomes
3.6.1. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells (BMSCs)
3.6.2. Exosomes Derived from NPCs
3.6.3. Exosomes Derived from Human Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells (PLMSCs/ULMSCs)
3.7. Nanocomposite Hydrogels Improve the Mechanical Properties of the IVD
3.7.1. Synthetic Material-Reinforced Hydrogels
3.7.2. Natural–Synthetic Composite Hydrogels
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Su, Z.; So, K.K.H.; Lu, Q.; Lyu, M.; Zuo, J.; Huang, Y.; Guan, Z.; Cheung, K.M.C.; et al. Fibrocyte enrichment and myofibroblastic adaptation causes nucleus pulposus fibrosis and associates with disc degeneration severity. Bone Res. 2025, 13, 10. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, B.; Luo, Z.-J. The Immune Privilege of the Intervertebral Disc: Implications for Intervertebral Disc Degeneration Treatment. Int. J. Med. Sci. 2020, 17, 685–692. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef]
- Murphy, K.; Lufkin, T.; Kraus, P. Development and Degeneration of the Intervertebral Disc—Insights from Across Species. Vet. Sci. 2023, 10, 540. [Google Scholar] [CrossRef]
- Crump, K.B.; Alminnawi, A.; Bermudez-Lekerika, P.; Compte, R.; Gualdi, F.; McSweeney, T.; Muñoz-Moya, E.; Nüesch, A.; Geris, L.; Dudli, S.; et al. Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research. JOR Spine 2023, 6, e1294. [Google Scholar] [CrossRef]
- Samanta, A.; Lufkin, T.; Kraus, P. Intervertebral disc degeneration—Current therapeutic options and challenges. Front. Public Health 2023, 11, 1156749. [Google Scholar] [CrossRef]
- Desmoulin, G.T.; Pradhan, V.; Milner, T.E. Mechanical Aspects of Intervertebral Disc Injury and Implications on Biomechanics. Spine 2020, 45, E457–E464. [Google Scholar] [CrossRef]
- Zhang, A.; Cheng, Z.; Chen, Y.; Shi, P.; Gan, W.; Zhang, Y. Emerging tissue engineering strategies for annulus fibrosus therapy. Acta Biomater. 2023, 167, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.; Hai, Y.; Cheng, Y. Regulatory Effect of Inflammatory Mediators in Intervertebral Disc Degeneration. Mediat. Inflamm. 2023, 2023, 6210885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Theologis, A.A.; O’Connell, G.D. Understanding the etiopathogenesis of lumbar intervertebral disc herniation: From clinical evidence to basic scientific research. JOR Spine 2024, 7, e1289. [Google Scholar] [CrossRef]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef]
- Ma, T.; Wu, J.; Chen, S.; Bian, J.; Gao, G.; Nong, L. pH-Responsive Modified HAMA Microspheres Regulate the Inflammatory Microenvironment of Intervertebral Discs. ACS Appl. Mater. Interfaces 2024, 16, 63295–63305. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wang, H.; Wang, Y.Y.; Li, L.; Ding, J.H.; Yuan, C.Y.; Xu, T.; Xu, H.R.; Xie, H.; Zhu, N.; et al. Co-delivery of luteolin and TGF-β1 plasmids with ROS-responsive virus-inspired nanoparticles for microenvironment regulation and chemo-gene therapy of intervertebral disc degeneration. Nano Res. 2022, 15, 8214–8227. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Z.; Wang, Z.; Lee, D.; Ma, Y.; Wilhelm, S.; Wang, H.; Kim, B.Y.S.; Jiang, W. Multi-omics approaches to decipher the interactions of nanoparticles and biological systems. Nat. Rev. Bioeng. 2024, 1–16. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, Y.; Liu, Y.; Sun, X.; Liu, X.; Li, B.; Yang, Q. Role of macrophage in intervertebral disc degeneration. Bone Res. 2025, 13, 15. [Google Scholar] [CrossRef]
- Fontana, G.; See, E.; Pandit, A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2015, 84, 146–158. [Google Scholar] [CrossRef]
- Mohd Isa, I.L.; Mokhtar, S.A.; Abbah, S.A.; Fauzi, M.B.; Devitt, A.; Pandit, A. Intervertebral Disc Degeneration: Biomaterials and Tissue Engineering Strategies toward Precision Medicine. Adv. Healthc. Mater. 2022, 11, e2102530. [Google Scholar] [CrossRef]
- Gansau, J.; Buckley, C.T. Priming as a strategy to overcome detrimental pH effects on cells for intervertebral disc regeneration. Eur. Cells Mater. 2021, 41, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Farhang, N.; Silverman, L.; Bowles, R.D. Improving Cell Therapy Survival and Anabolism in Harsh Musculoskeletal Disease Environments. Tissue Eng. Part B Rev. 2020, 26, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, K.; Pan, Q.; Huang, W.; Xiao, Y.; Lin, H.; Liu, S.; Chen, X.; Lv, X.; Feng, S.; et al. An Engineered Bionic Nanoparticle Sponge as a Cytokine Trap and Reactive Oxygen Species Scavenger to Relieve Disc Degeneration and Discogenic Pain. ACS Nano 2024, 18, 3053–3072. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Z.; Wang, Y.; Yang, J. Multiple nano-drug delivery systems for intervertebral disc degeneration: Current status and future perspectives. Bioact. Mater. 2023, 23, 274–299. [Google Scholar] [CrossRef]
- Shi, S.; Ou, X.; Liu, C.; Li, R.; Zheng, Q.; Hu, L. Nanotechnology-Enhanced Pharmacotherapy for Intervertebral Disc Degeneration Treatment. Int. J. Nanomed. 2024, 19, 14043–14058. [Google Scholar] [CrossRef]
- Elmounedi, N.; Bahloul, W.; Turki, M.; Amri, R.; Aoui, M.; Elbaya, W.; Keskes, H. Impact of Needle Size on the Onset and the Progression of Disc Degeneration in Rats. Pain Physician 2022, 25, 509–517. [Google Scholar]
- Mao, H.-j.; Chen, Q.-x.; Han, B.; Li, F.-c.; Feng, J.; Shi, Z.-l.; Lin, M.; Wang, J. The effect of injection volume on disc degeneration in a rat tail model. Spine 2011, 36, E1062–E1069. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhang, W.; Wei, K.; Pei, Y.; Zou, C.; Zhang, C.; Ding, J.; Fang, H.; Tan, S. Oxymatrine Liposomes for Intervertebral Disc Treatment: Formulation, in vitro and vivo Assessments. Drug Des. Dev. Ther. 2020, 14, 921–931. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Zhang, W.; Li, Y.; Liu, L.; Leng, T. Yeast Cell wall Particle mediated Nanotube-RNA delivery system loaded with miR365 Antagomir for Post-traumatic Osteoarthritis Therapy via Oral Route. Theranostics 2020, 10, 8479–8493. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Feng, M.; Zhang, W.; Li, Y. Yeast microcapsule-mediated oral delivery of IL-1β shRNA for post-traumatic osteoarthritis therapy. Mol. Ther. Nucleic Acids 2021, 23, 336–346. [Google Scholar] [CrossRef]
- Frapin, L.; Clouet, J.; Delplace, V.; Fusellier, M.; Guicheux, J.; Le Visage, C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv. Drug Deliv. Rev. 2019, 149–150, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Colella, F.; Garcia, J.P.; Sorbona, M.; Lolli, A.; Antunes, B.; D’Atri, D.; Barré, F.P.Y.; Oieni, J.; Vainieri, M.L.; Zerrillo, L.; et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: Selecting the optimal platform for the delivery of disease-modifying agents. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Huang, R.; Zhang, Y.; Li, T.; Dai, J.; Nannapuneni, N.; Chastanet, T.R.; Chen, M.; Shen, F.H.; Jin, L.; et al. A New Formyl Peptide Receptor-1 Antagonist Conjugated Fullerene Nanoparticle for Targeted Treatment of Degenerative Disc Diseases. ACS Appl. Mater. Interfaces 2019, 11, 38405–38416. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Pan, H.Y.; Haworth, K.; Mahoney, E.; Mercado-Shekhar, K.P.; Lin, C.Y.; Zhang, Z.; Park, Y.C. Multiple-Exposure Drug Release from Stable Nanodroplets by High-Intensity Focused Ultrasound for a Potential Degenerative Disc Disease Treatment. Ultrasound Med. Biol. 2019, 45, 160–169. [Google Scholar] [CrossRef]
- Shen, J.; Zhuo, N.; Xu, S.; Song, Z.; Hu, Z.; Hao, J.; Guo, X. Resveratrol delivery by ultrasound-mediated nanobubbles targeting nucleus pulposus cells. Nanomedicine 2018, 13, 1433–1446. [Google Scholar] [CrossRef]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef]
- Chang, K.Y.; Cheng, L.W.; Ho, G.H.; Huang, Y.P.; Lee, Y.D. Fabrication and characterization of poly(γ-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater. 2009, 5, 1937–1947. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Teixeira, G.Q.; Silva, R.V.; Caldeira, J.; Grad, S.; Gonçalves, R.M.; Barbosa, M.A. Poly(γ-glutamic acid) and poly(γ-glutamic acid)-based nanocomplexes enhance type II collagen production in intervertebral disc. J. Mater. Sci. Mater. Med. 2017, 28, 6. [Google Scholar] [CrossRef]
- Zeckser, J.; Wolff, M.; Tucker, J.; Goodwin, J. Multipotent Mesenchymal Stem Cell Treatment for Discogenic Low Back Pain and Disc Degeneration. Stem Cells Int. 2016, 2016, 3908389. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Yamamoto, Y.; Nomura, T.; Okuma, M.; Nishimura, K.; Nakai, T.; Ando, K.; Hotta, T. Transplantation of mesenchymal stem cells embedded in Atelocollagen® gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials 2003, 24, 3531–3541. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S.; Zhao, X.; Mao, Z.; Gao, C. Stromal cell-derived factor-1α-encapsulated albumin/heparin nanoparticles for induced stem cell migration and intervertebral disc regeneration in vivo. Acta Biomater. 2018, 72, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, S.; Li, P.; Xu, Y.; Wang, L.; Zhao, C.; Ouyang, B.; Tu, B.; Zhang, C.; Luo, L.; et al. A Controlled Release Codelivery System of MSCs Encapsulated in Dextran/Gelatin Hydrogel with TGF-β3-Loaded Nanoparticles for Nucleus Pulposus Regeneration. Stem Cells Int. 2016, 2016, 9042019. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Wallach, C.J.; Kim, J.S.; Sobajima, S.; Lattermann, C.; Oxner, W.M.; McFadden, K.; Robbins, P.D.; Gilbertson, L.G.; Kang, J.D. Safety assessment of intradiscal gene transfer: A pilot study. Spine J. Off. J. N. Am. Spine Soc. 2006, 6, 107–112. [Google Scholar] [CrossRef]
- Raftery, R.M.; Walsh, D.P.; Castano, I.M.; Heise, A.; Duffy, G.P.; Cryan, S.A.; O’Brien, F.J. Delivering Nucleic-Acid Based Nanomedicines on Biomaterial Scaffolds for Orthopedic Tissue Repair: Challenges, Progress and Future Perspectives. Adv. Mater. 2016, 28, 5447–5469. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T. Design of Polymeric Gene Carriers for Effective Intracellular Delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef]
- Feng, G.; Chen, H.; Li, J.; Huang, Q.; Gupte, M.J.; Liu, H.; Song, Y.; Ge, Z. Gene therapy for nucleus pulposus regeneration by heme oxygenase-1 plasmid DNA carried by mixed polyplex micelles with thermo-responsive heterogeneous coronas. Biomaterials 2015, 52, 1–13. [Google Scholar] [CrossRef]
- Au, T.Y.K.; Lam, T.K.; Peng, Y.; Wynn, S.L.; Cheung, K.M.C.; Cheah, K.S.E.; Leung, V.Y.L. Transformation of resident notochord-descendent nucleus pulposus cells in mouse injury-induced fibrotic intervertebral discs. Aging Cell 2020, 19, e13254. [Google Scholar] [CrossRef]
- Ohta, R.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Nakamae, T.; Izumi, B.; Fujioka, Y.; Ochi, M. Heme oxygenase-1 modulates degeneration of the intervertebral disc after puncture in Bach 1 deficient mice. Eur. Spine J. 2012, 21, 1748–1757. [Google Scholar] [CrossRef][Green Version]
- Feng, G.; Zhang, Z.; Dang, M.; Zhang, X.; Doleyres, Y.; Song, Y.; Chen, D.; Ma, P.X. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials 2017, 131, 86–97. [Google Scholar] [CrossRef]
- Feng, G.; Zha, Z.; Huang, Y.; Li, J.; Wang, Y.; Ke, W.; Chen, H.; Liu, L.; Song, Y.; Ge, Z. Sustained and Bioresponsive Two-Stage Delivery of Therapeutic miRNA via Polyplex Micelle-Loaded Injectable Hydrogels for Inhibition of Intervertebral Disc Fibrosis. Adv. Healthc. Mater. 2018, 7, e1800623. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, Z.; Dang, M.; Rambhia, K.J.; Ma, P.X. Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification. Biomaterials 2020, 256, 120213. [Google Scholar] [CrossRef] [PubMed]
- Whiffen, J.D.; Young, W.P.; Gott, V.L. Stability of the thrombus-resistant graphite-benzalkonium-heparin surface in an anti-heparin environment. J. Thorac. Cardiovasc. Surg. 1964, 48, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, X.; Ma, R.; Hou, Y.; Qian, Y.; Fan, C. Biological and biocompatible characteristics of fullerenols nanomaterials for tissue engineering. Histol. Histopathol. 2021, 36, 725–731. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, L.; Mahon, B.H.; Chordia, M.D.; Shen, F.H.; Li, X. Novel treatment of neuroinflammation against low back pain by soluble fullerol nanoparticles. Spine 2013, 38, 1443–1451. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, L.; Shen, F.H.; Balian, G.; Li, X.J. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells—A potential novel treatment for intervertebral disc degeneration. Spine J. Off. J. N. Am. Spine Soc. 2013, 13, 1571–1580. [Google Scholar] [CrossRef]
- Yang, X.; Jin, L.; Yao, L.; Shen, F.H.; Shimer, A.L.; Li, X. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int. J. Nanomed. 2014, 9, 2419–2430. [Google Scholar] [CrossRef]
- Dong, Z.; Meng, X.; Yang, W.; Zhang, J.; Sun, P.; Zhang, H.; Fang, X.; Wang, D.A.; Fan, C. Progress of gelatin-based microspheres (GMSs) as delivery vehicles of drug and cell. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111949. [Google Scholar] [CrossRef]
- Andres-Guerrero, V.; Zong, M.; Ramsay, E.; Rojas, B.; Sarkhel, S.; Gallego, B.; de Hoz, R.; Ramirez, A.I.; Salazar, J.J.; Trivino, A.; et al. Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology. J. Control. Release Off. J. Control. Release Soc. 2015, 211, 105–117. [Google Scholar] [CrossRef]
- Willems, N.; Mihov, G.; Grinwis, G.C.M.; van Dijk, M.; Schumann, D.; Bos, C.; Strijkers, G.J.; Dhert, W.J.A.; Meij, B.P.; Creemers, L.B.; et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 707–714. [Google Scholar] [CrossRef]
- Tellegen, A.R.; Rudnik-Jansen, I.; Beukers, M.; Miranda-Bedate, A.; Bach, F.C.; de Jong, W.; Woike, N.; Mihov, G.; Thies, J.C.; Meij, B.P.; et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Control. Release Off. J. Control. Release Soc. 2018, 286, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Rudnik-Jansen, I.; Tellegen, A.; Beukers, M.; Öner, F.; Woike, N.; Mihov, G.; Thies, J.; Meij, B.; Tryfonidou, M.; Creemers, L. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, W.; Chen, T.; Chen, X.; Liang, J.; Chen, H.; Shen, H.; Deng, L.; Ruan, H.; Cui, W. Hydrogen Ion Capturing Hydrogel Microspheres for Reversing Inflammaging. Adv. Mater. 2024, 36, e2306105. [Google Scholar] [CrossRef] [PubMed]
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin Matrices for Growth Factor Sequestration. Trends Biotechnol. 2020, 38, 546–557. [Google Scholar] [CrossRef]
- Xia, K.; Zhu, J.; Hua, J.; Gong, Z.; Yu, C.; Zhou, X.; Wang, J.; Huang, X.; Yu, W.; Li, L.; et al. Intradiscal Injection of Induced Pluripotent Stem Cell-Derived Nucleus Pulposus-Like Cell-Seeded Polymeric Microspheres Promotes Rat Disc Regeneration. Stem Cells Int. 2019, 2019, 6806540. [Google Scholar] [CrossRef]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef]
- Yuan, M.; Leong, K.W.; Chan, B.P. Three-dimensional culture of rabbit nucleus pulposus cells in collagen microspheres. Spine J. Off. J. N. Am. Spine Soc. 2011, 11, 947–960. [Google Scholar] [CrossRef]
- Li, Y.Y.; Diao, H.J.; Chik, T.K.; Chow, C.T.; An, X.M.; Leung, V.; Cheung, K.M.C.; Chan, B.P. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: Reduced risk of osteophyte formation. Tissue Eng. Part A 2014, 20, 1379–1391. [Google Scholar] [CrossRef]
- Liang, C.Z.; Li, H.; Tao, Y.Q.; Zhou, X.P.; Yang, Z.R.; Xiao, Y.X.; Li, F.C.; Han, B.; Chen, Q.X. Dual delivery for stem cell differentiation using dexamethasone and bFGF in/on polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J. Mater. Sci. Mater. Med. 2012, 23, 1097–1107. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, e1802896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, Y.; Liu, L.; Wang, H.; Shen, P.; Yang, H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 2020, 19, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, X.; Peng, C.; Yu, L.; Hao, Y. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp. Cell Res. 2020, 393, 112109. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, Y.; Liu, W.; Ni, W.; Huang, X.; Yuan, J.; Zhao, B.; Xiao, H.; Xue, F. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell. Mol. Med. 2020, 24, 11742–11754. [Google Scholar] [CrossRef]
- Su, K.-K.; Yu, D.-C.; Cao, X.-F.; Li, P.; Chang, L.; Yu, X.-L.; Li, Z.-Q.; Li, M. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Nuclear Pulposus Cells Degeneration Through the miR-145a-5p/USP31/HIF-1α Signaling Pathway. Stem Cell Rev. Rep. 2024, 20, 2268–2282. [Google Scholar] [CrossRef]
- Lan, W.R.; Pan, S.; Li, H.Y.; Sun, C.; Chang, X.; Lu, K.; Jiang, C.Q.; Zuo, R.; Zhou, Y.; Li, C.Q. Inhibition of the Notch1 Pathway Promotes the Effects of Nucleus Pulposus Cell-Derived Exosomes on the Differentiation of Mesenchymal Stem Cells into Nucleus Pulposus-Like Cells in Rats. Stem Cells Int. 2019, 2019, 8404168. [Google Scholar] [CrossRef]
- Moen, A.; Jacobsen, D.; Phuyal, S.; Legfeldt, A.; Haugen, F.; Røe, C.; Gjerstad, J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J. Transl. Med. 2017, 15, 89. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, B.; Mu, K.; Feng, S.Q.; Dong, Z.Y.; Ning, G.Z.; Li, H.R.; Liu, S.; Zhao, L.; Li, Y.; et al. Circular RNA GRB10 as a competitive endogenous RNA regulating nucleus pulposus cells death in degenerative intervertebral disk. Cell Death Dis. 2018, 9, 319. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, X.; Liu, L.; Cai, Y.; Zhao, X.; Ma, H.; Zhang, Y. Exosomes Derived from Human Placental Mesenchymal Stromal Cells Carrying AntagomiR-4450 Alleviate Intervertebral Disc Degeneration Through Upregulation of ZNF121. Stem Cells Dev. 2020, 29, 1038–1058. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, T.; Gao, S.; Bai, L.; Zhu, Z.; Zhao, S.; Wang, Y.; Liang, X.; Li, Y.; Gao, L.; et al. Exosomes from umbilical cord mesenchymal stem cells ameliorate intervertebral disc degeneration via repairing mitochondrial dysfunction. J. Orthop. Transl. 2024, 46, 103–115. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Wismer, N.; Grad, S.; Fortunato, G.; Ferguson, S.J.; Alini, M.; Eglin, D. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: Effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Eng. Part A 2014, 20, 672–682. [Google Scholar] [CrossRef]
- Collin, E.C.; Grad, S.; Zeugolis, D.I.; Vinatier, C.S.; Clouet, J.R.; Guicheux, J.J.; Weiss, P.; Alini, M.; Pandit, A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 2011, 32, 2862–2870. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Milani, A.H.; Jennings, J.; Adlam, D.J.; Freemont, A.J.; Hoyland, J.A.; Saunders, B.R. Highly compressive and stretchable poly(ethylene glycol) based hydrogels synthesised using pH-responsive nanogels without free-radical chemistry. Nanoscale 2019, 11, 7921–7930. [Google Scholar] [CrossRef]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 897–908. [Google Scholar] [CrossRef]
- Schmocker, A.; Khoushabi, A.; Frauchiger, D.A.; Gantenbein, B.; Schizas, C.; Moser, C.; Bourban, P.-E.; Pioletti, D.P. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials 2016, 88, 110–119. [Google Scholar] [CrossRef]
- Murab, S.; Samal, J.; Shrivastava, A.; Ray, A.R.; Pandit, A.; Ghosh, S. Glucosamine loaded injectable silk-in-silk integrated system modulate mechanical properties in bovine ex-vivo degenerated intervertebral disc model. Biomaterials 2015, 55, 64–83. [Google Scholar] [CrossRef]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite hydrogel of chitosan-poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, Z.; Huang, Z.; Luo, D.; Chen, Z.; Xie, L.; Zhu, L.; Liu, H.; Lian, K.; Alberton, P.; et al. A mouse coccygeal intervertebral disc degeneration model with tail-looping constructed using a suturing method. Anim. Model. Exp. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Kim, H.; Jeon, W.-J.; Yeo, C.; Kim, H.; Lee, J.; Lee, Y.J.; Ha, I.-H. Animal Models of Intervertebral Disc Diseases: Advantages, Limitations, and Future Directions. Neurol. Int. 2024, 16, 1788–1818. [Google Scholar] [CrossRef]

| Source of Exosome | Therapeutic Agent | Signal Pathway | Effect | Tests | Animal Model | Reference |
|---|---|---|---|---|---|---|
| Bone marrow-derived MSCs (BMSCs) | miR-142-3P | MLK3/MAPK | Inhibit NPs apoptosis | In vitro | - | [72] |
| BMSCs | miR-532-5p | RASSF5 | Inhibit NPs apoptosis and fibrosis | In vitro | - | [73] |
| BMSCs | miR-21 | PTEN/PI3K/Akt | Inhibit NPs apoptosis | In vitro/in vivo | SD Rat | [74] |
| BMSCs | Mitochondria-related proteins | TXNIP/NLRP3 | Inhibit NPs inflammation and oxidative stress | In vitro/in vivo | Rabbit | [75] |
| BMSCs | miR-410 | NLRP3 | Inhibit NPs pyroptosis | In vitro/in vivo | C57BL/6 | [76] |
| Human placental MSCs (PLMSCs) | AntagomiR-4450 | miR-4450/ZNF121 | Inhibit NPs inflammation and apoptosis | In vitro/in vivo | SD Rat | [81] |
| Human umbilical cord MSCs (ULMSCs) | - | miR-194-5p/TFAM | Inhibit mitochondrial oxidative stress | In vitro/in vivo | SD Rat | [82] |
| NPCs | Rab27a | Notch1 | Promote differentiation of MSCs into NPs | In vitro | - | [78] |
| NPCs | miR-223 | - | Inhibit lumbar neuronal pain | In vitro/in vivo | SD Rat/Human | [79] |
| NPCs | circRNA_0000253 | miRNA-141-5p/SIRT1 | Promote IDD | In vitro/in vivo | SD rat | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Li, F.; Wang, Y.; Pan, W.; Fu, X.; Tan, S. Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside. Pharmaceutics 2025, 17, 313. https://doi.org/10.3390/pharmaceutics17030313
Ding Y, Li F, Wang Y, Pan W, Fu X, Tan S. Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside. Pharmaceutics. 2025; 17(3):313. https://doi.org/10.3390/pharmaceutics17030313
Chicago/Turabian StyleDing, Yifan, Fan Li, Yunyun Wang, Weizhen Pan, Xiangning Fu, and Songwei Tan. 2025. "Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside" Pharmaceutics 17, no. 3: 313. https://doi.org/10.3390/pharmaceutics17030313
APA StyleDing, Y., Li, F., Wang, Y., Pan, W., Fu, X., & Tan, S. (2025). Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside. Pharmaceutics, 17(3), 313. https://doi.org/10.3390/pharmaceutics17030313









