An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans
Abstract
:1. Introduction
1.1. Cannabis
1.2. Cannabis Products
1.3. Epidemiology
1.4. Pharmacokinetics
1.4.1. Absorption
1.4.2. Distribution
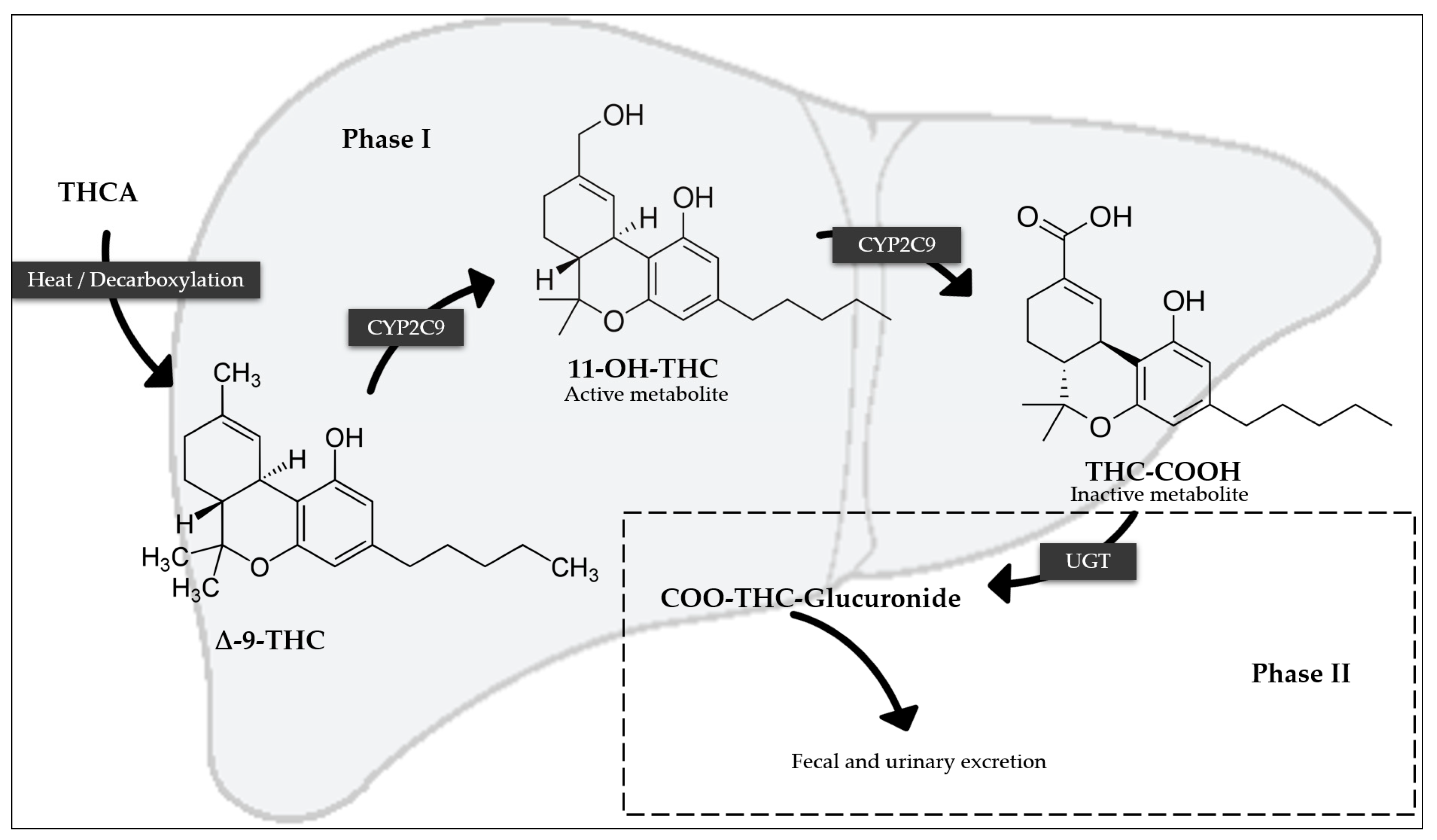
1.4.3. Metabolism
1.4.4. Elimination
1.5. Pharmacogenetics/Pharmacogenomics
1.6. Acute, Chronic Effects and Intoxication
2. Data Search and Selection Methods
- Strong: the interaction is supported by evidence from at least one meta-analysis, systematic review, or a clinical trial (randomized or non-randomized);
- Moderate: the interaction is supported by evidence from at least one observational study (cohort or case-control) or a minimum of three case reports;
- Weak: the interaction is supported by evidence from fewer than three case reports.
- Major: the interaction poses a risk of harm or injury. The parameter variation is 400% or more [Internationalized Normalized Ratio (INR), transaminases, Area Under the Curve (AUC)] or at least 80% (clearance);
- Significant: the interaction requires closer monitoring. The parameter variation is between 100 and 400% (INR, transaminases, AUC) or between 50% and 80% (clearance);
- Minor: the interaction causes little to no harm. The parameter variation ranges between 25% and 100% (INR, transaminases, AUC) or between 20% and 50% (clearance);
- Lack of relevance and/or not applicable (not classified in previous definitions).
3. Cannabis Products’ Drug Interactions
3.1. Interactions with Medicines
3.1.1. Anticoagulants
3.1.2. Antiepileptics
3.1.3. Analgesics
3.1.4. Opioids
3.1.5. Benzodiazepines
3.1.6. Immunosuppressants and Proliferation Inhibitors
3.1.7. Selective Serotonin Reuptake Inhibitors (SSRIs)
3.1.8. Tricyclic Antidepressants
3.1.9. Anti-Infectives
3.1.10. Antiretrovirals
3.1.11. Proton Pump Inhibitors (PPIs)
3.1.12. Psychostimulants
3.1.13. Antipsychotics
3.1.14. Methylxanthine Derivatives
3.2. Interactions with Drugs of Abuse
3.3. Pharmacodynamic Interactions (Medicines and Drugs of Abuse)
| Drug | Cannabinoid and Pharmacokinetics Mechanism | Observed Pharmacokinetic Findings and Effects | Clinical Effects | Recommendation | Risk Rating/PI | Evidence | Clinical Relevance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Anticoagulants | ||||||||
| Warfarin | CBD, Nabilone, Dronabiol: inhibits the CYP2C9 and CYP3A4 enzymes/Highly protein bound | -No clinical trial with cannabis has been conducted. Out of a total of 10 reported cases, 9 describe variations in INR (90%). A maximum INR variation of +0.4 to +9.61 was described in 8 of the 10 reports. | May increase the risk of bleeding. | Adjust warfarin dosage and closely monitor INR. | C/No | Moderate | Major | [62,63,95] |
| Antiepileptics | ||||||||
| Eslicarbazepine | CBD: Unknown | Eslicarbazepine as an object and CBD as a precipitant | Adverse effects were reported without clear evidence linking them to eslicarbazepine | Adjust eslicarbazepine dosage and, if it is possible, monitor plasma levels | C/No | Strong | Minor | [66,96] |
| -In adult subjects, trough concentrations of eslicarbazepine were elevated. The mean baseline concentration was 14.4 ± 7.4 μg/mL, increasing with CBD in subsequent measurements to 16.8 ± 7.9 μg/mL and 17.8 ± 9.1 μg/mL, demonstrating statistically significant changes. -Co-administration of eslicarbazepine and CBD increased eslicarbazepine level by 23% in one patient, while no changes were observed in the other patient. | ||||||||
| Stiripentol | CBD: CYP2C19 inhibition | Stiripentol as an object and CBD as a precipitant | Additive CNS depressant effects | Adjust stiripentol dosage and if it is possible monitor plasma levels | C/Yes | Strong | Minor | [39,65,74] |
| -CBD slight increase in stiripentol levels, with Cmax and the area under the concentration-time curve over the dosing interval (AUCtau) elevated by 2500 ng/mL (32.1%) and 27,000 ng · h/mL (57.6%), respectively. -CBD may increase stiripentol levels by 28% for Cmax and 55% for AUC, potentially leading to diverse adverse effects such as rashes and elevated liver enzymes (AST/ALT). | ||||||||
| CBD as an object and stiripentol as a precipitant medication | ||||||||
| -Minor reductions in CBD major metabolites, 7-OH-CBD which a reduction of 29% and 7-COOH-CBD which decreased by 13%. However, CBD was not affected. | ||||||||
| Valproate | CBD: Unknown/Possible UGT1A9 and UGT2B7 inhibition/Highly protein bound | -AST/ALT levels were significantly higher in participants co-administered with valproate and CBD, with mean AST and ALT levels of 37.1 U/L and 35.3 U/L, respectively. In contrast, participants not taking valproate had lower AST and ALT levels of 23.97 U/L and 23.7 U/L. | May increase ALT and AST levels | Assess liver function before starting CBD and monitor liver function | C/No | Strong | Minor | [66] |
| Analgesics—Opioids | ||||||||
| Buprenorphine | Cannabis recreational: CYP3A4 inhibition | Buprenorphine as an object and CBD as a precipitant | May increase the risk of sedation and somnolence | Adjust buprenorphine dosage and monitor plasma levels of buprenorphine | C/No | Moderate | Significant | [70] |
| -Buprenorphine concentrations were found to be 170% higher in individuals who concurrently use cannabis recreationally. In one case report, a patient experienced a 95% decrease in serum buprenorphine levels upon discontinuing cannabis use. | ||||||||
| Methadone | CBD: CYP3A4 and CYP2C19 inhibition | Methadone as an object and CBD as a precipitant | May increase fatigue and somnolence | Adjust methadone dosage and if it is possible monitor plasma levels | C/No | Weak | Significant | [97] |
| -CBD can inhibit methadone metabolism. The serum methadone levels were measured at 271 ng/mL (2 days after discontinuing CBD), 149 ng/mL (7 days after discontinuing CBD), and 125 ng/mL (14 days after discontinuing CBD) | ||||||||
| Fentanyl | CBD: unknown | CBD as an object and fentanyl as a precipitant medication | Probably not clinically significant | Adjust fentanyl dosage and, if it is possible, monitor plasma levels | C/No | Strong | Lack of relevance | [72] |
| -Plasma concentrations of CBD were not significantly affected. | ||||||||
| Benzodiazepines | ||||||||
| Clobazam | CBD: CYP2C19 inhibition | Clobazam as an object and CBD as a precipitant | May increase the incidence of sedation and somnolence | Adjust clobazam dosage and it is possible, monitor plasma levels | C/Yes | Strong | Minor | [39,53,66,74,75,76,77,78] |
| -CBD increase mean concentrations of N-desmethylclobazam (10–526%). -In the general group, there was a small increase in exposure to steady-state clobazam (Cmax = 20%, AUCτ = 21%) and a notable increase in exposure to N-desmethylclobazam (Cmax = 3.4-fold [239%], AUCτ = 3.4-fold [238%]). In the epilepsy volunteer group, there were no effects on exposure to clobazam, but there was an increase in exposure to N-desmethylclobazam (Cmax = 2.2-fold [122%], AUCτ = 2.6-fold [164%]) -Slight increase in Clobazam exposure, with a trough Cmax of 1.20 ng/mL, and an increase in N-desmethylclobazam exposure, where the mean Cmax increased 3.39-fold. -Elevated levels (3- to 4-fold) of N-desmethylclobazam (substrate of CYP2C19) can occur when combined with CBD. | ||||||||
| CBD as an object and clobazam as a precipitant medication | ||||||||
| -Clobazam may increase exposure to 7-OH-CBD, for which plasma AUC increased by 47%. Clobazam showed a slight increase in CBD exposure, with a trough Cmax of 1.34 ng/mL | ||||||||
| Brivaracetam | CBD: CYP2C19 inhibition | Brivaracetam as an object and CBD as a precipitant | Mild effects, such as diarrhea and somnolence, may occur | Adjust brivaracetam dosage and, if it is possible, monitor plasma levels | C/No | Strong | Significant | [96] |
| -Brivaracetam levels increased by 107% to 280% in patients receiving co-medication of brivaracetam and CBD. | ||||||||
| Immunosuppressants and Proliferation Inhibitors | ||||||||
| Tacrolimus | CBD: CYP3A4 inhibition/Highly protein bound | Tacrolimus as an object and CBD as a precipitant | Nausea, dizziness, and somnolence may appear. Creatinine levels may also increase | Adjust tacrolimus dosage and monitor blood levels of tacrolimus. Dose reduction is warranted when creatinine levels increase | C/No | Strong | Significant | [83,84,85] |
| -A case report showed baseline tacrolimus levels ranged from 3.9 to 8.4 ng/mL. By Day 164, the dose-adjusted tacrolimus level had increased approximately threefold to 13.3 ng/mL. Serum creatinine levels rose to 2.4 mg/dL by Day 124 (baseline 1.2 mg/dL), prompting discontinuation of tacrolimus for a week, after which creatinine levels decreased to 1.5 mg/dL. Attempts to maximize tacrolimus dosage led to a subsequent increase in creatinine levels by Day 282. -Increased tacrolimus’ Cmax 4.2-fold and AUC0-∞ 3.1-fold | ||||||||
| Everolimus | CBD: CYP3A4 inhibition | Everolimus as an object and CBD as a precipitant | May increase the incidence of diarrhea | Adjust everolimus dosage and monitor blood levels | C/No | Moderate | Minor | [79] |
| -Median increase of everolimus AUC by 9.8 ng/mL as compared with baseline | ||||||||
| Sirolimus (convencional) | CBD: CYP3A4 inhibition | Sirolimus as an object and CBD as a precipitant | May increase the incidence of diarrhea | Adjust sirolimus dosage and, if it is possible, monitor blood levels | D/No | Moderate | Minor | [79] |
| -Median increase of sirolimus AUC by 5.1 ng/mL as compared with baseline | ||||||||
| Cyclosporine | CBD: CYP3A4 inhibition/Highly protein bound drugs | Inconclusive findings: -CBD might increase and displace the free fraction of other concomitantly administered protein-bound drugs (not confirmed in vivo). -The levels of cyclosporine are stable in co-administration with CBD | Probably not clinically significant | Adjust cyclosporine dosage and if it is possible monitor blood levels | C/Yes | Strong | Lack of relevance | [44,45,46,83] |
| Selective serotonin reuptake inhibitors (SSRIs) | ||||||||
| Citalopram | CBD: CYP2C19 and CYP3A4 inhibition | Citalopram as an object and CBD as a precipitant | The reported adverse events were mild | Adjust citalopram or escitalopram dosage and, if it is possible, monitor plasma levels | D/No | Moderate | Minor | [86] |
| -Citalopram plasma concentrations increased from baseline (42 ng/mL) to Week 8 (79 ng/mL). | ||||||||
| Tricyclic antidepressants | ||||||||
| Imipramine | Smoked a marijuana cigarette: unknown | -Serum levels were not measured | Disorientation, restlessness, dizziness, and palpitations may appear, suggestive of tricyclic antidepressants toxicity | Adjust imipramine dosage and, if it is possible, monitor plasma levels | C/No | Strong | NA | [53,88] |
| Antipsychotics | ||||||||
| Clozapine | Cannabis and cigarettes: CYP1A2 induction | Clozapine as an object and cannabis and cigarettes as a precipitant | May increase the risk of hallucinations. | Adjust clozapine dosage and if it is possible, monitor plasma levels | C/No | Weak | Significant | [92,98] |
| -In one case report, a patient stopped the consumption of cannabis and cigarettes, and the plasma levels of clozapine increased by 230%. | ||||||||
| Chlorpromazine | Cannabis and cigarettes: CYP1A2 induction | Chlorpromazine as an object and cannabis and cigarettes as a precipitant | May increase the risk of sedation and somnolence | Adjust chlorpromazine dosage and if it is possible, monitor plasma levels | C/No | Strong | Minor | [93] |
| -Increase the clearance of chlorpromazine by 50%, while co-administration of tobacco and cannabis further increases this clearance by 107%. | ||||||||
| Pimozide | CBD: CYP3A4 inhibitors | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May produce potentially serious clinical outcomes, increasing the risk of CNS depression | Avoid combination | X/No | NA | NA | [99] |
| Bromperidol | Nabilone: Unknown | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May produce potentially serious clinical outcomes, increasing the risk of CNS depression | Avoid combination | X/No | NA | NA | [99] |
| Methotrimeprazine | Dronabinol: Unknown | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May increase the risk of CNS depression | Avoid combination | X/No | NA | NA | [99] |
| Anti-infectives | ||||||||
| Ketoconazole | Nabiximols: CYP3A4 inhibition | Nabiximols as an object and ketoconazole as a precipitant | Somnolence, malaise, dizziness, anxiety, and disorientation may appear | Adjust ketoconazole dosage and, if it is possible, monitor plasma levels | C/No | Strong | Minor | [65] |
| -In subjects using THC/CBD oromucosal spray alongside ketoconazole, there were increases in Cmax levels of THC from 2.65 to 3.36 ng/mL, CBD from 0.66 to 1.25 ng/mL, and 11-OH-THC from 3.59 to 10.92 ng/mL. Overall, Cmax levels increased by 36% to 87%. | ||||||||
| Rifampicin | Nabiximols: CYP3A4 induction, CYP2C19 inducers | Nabiximols as an object and rifampicin as a precipitant | Few clinical manifestations; headache can occur | Adjust rifampicin dosage and, if it is possible, monitor plasma levels | C/No | Strong | Minor | [65] |
| -When using THC/CBD oromucosal spray alone, Cmax levels were observed as 2.94 ng/mL for THC, 1.03 ng/mL for CBD, and 3.38 ng/mL for 11-hydroxy-THC (11-OH-THC). With co-administration of THC/CBD spray and rifampicin, Cmax levels decreased to 1.88 ng/mL for THC, 0.50 ng/mL for CBD, and 0.45 ng/mL for 11-OH-THC. Overall, Cmax levels were reduced by 26% to 87%. | ||||||||
| Fusidic acid | Dronabinol, Nabilone: CYP3A4 substrates | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May increase the risk of toxicity of these medications | Modify the regimen of fusidic acid to include aggressive monitoring, empirical adjustments, and consideration of alternative agents | D/No | NA | NA | [99] |
| Fluconazole | Dronabinol: CYP2C9 and CYP3A4 inhibitor | Dronabinol as an object and fluconazole as a precipitant | Can increase cannabis-product-related adverse reactions | Adjust fluconazole dosage and, if it is possible, monitor plasma levels. | C/Yes | Weak | Minor | [39,45,46] |
| -May increase the systemic exposure of dronabinol and/or its active metabolite, increasing THC Cmax by 22% and AUC by 32%. Exposure to the metabolite 11-OH-THC also increased approximately 2.1-fold for Cmax and 2.5-fold for AUC. -The Cmax of CBD increased by approximately 40% with fluconazole. | ||||||||
| Metronidazole | Dronabinol (Syndros®): Unknown | Dronabinol as an object and metronidazole as a precipitant | Disulfiram-like reaction. May produce potentially serious clinical outcomes | Avoid combination | X/Yes | NA | NA | [45] |
| -The liquid formulation (Syndros®) of dronabinol contains alcohol and Metronidazole can inhibit the metabolism of alcohol. | ||||||||
| Itraconazole | Nabilone, dronabinol: Unknown | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | Can increase cannabis-product-related adverse reactions | Adjust itraconazole dosage and, if it is possible, monitor plasma levels | C/No | NA | NA | [99] |
| Fexinidazole | Dronabinol, Nabilone: CYP3A4 substrates | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May produce potentially serious clinical outcomes | Avoid combination | X/No | NA | NA | [99] |
| Ordinazole, Secnidazole | Dronabinol: Unknown | No clinical trials with cannabis have been conducted. Considering the metabolism and theoretical principles, a significant interaction may be possible. | May increase the risk of toxicity of these medications | Avoid combination | X/No | NA | NA | [99] |
| Antiretrovirals | ||||||||
| Indinavir | THC cigarettes: Possible CYP3A4 induction | Indinavir as an object and cannabis and THC cigarettes as a precipitant | Probably not clinically significant | Adjust indinavir dosage and, if it is possible, monitor plasma levels | C/No | Strong | Lack of relevance | [89] |
| -Decrease in Cmax of indinavir by 17.4% (n = 11) | ||||||||
| Nelfinavir | THC cigarettes: Possible CYP3A4 induction | Nelfinavir as an object and cannabis and THC cigarettes as a precipitant | Probably not clinically significant | Adjust nelfinavir dosage and, if it is possible, monitor plasma levels | C/No | Strong | Lack of relevance | [89] |
| -Decrease in Cmax of nelfinavir by 14.1% (n = 14) | ||||||||
| Mehtylxanthine derivates | ||||||||
| Caffeine | CBD: CYP1A2 inhibition | Caffeine as an object and CBD as a precipitant | Diarrhea and elevated liver enzymes (AST, ALT, GGT) may appear | No action needed | B/Yes | Strong | Minor | [39,94] |
| -CBD and caffeine co-administration may increase caffeine exposure by +15% for Cmax and +95% for AUC compared to a single administration of caffeine. | ||||||||
| Proton pump inhibitor (PPIs) | ||||||||
| Omeprazole | THC and CBD: CYP3A4 inhibition | THC and CBD as an object and omeprazole as a precipitant | Dizziness may appear | No action needed | B/No | Strong | Lack of relevance | [61,65] |
| -No significant alteration in plasma levels of THC and CBD. | ||||||||
| Psychostimulants | ||||||||
| Methylphenidate (MPH) | CBD: possible serine hydrolase inhibition | MPH as an object and CBD as a precipitant | Probably not clinically significant | No action needed | B/No | Strong | Lack of relevance | [65,91,100] |
| -The geometric mean ratios (GMR) for AUC from time 0 to infinity (AUCinf) and Cmax with CBD co-administration, compared to MPH monotherapy were 1.08 (0.85, 1.37) and 1.09 (0.89, 1.32), respectively. Tmax was longer for CBD (4 h) than MPH (1.25 h). | ||||||||
| Other central nervous system depressants | ||||||||
| Hexobarbital | CBD: possible CYP3A4 inhibition | Hexobarbital as an object and CBD as a precipitant | May increase the risk of sedation and somnolence | Adjust hexobarbital dosage and, if it is possible, monitor plasma levels | Not found/No | Strong | Minor | [101] |
| -CBD reduced hexobarbital clearance by 35%, compared to when it was not administrated, in subjects who consume recreational cannabis regularly. | ||||||||
| Phenobarbital | Dronabinol: possible CYP3A4, CYP2C9 and CYP2C19 induction | Phenobarbital as an object and CBD as a precipitant | Loss of efficacy of cannabis product | Adjust phenobarbital dosage and, if it is possible, monitor plasma levels | C/Yes | Strong | Lack of relevance | [39,58,66] |
| -In co-administration with CBD, no significant changes were found in plasma concentrations of phenobarbital. | ||||||||
| Dronabinol as an object and phenobarbital as a precipitant | ||||||||
| -May reduce the systemic exposure of dronabinol parent drug and/or its active metabolite | ||||||||
| Propofol | CBD: UGT1A9 and UGT2B7 | Inconclusive findings: -Concentrations of propofol may increase by enzyme inhibition. -Some studies reported that an increased dose is required to induce anesthesia in cannabis users. | May increase the risk of sedation and somnolence | Adjust propofol dosage and, if it is possible, monitor plasma levels | C/Yes | Moderate | NA | [39,102] |
| Other unclassified drugs | ||||||||
| Disulfiram | Dronabinol (Syndros®): Unknown | Dronabinol as an object and Disulfiram as a precipitant | Disulfiram-like reaction. May produce potentially serious clinical outcomes | Avoid combination | X/Yes | NA | NA | [45] |
| -The liquid formulation (Syndros®) of dronabinol contains alcohol, and disulfiram can inhibit the metabolism of alcohol. | ||||||||
| Colchicine, Pralsetinib, Relugolix, Estradiol, Norethindrone, Rimegepant, Tizanidine, Ubrogepant, Venetoclax, Digoxin, Lefamulin, Lemborexant, Afatinib, Berotralstat | CBD: P-glycoprotein/ABCB1 inhibitor | No clinical trials with cannabis have been conducted, but several pharmacokinetic studies have found that the AUC and Cmax of these drugs increase during co-administration with P-glycoprotein inhibitors. | May increase the risk of toxicity of these medications | Modify the regimen of these drugs to include aggressive monitoring, empirical adjustments, and consideration of alternative agents | D/No | NA | NA | [99,103] |
| Bilastine, Doxorubicin (conventional) Pazopanib, Repotrectinib, Topotecan, Vincristine (liposomal) | CBD: P-glycoprotein/ABCB1 inhibitors | No clinical trials with cannabis have been conducted. but several pharmacokinetic studies have found that the AUC and Cmax of these drugs increase during co-administration with P-glycoprotein inhibitors. | May produce potentially serious clinical outcomes | Avoid combination | X/No | NA | NA | [99] |
| Amifostine, Obinutuzumab | Nabilone: Unknown | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible | May increase the risk of toxicity of these medications | Modify the regimen of these drugs to include aggressive monitoring, empirical adjustments, and consideration of alternative agents | D/No | NA | NA | [99] |
| Lomitapide | CBD: CYP3A4 inhibitors | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May increase the risk of toxicity of these medications | Modify the regimen of lomitapide to include aggressive monitoring, empirical adjustments, and consideration of alternative agents | D/No | NA | NA | [99] |
| Cilostazol | CBD: CYP2C19 inhibitors | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May increase the risk of toxicity of these medications | Modify the regimen of cilostazol to include aggressive monitoring, empirical adjustments, and consideration of alternative agents | D/No | NA | NA | [99] |
| Mavacamten | CBD: CYP2C19 inhibitors | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May produce potentially serious clinical outcomes | Avoid combination | X/No | NA | NA | [99] |
| Fezolinetant | CBD: CYP1A2 inhibitors | No clinical trials with cannabis have been conducted. Considering mechanisms, metabolism, and theoretical principles, a significant interaction may be possible. | May produce potentially serious clinical outcomes | Avoid combination | X/No | NA | NA | [99] |
| Drug | Cannabinoid and Pharmacokinetics Mechanism | Observed Pharmacokinetic Findings and Effects | Clinical Effects | References |
|---|---|---|---|---|
| Alcohol | THC, CBN and dronabinol: Unknown | THC as an object and alcohol as a precipitant | May increase HR May increase subjective “like” and its duration, and euphoria May increase subjective “drunkenness” May impair driving lateral control performance May impair horizontal gaze nystagmus and one-leg study May produce additional decremental effects on performance | [104,105,106,107,108,109,110,111] |
| ||||
| Cocaine | Smoked cannabis: Unknown | Cocaine as an object and smoked cannabis as a precipitant | May increase MAP and HR May increase subjective “high” May reduce reaction time and proficiency of impulse control May increase errors May decrease subjective “hunger” and “calm” May reduce the latency to cocaine effects and decrease the duration of dysphoric or bad effects | [112,113,114,115,116] |
| ||||
| Dextroamphetamine | THC: Unknown | No available information | May increase BP and HR May increase tremors, intensity of symptoms, and their duration. May increase subjective “high” May reduce flexibility to closure | [117,118] |
| 3,4-metilendioximetanfetamina (MDMA) | THC: Unknown | No pharmacokinetics modifications | May increase BP, HR, and T May increase subjective drug effects and drug strength May impair task performance (EEG oscillations) | [119,120,121] |
| Methylphenidate (MPH) | CBD: possible serine hydrolase inhibition | No pharmacokinetics modifications (for more information, see the Psychostimulants section of Table 5). | May increase HR and BP May increase subjective “feel drug”, “good effect”, and “take drug again” | [65,100] |
| Nicotine | THC: Unknown | No pharmacokinetics modifications | May increase HR May increased subjective “euphoria” and “high” and its duration | [122,123,124] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (Internet). EU Drug Market: Cannabis—Production. Available online: https://www.emcdda.europa.eu/publications/eu-drug-markets/cannabis/production_en#level-2-section1 (accessed on 1 April 2024).
- Camí, J.; Farré, M. Drug Addiction. N. Engl. J. Med. 2003, 349, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A. Cannabis-Related Disorders and Toxic Effects. N. Engl. J. Med. 2023, 389, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Watson, C.J.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Sainsbury, B.; Bloxham, J.; Pour, M.H.; Padilla, M.; Enciso, R. Efficacy of cannabis-based medications compared to placebo for the treatment of chronic neuropathic pain: A systematic review with meta-analysis. J. Dent. Anesthesia Pain Med. 2021, 21, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC) (Internet). Herbal Cannabis for Medical Use: A Spectrum of Regulatory Approaches. 2023. Available online: https://www.unodc.org/res/WDR-2023/WDR23_B3_CH3_Medical_Cannabis.pdf (accessed on 1 May 2024).
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (Internet). Medical Use of Cannabis and Cannabinoids: Questions and Answers for Policymaking. 2018. Available online: https://www.emcdda.europa.eu/publications/rapid-communications/medical-use-of-cannabis-and-cannabinoids-questions-and-answers-for-policymaking_en (accessed on 12 January 2024).
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine (Internet). Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425767 (accessed on 12 January 2024).
- Food and Drug Administration (FDA) (Internet). Regulation and Quality Considerations for Cannabis and Cannabis-Derived Compounds. Available online: https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/fda-regulation-and-quality-considerations-cannabis-and-cannabis-derived-compounds (accessed on 12 January 2024).
- Arellano, A.L.; Papaseit, E.; Romaguera, A.; Torrens, M.; Farré, M. Neuropsychiatric and General Interactions of Natural and Synthetic Cannabinoids with Drugs of Abuse and Medicines. CNS Neurol. Disord.—Drug Targets 2017, 16, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; Verduin, M.L. (Eds.) Substance Use and Addictive Disorders: Cannabis-Related disorders. In Kaplan & Sadock’s Synopsis of Psychiatry, 12th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 930–941. [Google Scholar]
- Plan Nacional sobre Drogas (PNSD) (National Drug Plan) (Internet). Technical Report on Cannabis. Use Consequences. 2022. Available online: https://pnsd.sanidad.gob.es/profesionales/publicaciones/catalogo/catalogoPNSD/publicaciones/pdf/2022_Technical_Report_Alcohol_2021.pdf (accessed on 12 January 2024).
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- World Health Organization (WHO) (Internet). The Health and Social Effects of Nonmedical Cannabis Use. 2016. Available online: https://www.who.int/publications/i/item/9789241510240 (accessed on 15 January 2024).
- United Nations Office on Drugs and Crime (UNODC) (Internet). World Drug Report 2024. Available online: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2024.html (accessed on 9 May 2024).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (Internet). EU Drug Market: Cannabis—Key Findings and Threat Assessment. Available online: https://www.emcdda.europa.eu/publications/eu-drug-markets/cannabis/key-findings-and-threat-assessment_en (accessed on 1 April 2024).
- Food and Drug Administration (FDA) (Internet). Cannabis Derived Products Data Acceleration Plan. Available online: https://www.fda.gov/media/153183/download (accessed on 2 April 2024).
- Matson, T.E.; Carrell, D.S.; Bobb, J.F.; Cronkite, D.J.; Oliver, M.M.; Luce, C.; Ghitza, U.E.; Hsu, C.W.; Campbell, C.I.; Browne, K.C.; et al. Prevalence of Medical Cannabis Use and Associated Health Conditions Documented in Electronic Health Records Among Primary Care Patients in Washington State. JAMA Netw. Open 2021, 4, e219375. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Chan, G.; Stjepanović, D.; Chung, J.Y.C.; Hall, W.; Hammond, D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology 2022, 239, 1509–1519. [Google Scholar] [CrossRef]
- Mahabir, V.K.; Merchant, J.J.; Smith, C.; Garibaldi, A. Medical cannabis use in the United States: A retrospective database study. J. Cannabis Res. 2020, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.G.; Marti, C.N.; Choi, B.Y. Prevalence of cannabidiol use and correlates in U.S. adults. Drug Alcohol Depend. Rep. 2024, 13, 100289. [Google Scholar] [CrossRef] [PubMed]
- Chapin, M.R.; Kane-Gill, S.L.; Li, X.; Abanyie, K.; Taneja, S.B.; Egbert, S.; Paine, M.F.; Boyce, R.D. Part 1: Evaluation of Pediatric Cannabis–Drug Interaction Reports. Pharmacol. Res. Perspect. 2025, 13, e70046. [Google Scholar] [CrossRef]
- Chapin, M.R.; Kane-Gill, S.L.; Li, X.; Abanyie, K.; Taneja, S.B.; Egbert, S.; Paine, M.F.; Boyce, R.D. Part 2: Drug Interactions Involving Cannabis Products in Persons Aged 18 and Over: A Summary of Published Case Reports and Analysis of the FDA Adverse Event Reporting System. Pharmacol. Res. Perspect. 2025, 13, e70047. [Google Scholar] [CrossRef]
- Bardhi, K.; Coates, S.; Watson, C.J.; Lazarus, P. Cannabinoids and drug metabolizing enzymes: Potential for drug-drug interactions and implications for drug safety and efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.J.; Maharao, N.; Patilea-Vrana, G.; Unadkat, J.D.; Rettie, A.E.; McCune, J.S.; Paine, M.F. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019, 201, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Health Canada: Government of Canada (Internet). Information for Health Care Professionals: Cannabis (Marihuana, Mariju-Ana) and the Cannabinoids. 2018. Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html (accessed on 20 September 2024).
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human skin permeation of Δ8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Valiveti, S.; Hammell, D.C.; Earles, D.C.; Stinchcomb, A.L. In vitro/in vivo correlation studies for transdermal Δ8-THC development. J. Pharm. Sci. 2004, 93, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- McGilveray, I.J. Pharmacokinetics of Cannabinoids. Pain Res. Manag. 2005, 10, 15A–22A. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2021, 25, 19.200. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española del Medicamento y Productos Sanitarios (AEMPS) (Spanish Agency for Medicines and Health Products) (Internet) Product Information Sativex®, Epidyolex®. Available online: https://cima.aemps.es/cima/publico/home.html (accessed on 12 January 2024).
- Huestis, M.A.; Cone, E.J. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J. Anal. Toxicol. 1998, 22, 445–454. [Google Scholar] [CrossRef]
- Heuberger, J.A.A.C.; Guan, Z.; Oyetayo, O.-O.; Klumpers, L.; Morrison, P.D.; Beumer, T.L.; van Gerven, J.M.A.; Cohen, A.F.; Freijer, J. Population Pharmacokinetic Model of THC Integrates Oral, Intravenous, and Pulmonary Dosing and Characterizes Short- and Long-term Pharmacokinetics. Clin. Pharmacokinet. 2015, 54, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef]
- Wright, J.A.; Huang, L.; Katamesh, B.E.; Yadav, S.; Singla, A.; Vincent, A. Hypothesized pharmacogenomic and medication influences on tetrahydrocannabinol and cannabidiol metabolism in a cohort of unselected oral cannabis users. J. Cannabis Res. 2025, 7, 1. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA) (Internet). Label Cesamet®, Syndros®, Marinol®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf (accessed on 12 January 2024).
- Food and Drug Administration (FDA) (Internet). Label Syndros®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/205525Orig1s009lbledt.pdf (accessed on 12 January 2024).
- Food and Drug Administration (FDA) (Internet). Label Marinol®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 12 January 2024).
- Grotenhermen, F.; Müller-Vahl, K. The therapeutic potential of cannabis and cannabinoids. Dtsch. Arztebl. Int. 2012, 109, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.C.; Abramovici, H.; Harris, C.S. Cannabis and Cannabinoids: Kinetics and Interactions. Am. J. Med. 2019, 132, 1266–1270. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM V; American Psychiatric Association: Washington, DC, USA, 2013; pp. 509–519. [Google Scholar]
- Chu, F.; Cascella, M. Cannabinoid Hyperemesis Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549915 (accessed on 3 July 2024).
- Lawn, W.; Trinci, K.; Mokrysz, C.; Borissova, A.; Ofori, S.; Petrilli, K.; Bloomfield, M.; Haniff, Z.R.; Hall, D.; Fernandez-Vinson, N.; et al. The acute effects of cannabis with and without cannabidiol in adults and adolescents: A randomised, double-blind, placebo-controlled, crossover experiment. Addiction 2023, 118, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Vandrey, R.; Budney, A.; Hughes, J.; Liguori, A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008, 92, 48–54. [Google Scholar] [CrossRef]
- Nachnani, R.; Knehans, A.; Neighbors, J.D.; Kocis, P.T.; Lee, T.; Tegeler, K.; Trite, T.; Raup-Konsavage, W.M.; Vrana, K.E. Systematic review of drug-drug interactions of delta-9-tetrahydrocannabinol, cannabidiol, and Cannabis. Front. Pharmacol. 2024, 15, 1282831. [Google Scholar] [CrossRef] [PubMed]
- Lopera, V.; Rodríguez, A.; Amariles, P. Clinical Relevance of Drug Interactions with Cannabis: A Systematic Review. J. Clin. Med. 2022, 11, 1154. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Wang, J.-S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; DeVane, C.L. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Kocis, P.T.; Vrana, K.E. Delta-9-Tetrahydrocannabinol and Cannabidiol Drug-Drug Interactions. Med. Cannabis Cannabinoids 2020, 3, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Zamarripa, C.A.; Spindle, T.R.; Surujunarain, R.; Weerts, E.M.; Bansal, S.; Unadkat, J.D.; Paine, M.F.; Vandrey, R. Assessment of Orally Administered Δ9-Tetrahydrocannabinol When Coadministered with Cannabidiol on Δ9-Tetrahydrocannabinol Pharmacokinetics and Pharmacodynamics in Healthy Adults: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2254752. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.; Kulpa, J.; Paulionis, L. Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs. Front. Veter. Sci. 2020, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Arkell, T.R.; Kevin, R.C.; Stuart, J.; Lintzeris, N.; Haber, P.S.; Ramaekers, J.G.; McGregor, I.S. Detection of Δ9 THC in oral fluid following vaporized cannabis with varied cannabidiol (CBD) content: An evaluation of two point-of-collection testing devices. Drug Test. Anal. 2019, 11, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Ertl, N.; Freeman, T.P.; Mokrysz, C.; Ofori, S.; Borissova, A.; Petrilli, K.; Curran, H.V.; Lawn, W.; Wall, M.B. Acute effects of different types of cannabis on young adult and adolescent resting-state brain networks. Neuropsychopharmacology 2024, 49, 1640–1651. [Google Scholar] [CrossRef]
- MacDonald, E.; Adams, A. The Use of Medical Cannabis with Other Medications: A Review of Safety and Guidelines—An Update (Internet): Canadian Agency for Drugs and Technologies in Health. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549545/ (accessed on 8 June 2024).
- Damkier, P.; Lassen, D.; Christensen, M.M.H.; Madsen, K.G.; Hellfritzsch, M.; Pottegård, A. Interaction between warfarin and cannabis. Basic Clin. Pharmacol. Toxicol. 2019, 124, 28–31. [Google Scholar] [CrossRef]
- Smythe, M.A.; Wu, W.; Garwood, C.L. Anticoagulant drug–drug interactions with cannabinoids: A systematic review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 1327–1338. [Google Scholar] [CrossRef]
- Treyer, A.; Reinhardt, J.K.; Eigenmann, D.E.; Oufir, M.; Hamburger, M. Phytochemical Comparison of Medicinal Cannabis Extracts and Study of Their CYP-Mediated Interactions with Coumarinic Oral Anticoagulants. Med. Cannabis Cannabinoids 2023, 6, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Stott, C.; White, L.; Wright, S.; Wilbraham, D.; Guy, G. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. SpringerPlus 2013, 2, 236. [Google Scholar] [CrossRef]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; The Uab Cbd Program. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.Y.; Goh, C.; Leong, C.S.A.; Ng, K.Y.; Bakhtiar, A. Evaluation of potential drug–drug interactions with medical cannabis. Clin. Transl. Sci. 2024, 17, e13812. [Google Scholar] [CrossRef]
- Critchley, D.; Szaflarski, J.; Patsalos, P.; Gidal, B.; VanLandingham, K.; Morrison, G. Drug-drug Interaction Studies with Coadministration of Cannabidiol (CBD) and Clobazam, Valproate, Stiripentol or Midazolam in Healthy Volunteers and Adults with Epilepsy. Epilepsy Behav. 2019, 101, 106735. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Jones, R.T. Effects of delta-9-tetrahydrocannabinol on drug distribution and metabolism Antipyrine, pentobarbital, and ethanol. Clin. Pharmacol. Ther. 1977, 22, 259–268. [Google Scholar] [CrossRef]
- Vierke, C.; Marxen, B.; Boettcher, M.; Hiemke, C.; Havemann-Reinecke, U. Buprenorphine–cannabis interaction in patients undergoing opioid maintenance therapy. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Couey, P.; Shade, S.B.; Kelly, M.E.; Benowitz, N.L. Cannabinoid–Opioid Interaction in Chronic Pain. Clin. Pharmacol. Ther. 2011, 90, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Manini, A.F.; Yiannoulos, G.; Bergamaschi, M.M.; Hernandez, S.; Olmedo, R.; Barnes, A.J.B.; Winkel, G.; Sinha, R.; Jutras-Aswad, D.; Huestis, M.A.; et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J. Addict. Med. 2015, 9, 204–210. [Google Scholar] [CrossRef]
- Schulze Westhoff, M.S.; Massarou, C.; Bleich, S.; Heck, J.; Jendretzky, K.F.; Glahn, A.; Schröder, S. Drug interactions in a sample of inpatients diagnosed with cannabis use disorder. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; GWPCARE1 Part A Study Group; et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical implications of trials investigating drug-drug interactions between cannabidiol and enzyme inducers or inhibitors or common antiseizure drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef] [PubMed]
- VanLandingham, K.E.; Crockett, J.; Taylor, L.; Morrison, G. A Phase 2, Double-Blind, Placebo-Controlled Trial to Investigate Potential Drug-Drug Interactions Between Cannabidiol and Clobazam. J. Clin. Pharmacol. 2020, 60, 1304–1313. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Agricola, K.D.; Tudor, C.; Krueger, D.; Franz, D.N. Cannabidiol Elevates Mechanistic Target of Rapamycin Inhibitor Levels in Patients With Tuberous Sclerosis Complex. Pediatr. Neurol. 2020, 105, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.; Berwaerts, J.; Critchley, D.; Hyland, K.; Chen, C.; Thai, C.; Tayo, B. Pharmacokinetic Drug-Drug Interaction With Coadministration of Cannabidiol and Everolimus in a Phase 1 Healthy Volunteer Trial. Clin. Pharmacol. Drug Dev. 2023, 12, 911–919. [Google Scholar] [CrossRef]
- Moadel, D.; Chism, K. Medical Marijuana-Induced Tacrolimus Toxicity. Psychosomatics 2019, 60, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Hauser, N.; Sahai, T.; Richards, R.; Roberts, T. High on Cannabis and Calcineurin Inhibitors: A Word of Warning in an Era of Legalized Marijuana. Case Rep. Transplant. 2016, 2016, 4028492. [Google Scholar] [CrossRef]
- Cuñetti, L.; Manzo, L.; Peyraube, R.; Arnaiz, J.; Curi, L.; Orihuela, S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transplant. Proc. 2018, 50, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Leino, A.D.; Emoto, C.; Fukuda, T.; Privitera, M.; Vinks, A.A.; Alloway, R.R. Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am. J. Transplant. 2019, 19, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- So, G.C.; Lu, J.B.L.; Koyama, S.; Cheng, Y.; Gisch, D.L.; McClara, K.; Dexter, P.R.; Sharfuddin, A.A.; Etkins, J.; Tillman, E.M.; et al. A Phase I Trial of the Pharmacokinetic Interaction Between Cannabidiol and Tacrolimus. Clin. Pharmacol. Ther. 2024, 117, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Doohan, P.T.B.; Oldfield, L.; Kevin, R.C.; Arnold, J.C.; Berger, M.; Amminger, G.P.; McGregor, I.S. Citalopram and Cannabidiol. J. Clin. Psychopharmacol. 2021, 41, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Biederman, J.; Spencer, T.J. Case study: Adverse effects of smoking marijuana while receiving tricyclic antidepressants. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kizer, K.W. Possible interaction of TCA and marijuana. Ann. Emerg. Med. 1980, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Kosel, B.W.; Aweeka, F.T.; Benowitz, N.L.; Shade, S.B.; Hilton, J.F.; Lizak, P.S.; Abrams, D.I. The effects of cannabinoids on the pharmacokinetics of indinavir and nelfinavir. AIDS 2002, 16, 543–550. [Google Scholar] [CrossRef]
- Drugs.com: Drugs Interaction Checker 2021. Available online: https://www.drugs.com/drug_interactions.html (accessed on 2 September 2024).
- Markowitz, J.S.; De Faria, L.; Zhang, Q.; Melchert, P.W.; Frye, R.F.; Klee, B.O.; Qian, Y. The Influence of Cannabidiol on the Pharmacokinetics of Methylphenidate in Healthy Subjects. Med. Cannabis Cannabinoids 2022, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Preston, C. Stockley’s Drug Interactions, 12th ed.; Pharmaceutical Press: London, UK, 2019. [Google Scholar]
- Chetty, M.; Miller, R.; Moodley, S.V. Smoking and body weight influence the clearance of chlorpromazine. Eur. J. Clin. Pharmacol. 1994, 46, 523–526. [Google Scholar] [CrossRef]
- Thai, C.; Tayo, B.; Critchley, D. A Phase 1 Open-Label, Fixed-Sequence Pharmacokinetic Drug Interaction Trial to Investigate the Effect of Cannabidiol on the CYP1A2 Probe Caffeine in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2021, 10, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Udomuksorn, W.; Saowaneepitak, N.; Dolthammasiri, P.; Ruanglertboon, W.; Limsuwanchote, S.; Wiriyapongsukit, S.; Tungsukruthai, S. Unveiling the impact of water-boiled cannabis on warfarin: A case report of atrial fibrillation patients after cannabis legalization in Thailand. Toxicol. Rep. 2024, 13, 101838. [Google Scholar] [CrossRef] [PubMed]
- Klotz, K.A.; Grob, D.; Hirsch, M.; Metternich, B.; Schulze-Bonhage, A.; Jacobs, J. Efficacy and Tolerance of Synthetic Cannabidiol for Treatment of Drug Resistant Epilepsy. Front. Neurol. 2019, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.; Tanco, K.; Bruera, E. Clinically Significant Drug-Drug Interaction Between Methadone and Cannabidiol. Pediatrics 2020, 145, e20193256. [Google Scholar] [CrossRef] [PubMed]
- Zullino, D.; Delessert, D.; Eap, C.; Preisig, M.; Baumann, P. Tobacco and cannabis smoking cessation can lead to intoxication with clozapine or olanzapine. Int. Clin. Psychopharmacol. 2002, 17, 141–143. [Google Scholar] [CrossRef] [PubMed]
- UpToDate. Drug Interactions, Search Results in UpToDate; UpToDate, Inc.: Waltham, MA, USA, 2024; Available online: https://www.uptodate.com (accessed on 2 September 2024).
- Kollins, S.H.; Schoenfelder, E.N.; English, J.S.; Holdaway, A.; Van Voorhees, E.; O’brien, B.R.; Dew, R.; Chrisman, A.K. An exploratory study of the combined effects of orally administered methylphenidate and delta-9-tetrahydrocannabinol (THC) on cardiovascular function, subjective effects, and performance in healthy adults. J. Subst. Abus. Treat. 2015, 48, 96–103. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Nguyen, T.-L.; Jones, R.T.; Herning, R.I.; Bachman, J. Metabolic and psychophysiologic studies of cannabidiol-hexobarbital interaction. Clin. Pharmacol. Ther. 1980, 28, 115–120. [Google Scholar] [CrossRef]
- Imasogie, N.; Rose, R.V.; Wilson, A. High quantities: Evaluating the association between cannabis use and propofol anesthesia during endoscopy. PLoS ONE 2021, 16, e0248062. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA) (Internet). Colchicine (Colcrys®), Label Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022351lbl.pdf (accessed on 12 September 2024).
- Hartman, R.L.; Brown, T.L.; Milavetz, G.; Spurgin, A.; Pierce, R.S.; Gorelick, D.A.; Gaffney, G.; Huestis, M.A. Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend. 2015, 154, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Bosker, W.M.; Theunissen, E.L.; Conen, S.; Kuypers, K.P.C.; Jeffery, W.K.; Walls, H.C.; Kauert, G.F.; Toennes, S.W.; Moeller, M.R.; Ramaekers, J.G. A placebo-controlled study to assess Standardized Field Sobriety Tests performance during alcohol and cannabis intoxication in heavy cannabis users and accuracy of point of collection testing devices for detecting THC in oral fluid. Psychopharmacology 2012, 223, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, J.G.; Theunissen, E.L.; de Brouwer, M.; Toennes, S.W.; Moeller, M.R.; Kauert, G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology 2011, 214, 391–401. [Google Scholar] [CrossRef]
- Lenné, M.G.; Dietze, P.M.; Triggs, T.J.; Walmsley, S.; Murphy, B.; Redman, J.R. The effects of cannabis and alcohol on simulated arterial driving: Influences of driving experience and task demand. Accid. Anal. Prev. 2010, 42, 859–866. [Google Scholar] [CrossRef]
- Chait, L.D.; Perry, J.L. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology 1994, 115, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.J.; Brown, B.; Haegerstrom-Portnoy, G.; Flom, M.C.; Jones, R.T. Marijuana, alcohol, and combined drug effects on the time course of glare recovery. Psychopharmacology 1978, 56, 81–86. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Hicks, R.E.; Bumberry, J.; Jeffcoat, A.R.; Cook, C.E. Interaction between marihuana and ethanol: Effects on psychomotor performance. Alcohol. Clin. Exp. Res. 1988, 12, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Toennes, S.W.; Schneider, K.; Kauert, G.F.; Wunder, C.; Moeller, M.R.; Theunissen, E.L.; Ramaekers, J.G. Influence of ethanol on cannabinoid pharmacokinetic parameters in chronic users. Anal. Bioanal. Chem. 2010, 400, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Foltin, R.W.; Fischman, M.W.; Pedroso, J.J.; Pearlson, G.D. Marijuana and cocaine interactions in humans: Cardiovascular consequences. Pharmacol. Biochem. Behav. 1987, 28, 459–464. [Google Scholar] [CrossRef]
- Van Wel, J.H.P.; Kuypers, K.P.C.; Theunissen, E.L.; Toennes, S.W.; Spronk, D.B.; Verkes, R.J.; Ramaekers, J.G. Single doses of THC and cocaine decrease proficiency of impulse control in heavy cannabis users. Br. J. Pharmacol. 2013, 170, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Foltin, R.W.; Fischman, M.W. Effects of the combination of cocaine and marijuana on the task-elicited physiological response. NIDA Res. Monogr. 1989, 95, 359–360. [Google Scholar] [PubMed]
- Murray, C.H.; Haney, M.; Foltin, R.W.; Manubay, J.; Bedi, G.; Cooper, Z.D. Smoked cannabis reduces peak cocaine plasma levels and subjective effects in a controlled drug administration study of polysubstance use in men. Drug Alcohol Depend. 2022, 243, 109757. [Google Scholar] [CrossRef] [PubMed]
- Lukas, S.E.; Sholar, M.; Kouri, E.; Fukuzako, H.; Mendelson, J.H. Marihuana smoking increases plasma cocaine levels and subjective reports of euphoria in male volunteers. Pharmacol. Biochem. Behav. 1994, 48, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Martz, R.; Rodda, B.E.; Lemberger, L.; Forney, R.B. Effects of marihuana-dextroamphetamine combination. Clin. Pharmacol. Ther. 1976, 20, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, S.; Liskow, B.; Cadoret, R.; Goodwin, D. Marijuana and amphetamine: The question of interaction. Am. J. Psychiatry 1973, 130, 707–708. [Google Scholar] [CrossRef]
- Dumont, G.J.; Kramers, C.; Sweep, F.C.; Touw, D.J.; van Hasselt, J.G.; de Kam, M.; van Gerven, J.M.; Buitelaar, J.K.; Verkes, R.J. Cannabis coadministration potentiates the effects of “ecstasy” on heart rate and temperature in humans. Clin. Pharmacol. Ther. 2009, 86, 160–166. [Google Scholar] [CrossRef]
- Lansbergen, M.M.; Dumont, G.J.H.; van Gerven, J.M.A.; Buitelaar, J.K.; Verkes, R.-J. Acute effects of MDMA (3,4-methylenedioxymethamphetamine) on EEG oscillations: Alone and in combination with ethanol or THC (delta-9-tetrahydrocannabinol). Psychopharmacology 2011, 213, 745–756. [Google Scholar] [CrossRef]
- Dumont, G.; van Hasselt, J.; de Kam, M.; van Gerven, J.; Touw, D.; Buitelaar, J.; Verkes, R. Acute psychomotor, memory and subjective effects of MDMA and THC co-administration over time in healthy volunteers. J. Psychopharmacol. 2011, 25, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Penetar, D.M.; Kouri, E.M.; Gross, M.M.; McCarthy, E.M.; Rhee, C.K.; Peters, E.N.; Lukas, S.E. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 2005, 79, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; Mensinga, T.T.; de Vries, I.; Kelholt-Dijkman, H.H.; Hoek, J.; Kruidenier, M.; Leenders, M.E.C.; Meulenbelt, J. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology 2008, 201, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; Mensinga, T.T.; Böcker, K.B.E.; Schipper, C.M.A.; Kruidenier, M.; Leenders, M.E.C.; de Vries, I.; Meulenbelt, J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology 2009, 204, 85–94. [Google Scholar] [CrossRef] [PubMed]

| Cannabis-Based Products (Cannabis Preparations) | ||||
|---|---|---|---|---|
| No license for specific medical indications To use off-license for medicinal purposes Recommended quality control practices Approach to regulated cannabis-based products for medical use vary widely between countries | ||||
| Raw Cannabis | Compounding Preparations | Cannabis-Based Products with Unspecified Composition | Standardized Cannabis Based Medical Products * | |
|
Unheated or “non-activated” cannabis | Patient-specific products based on a physician’s prescription prepared by a pharmacist | Flowers Tincture Chewable Lozenge Oil Infusions Cream Crystal Sublingual drop Balm Salve Lotion Spray Ointment | Inflorescences | Bedrocan® (THC approx. 22%, CBD < 1%) Bedica® (THC approx. 14%, CBD < 1%) Bedrobinol® (THC approx. 13.5%, CBD < 1%) FM1 (THC 13–20%, CBD < 1%) FM2 (THC 5–8%, CBD7–12%) Bediol® (THC 6.3%, CBD 8%) Bedrolite® (THC < 1%, CBD 9%) Tilray THC 25® (THC 25%, CBD < 1%) Tilray THC 10® (THC 10%, CBD 10%) |
| Cannabis extract diluted in oil | T10/C2® (THC 10%, CBD 2%) T15/C3® (THC 15%, CBD3%) T10/C10® (THC 10%, CBD 10%) T5/C10® (THC 5%, CBD 10%) T3/C15® (THC 3%, CBD15%) T1/C20® (THC1%, CBD 20%) | |||
| Prescription Drugs | |||||
|---|---|---|---|---|---|
| Terminology | Cannabis-Derived Products | Cannabis-Related Products | |||
| Origin | Compounds occurring naturally in the plant that are extracted directly from the plant | Synthetic compounds created in a laboratory and can be used to manufacture drug products | |||
| Brand name | Epidiolex® | Sativex® | Marinol® | Syndros® | Cesamet® |
| Active principle/substance | CBD | Nabiximols (THC: CBD equal quantities) | Dronabinol | Dronabinol | Nabilone |
| Pharmaceutical form | Oral solution | Oromucosal spray solution | Oral capsules (Marinol®, Cesamet®) Oral solution (Syndros®) | ||
| Indication | Patients 2 years of age or older with Dravet syndrome. Seizures associated with Lennox–Gastaut syndrome. | Muscle spasticity associated with multiple sclerosis. | Anorexia associated with weight loss in patients with acquired immunodeficiency syndrome. Vomiting and nausea associated with cancer chemotherapy in patients who have failed to respond to conventional treatments. | ||
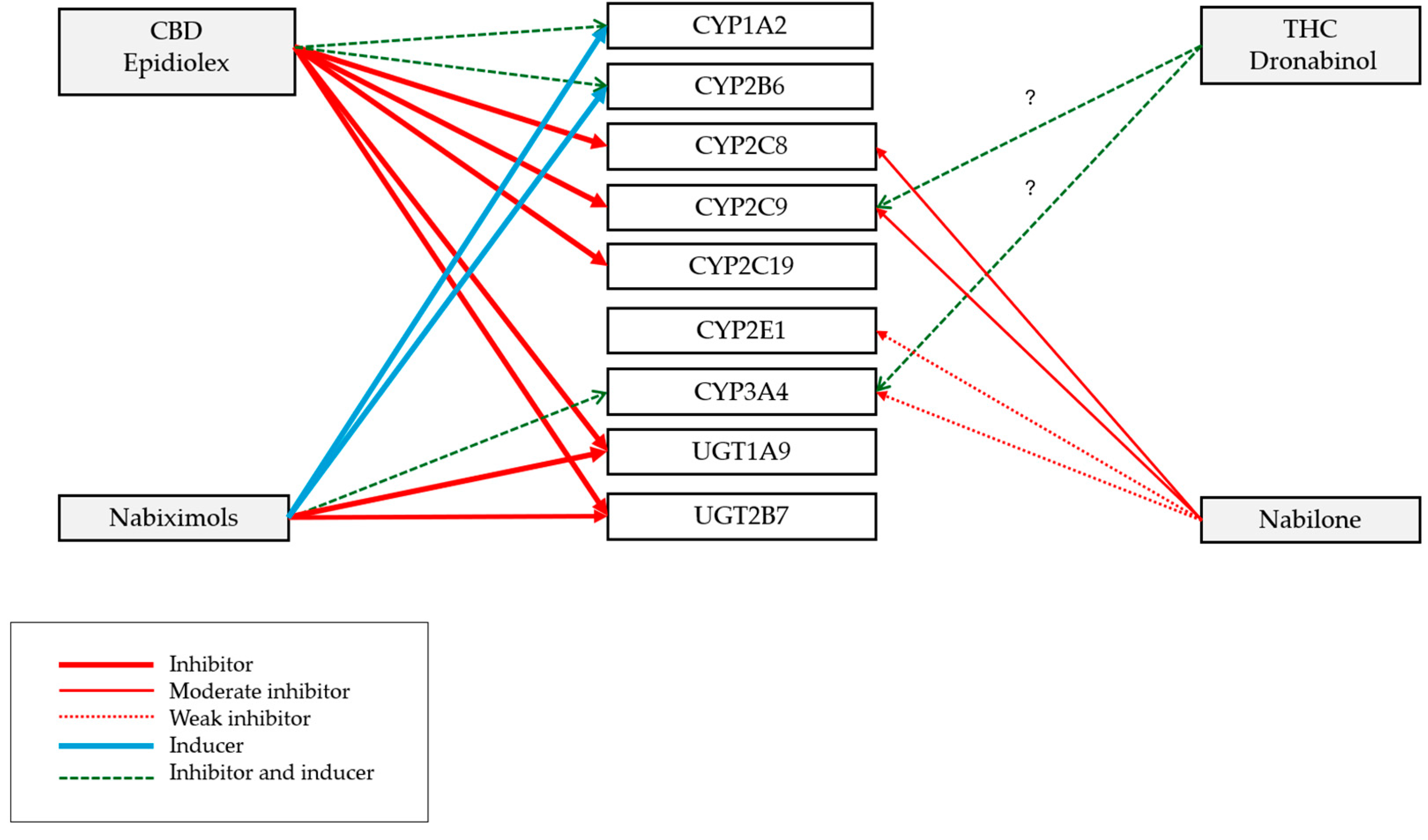
| Cannabinoid (Precipitant) | CBD (Epidiolex®) | Nabiximols (Sativex®, THC: CBD Equal Quantities) | Dronabinol (Marinol® and Syndros®, Synthetic Form of THC) | Nabilone (Cesamet®, Synthetic Cannabinoid) |
|---|---|---|---|---|
| Can affect the metabolism of substrate medication (object) with a NTI | Inhibits and induces: CYP1A2 and CYP2B6 substrates Inhibits: CYP2C9, CYP2C19, CYP2C8, UGT1A9, and UGT2B7 substrates | Inhibits: CYP3A4, UGT1A9, UGT2B7 substrates Induces: CYP2B6, CYP1A2, and CYP3A4 substrates | CYP2C9 and CYP3A4 substrates | Weak inhibitor: CYP3A4 and CYP2E1 substrates Moderate inhibitor: CYP2C8 and CYP2C9 substrates |
| Medication with Narrow Therapeutic Index (NTI) | |||
|---|---|---|---|
| Acenocuomarol | Dihydroergotamine | Mephenytoin | |
| Alfentanil | Diphenadione | Mycophenolic acid | |
| Aminophylline | Dofetilide | Nortriptyline | |
| Amiodarone | Dosulepin | Paclitaxel | |
| Amitriptyline | Doxepin | Phenobarbital | |
| Amphotericin B | Ergotamine | Phenprocoumon | |
| Argatroban | Esketamine | Phenytoin | |
| Busulfan | Ethinyl estradiol (oral contraceptives) | Pimozide | |
| Carbamazepine | Ethosuximide | Propofol | |
| Clindamycin | Ethyl biscoumacetate | Quinidine | |
| Clomipramine | Everolimus | Sirolimus | |
| Clonidine | Fentanyl | Tacrolimus | |
| Clorindione | Fluindione | Temsirolimus | |
| Cyclobenzaprine | Fosphenytoin | Theophylline | |
| Cyclosporine | Imipramine | Thiopental | |
| Dabigatran etexilate | Levothyroxine | Tianeptine | |
| Desipramine | Lofepramine | Trimipramine | |
| Dicoumarol | Melitracen | Valproic acid | |
| Digitoxin | Meperidine | Warfarin | |
| Cannabinoid Medication with Protein Binding ≥ 85% * | |||
| Cannabidiol | Nabilone | ||
| Dronabinol | Nabiximols | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, D.A.; Pérez-Mañá, C.; Farré, M.; Papaseit, E. An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans. Pharmaceutics 2025, 17, 319. https://doi.org/10.3390/pharmaceutics17030319
Caicedo DA, Pérez-Mañá C, Farré M, Papaseit E. An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans. Pharmaceutics. 2025; 17(3):319. https://doi.org/10.3390/pharmaceutics17030319
Chicago/Turabian StyleCaicedo, Dolly Andrea, Clara Pérez-Mañá, Magí Farré, and Esther Papaseit. 2025. "An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans" Pharmaceutics 17, no. 3: 319. https://doi.org/10.3390/pharmaceutics17030319
APA StyleCaicedo, D. A., Pérez-Mañá, C., Farré, M., & Papaseit, E. (2025). An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans. Pharmaceutics, 17(3), 319. https://doi.org/10.3390/pharmaceutics17030319







