Cyclodextrin-Based Drug Delivery Systems for Depression: Improving Antidepressant Bioavailability and Targeted Central Nervous System Delivery
Abstract
:1. Introduction
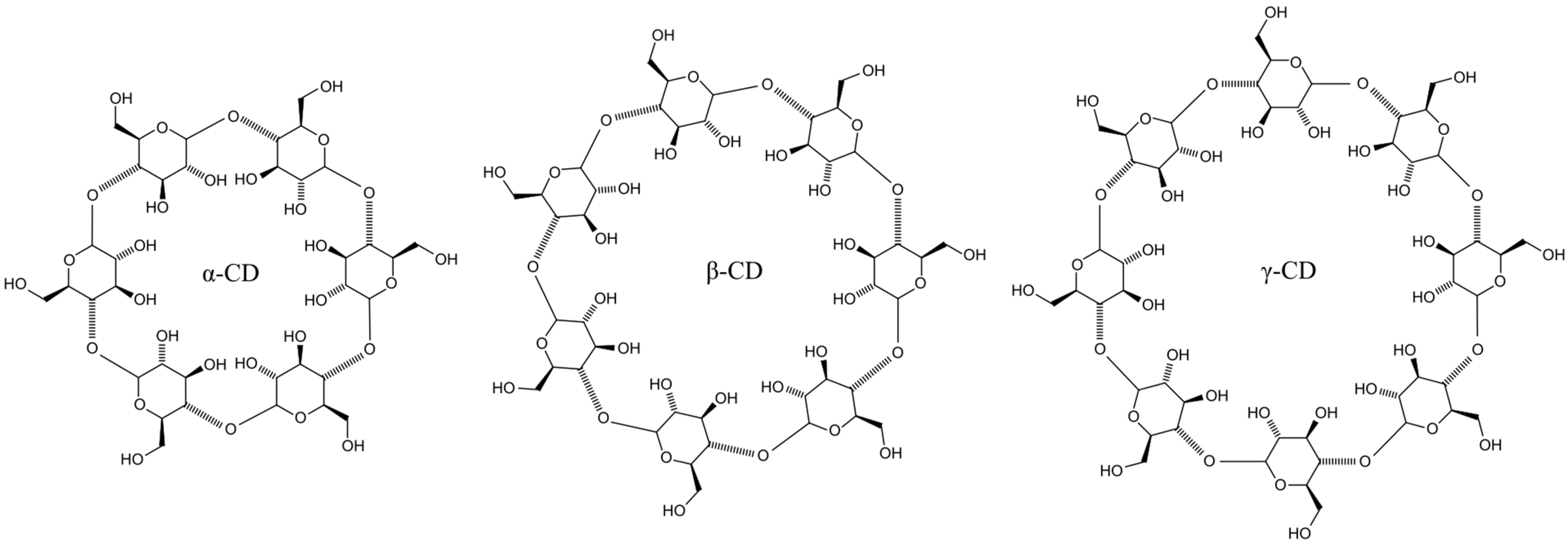
2. CDs
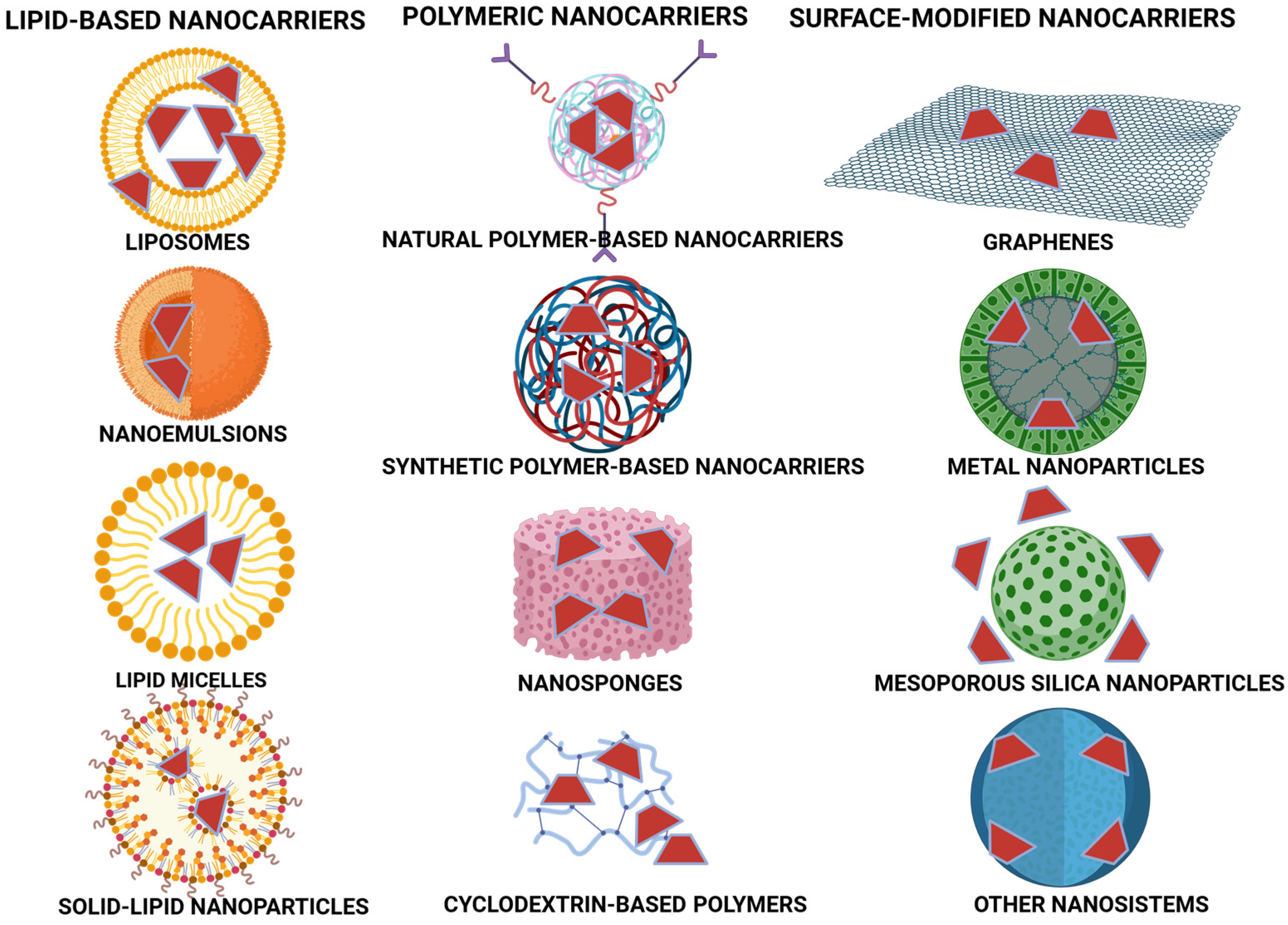
2.1. Comparison of CDs with Other Drug Delivery Systems
2.2. Molecular Mechanisms of CD-Antidepressant Complexation
2.3. Cyclodextrin-Based Controlled-Release Strategies in Antidepressant Therapy
3. Factors Affecting Depression
4. Overview of Different Antidepressant Drugs Combined with CDs
4.1. TCAs
4.2. SSRIs
4.3. Atypical Antidepressants
4.4. Other Antidepressant Drugs
4.5. Agents with Antidepressant Proprieties
4.6. Clinical Implications and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Depression. 2019. Available online: https://applications.emro.who.int/docs/EMRPUB_leaflet_2019_mnh_219_en.pdf?ua=1&ua=1 (accessed on 18 February 2025).
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Fellinger, M.; Waldhör, T.; Serretti, A.; Hinterbuchinger, B.; Pruckner, N.; König, D.; Gmeiner, A.; Vyssoki, S.; Vyssoki, B.; Fugger, G. Seasonality in major depressive disorder: Effect of sex and age. J. Affect. Disord. 2022, 296, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Zhang, G.-Z.; Li, B.; Li, M.; Woelfer, M.; Walter, M.; Wang, L. Role of inflammation in depression relapse. J. Neuroinflamm. 2019, 16, 90. [Google Scholar] [CrossRef]
- Rice, F.; Riglin, L.; Lomax, T.; Souter, E.; Potter, R.; Smith, D.J.; Thapar, A.K.; Thapar, A. Adolescent and adult differences in major depression symptom profiles. J. Affect. Disord. 2019, 243, 175–181. [Google Scholar] [CrossRef]
- Albert, K.M.; Newhouse, P.A. Estrogen, stress, and depression: Cognitive and biological interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef]
- Rogers, J.P.; Watson, C.J.; Badenoch, J.; Cross, B.; Butler, M.; Song, J.; Rooney, A.G. Neurology and neuropsychiatry of COVID-19: A systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J. Neurol. Neurosurg. Psychiatry. 2021, 92, 932–941. [Google Scholar] [CrossRef]
- Perlis, R.H.; Ognyanova, K.; Santillana, M.; Baum, M.A.; Lazer, D.; Druckman, J.; Della Volpe, J. Association of acute symptoms of COVID-19 and symptoms of depression in adults. JAMA Netw. Open 2021, 4, e213223. [Google Scholar] [CrossRef]
- Koski, A.; Vuori, E.; Ojanperä, I. Newer antidepressants: Evaluation of fatal toxicity index and interaction with alcohol based on Finnish postmortem data. Int. J. Leg. Med. 2005, 119, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Mutingwende, F.P.; Kondiah, P.P.D.; Ubanako, P.; Marimuthu, T.; Choonara, Y.E. Advances in nano-enabled platforms for the treatment of depression. Polymers 2021, 13, 1431. [Google Scholar] [CrossRef]
- Patel, R.B.; Rao, H.R.; Thakkar, D.V.; Patel, M.R. Comprehending the potential of metallic, lipid, and polymer-based nanocarriers for treatment and management of depression. Neurochem. Int. 2022, 153, 105259. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liang, Q.; Zhang, F.; Guo, S.; Fan, L.; Zhao, F. Efficacy of noninvasive brain stimulation combined with antidepressant medications for depression: A systematic review and meta-analysis of randomized controlled trials. Syst. Rev. 2024, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, J.; Awosika, A.O.; Aedma, K.K. Tricyclic Antidepressants [Updated 2023 Aug 17]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jagtiani, A. Novel treatments of depression: Bridging the gap in current therapeutic approaches. Explor. Neurosci. 2024, 3, 272–286. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Buckman, J.E.J.; Underwood, A.; Clarke, K.; Saunders, R.; Hollon, S.D.; Fearon, P.; Pilling, S. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin. Psychol. Rev. 2018, 64, 13–38. [Google Scholar] [CrossRef]
- Yuan, S.; Ma, T.; Zhang, Y.N.; Wang, N.; Baloch, Z.; Ma, K. Novel drug delivery strategies for antidepressant active ingredients from natural medicinal plants: The state of the art. J. Nanobiotechnol. 2023, 21, 391. [Google Scholar] [CrossRef]
- Monroe, S.M.; Harkness, K.L. Major depression and its recurrences: Life course matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Nanoporous polymers: New nanosponge absorbent media. Filtr. Sep. 1999, 36, 26–28. [Google Scholar]
- Ma, M.; Li, D.Q. New organic nanoporous polymers and their inclusion complexes. Chem. Mater. 1999, 11, 872–874. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Dalla Bella, M.; Szejtli, J. Cyclodextrins. Drugs Future 1983, 8, 391–394. [Google Scholar]
- Rajput, K.N.; Patel, K.C.; Trivedi, U.B. β-Cyclodextrin Production by Cyclodextrin Glucanotransferase from an Alkaliphile Microbacterium terrae KNR 9 Using Different Starch Substrates. Biotechnol. Res. Int. 2016, 2016, 2034359. [Google Scholar] [CrossRef] [PubMed]
- Osmani, R.A.; Kulkarni, P.; Manjunatha, S.; Gowda, V.; Hani, U.; Vaghela, R.; Bhosale, R. Cyclodextrin Nanosponges in Drug Delivery and Nanotherapeutics. In Environmental Nanotechnology. Environmental Chemistry for a Sustainable World, Volume 14; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 279–342. [Google Scholar]
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X.; et al. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 5995–6015. [Google Scholar] [CrossRef]
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.C.; Kadam, V.J. Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin. Drug Deliv. 2014, 11, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Felton, L.A.; Popescu, C.; Wiley, C.; Esposito, E.X.; Lefevre, P.; Hopfinger, A.J. Experimental and Computational Studies of Physicochemical Properties Influence NSAID-Cyclodextrin Complexation. AAPS PharmSciTech 2014, 15, 872–881. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Payamifar, S.; Foroozandeh, A.; Pourmadadi, M.; Abdouss, M.; Hasanzadeh, M. Cyclodextrin-based nanocarriers as promising scaffolds for overcoming challenges of doxorubicin delivery in cancer chemotherapy. Carbohydr. Polym. Technol. Appl. 2025, 9, 100677. [Google Scholar] [CrossRef]
- Ai, F.; Wang, J.; Li, Y.; Ma, Y. Effect of Drug Particle Size on Complexation, Physicochemical Properties and Dissolution of Cyclodextrin Inclusion Complexes. Indian J. Pharm. Sci. 2017, 79, 131–138. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Allahyari, S.; Trotta, F.; Valizadeh, H.; Jelvehgari, M.; Zakeri-Milani, P. Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opin. Drug Deliv. 2019, 16, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Puskás, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Várnai, B.; Béni, S.; Hazai, E. Sulfobutylether-betacyclodextrin-enabled antiviral remdesivir: Characterization of electrospun- and lyophilized formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Sursyakova, V.V.; Levdansky, V.A.; Rubaylo, A.I. Thermodynamic parameters for the complexation of water-soluble betulin derivatives with (2-hydroxypropyl)-β-cyclodextrin determined by affinity capillary electrophoresis. J. Mol. Liq. 2019, 283, 325–331. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Zhao, L.; Fu, Y.; Ye, F. Encapsulation of thiabendazole in hydroxypropyl-β-cyclodextrin nanofibers via polymer-free electrospinning and its characterization. Pest Manag. Sci. 2020, 76, 3264–3272. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Liu, Y.-X.; Kou, S.-B.; Wang, B.-L.; Shi, J.-H. Characterization of the inclusion interaction of ethinyloestradiol with β-cyclodextrin and hydroxypropyl-β-cyclodextrin: Multi-spectroscopic and molecular modeling methods. J. Mol. Liq. 2020, 311, 113290. [Google Scholar] [CrossRef]
- Briñez-Ortega, E.; De Almeida, V.L.; Lopes, J.C.D.; Burgos, A.E. Partial inclusion of bis(1,10-phenanthroline) silver(i) salicylate in β-cyclodextrin: Spectroscopic characterization, in vitro and in silico antimicrobial evaluation. An. Acad. Bras. Cienc. 2020, 92, e20181323. [Google Scholar] [CrossRef]
- Xavier-Júnior, F.H.; Tavares, C.T.; Rabello, M.M.; Hernandes, M.Z.; Bezerra, B.P.; Ayala, A.P.; Pessoa, O.D.L.; Ximenes, R.M.; Santos-Magalhães, N.S. Elucidation of the mechanism of complexation between oncocalyxone A and cyclodextrins by isothermal titration calorimetry and molecular modeling. J. Mol. Liq. 2019, 274, 165–172. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Hu, Y.; Zhang, X.; Ma, Y.; Lv, H.; Xu, S.; Wang, Y.; Jiang, Z. Fe3+ sensitivity fluorescence sensor from β-cyclodextrin-enhanced Eu3+ luminescence aggregates. J. Mater. Sci. 2021, 56, 10979–10989. [Google Scholar] [CrossRef]
- Sonaimuthu, M.; Balakrishnan, S.B.; Kuppu, S.V.; Veerakanellore, G.B.; Thambusamy, S. Spectral and proton transfer behavior of 1,4-dihydroxylanthraquinone in aqueous and confined media; molecular modelling strategy. J. Mol. Liq. 2018, 259, 186–198. [Google Scholar] [CrossRef]
- Bomzan, P.; Roy, N.; Sharma, A.; Rai, V.; Ghosh, S.; Kumar, A.; Roy, M.N. Molecular encapsulation study of indole 3-methanol in cyclodextrins: Effect on antimicrobial activity and cytotoxicity. J. Mol. Struct. 2021, 1225, 129093. [Google Scholar] [CrossRef]
- Aree, T. Supramolecular complexes of β-cyclodextrin with clomipramine and doxepin: Effect of the ring substituent and component of drugs on their inclusion topologies and structural flexibilities. Pharmaceuticals 2020, 13, 278. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W.; et al. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Gaálová, J.; Michel, M.; Bourassi, M.; Ladewig, B.P.; Kasal, P.; Jindřich, J.; Izák, P. Nafion membranes modified by cationic cyclodextrin derivatives for enantioselective separation. Sep. Purif. Technol. 2021, 266, 118538. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.; Guo, X.; Di, X.; Yu, J. Enantioseparation and modelling study of six proton pump inhibitors on a novel 3,5-dichlorophenylcarbamated β-cyclodextrin chemically bonded chiral stationary phase by high performance liquid chromatography. Microchem. J. 2021, 166, 106211. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; Den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey, and soy protein hydrolysates. LWT 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R.; Dahiya, L.; Sarwal, A. Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin complex for effective management of depression. Int. J. Pharm. 2021, 594, 120129. [Google Scholar] [CrossRef]
- Urcuk, A.; Karadurmus, L.; Bakirhan, N.K.; Ozkan, S.A. Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride. Open Chem. 2021, 19, 228–236. [Google Scholar] [CrossRef]
- Aree, T. β-Cyclodextrin inclusion complexation with tricyclic antidepressants desipramine and imipramine: A structural chemistry perspective. J. Pharm. Sci. 2020, 109, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Belica-Pacha, S.; Małecka, M.; Daśko, M.; Miłowska, K.; Bryszewska, M.; Budryn, G.; Oracz, J.; Pałecz, B. The interaction of heptakis (2,6-di-O-methyl)-β-cyclodextrin with mianserin hydrochloride and its influence on the drug toxicity. Int. J. Mol. Sci. 2021, 22, 9419. [Google Scholar] [CrossRef] [PubMed]
- Majewska, K.; Skwierawska, A.; Kamińska, B.; Prześniak-Welenc, M. Improvement of opipramol base solubility by complexation with β-cyclodextrin. Supramol. Chem. 2018, 30, 20–31. [Google Scholar] [CrossRef]
- Diniz, T.C.; Pinto, T.C.C.; Menezes, P.D.P.; Silva, J.C.; Teles, R.B.D.A.; Ximenes, R.C.C.; Guimarães, A.G.; Serafini, M.R.; Araújo, A.A.D.S.; Quintans Júnior, L.J.; et al. Cyclodextrins improving the physicochemical and pharmacological properties of antidepressant drugs: A patent review. Expert Opin. Ther. Pat. 2018, 28, 81–92. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Deshmane, A.B.; Singh, S.R.; Kulkarni, Y.A. Lurasidone-β-cyclodextrin complexes: Physicochemical characterization and comparison of their antidepressant, antipsychotic activities against that of self-microemulsifying formulation. J. Mol. Struct. 2018, 1157, 395–400. [Google Scholar] [CrossRef]
- Belica-Pacha, S.; Miłowska, K.; Ionov, M.; Bryszewska, M.; Buczkowski, A.; Budryn, G.; Oracz, J.; Zaczyńska, D.; Wróblewska, A.; Urbaniak, P.; et al. The impact of β-cyclodextrin on biological and chemical properties of mianserin hydrochloride in aqueous solution. J. Mol. Liq. 2020, 314, 113589. [Google Scholar] [CrossRef]
- Buko, V.; Zavodnik, I.; Lukivskaya, O.; Naruta, E.; Palecz, B.; Belica-Pacha, S.; Belonovskaya, E.; Kranc, R.; Abakumov, V. Cytoprotection of pancreatic β-cells and hypoglycemic effect of 2-hydroxypropyl-β-cyclodextrin: Sertraline complex in alloxan-induced diabetic rats. Chem. Biol. Interact. 2016, 244, 105–112. [Google Scholar] [CrossRef]
- Daraban, B.S.; Popa, A.S.; Stan, M.S. Latest Perspectives on Alzheimer’s Disease Treatment: The Role of Blood-Brain Barrier and Antioxidant-Based Drug Delivery Systems. Molecules 2024, 29, 4056. [Google Scholar] [CrossRef]
- Waqar, M.A.; Zaman, M.; Hameed, H.; Jamshaid, M.; Irfan, A.; Shazly, G.A.; Paiva-Santos, A.C.; Bin Jardan, Y.A. Formulation, characterization, and evaluation of β-cyclodextrin functionalized hypericin loaded nanocarriers. ACS Omega 2023, 8, 38191–38203. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of polymer-based nanoformulations for glioblastoma brain cancer therapy and diagnosis: An update. Polymers 2021, 13, 4114. [Google Scholar] [CrossRef]
- Miranda, G.M.; Santos, V.O.R.e.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.C.M.A.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Vallejo, Á.; Caja, M.d.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Sammeta, S.M.; Vaka, S.R.K.; Murthy, S.N. Transcutaneous electroporation mediated delivery of doxepin-HPCD complex: A sustained release approach for treatment of postherpetic neuralgia. J. Control. Release 2010, 142, 361–367. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R.; Dahiya, L.; Singh, G.; Sarwal, A. Impact of cyclodextrin derivatives on systemic release of duloxetine HCl via buccal route. Drug Dev. Ind. Pharm. 2020, 46, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and Pharmacodynamics: A Comprehensive Analysis of the Absorption, Distribution, Metabolism, and Excretion of Psychiatric Drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef]
- Ambwani, S.; Dutta, S.; Mishra, G.; Lal, H.; Singh, S.; Charan, J. Adverse Drug Reactions Associated with Drugs Prescribed in Psychiatry: A Retrospective Descriptive Analysis in a Tertiary Care Hospital. Cureus 2021, 13, e19493. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Mamoon, B.; Batool, T.; Khattak, M.I.; Amir, F.; Akbar, A.; Khan, S. Advances in Antidepressant Therapy: Comparing the Efficacy of Selective Serotonin Reuptake Inhibitors (SSRIs), Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), and Novel Agents. Cureus 2024, 16, e76318. [Google Scholar] [CrossRef]
- Passos, J.J.; De Sousa, F.B.; Lula, I.S.; Barretto, E.A.; Lopes, J.F.; De Almeida, W.B.; Sinisterra, R.D. Multi-equilibrium system based on sertraline and β-cyclodextrin supramolecular complex in aqueous solution. Int. J. Pharm. 2011, 421, 24–33. [Google Scholar] [CrossRef]
- Volkova, T.; Simonova, O.; Perlovich, G. Cyclodextrin’s Effect on Permeability and Partition of Nortriptyline Hydrochloride. Pharmaceuticals 2023, 16, 1022. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Jin, Z.; Han, Y.; Zhang, D.; Li, Z.; Jing, Y.; Hu, B.; Sun, S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics 2022, 14, 2070. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Fialho, S.; Cunha, S.; Falcão, A.; Fortuna, A. Intranasal delivery of paroxetine: A preclinical study on pharmacokinetics, depressive-like behaviour, and neurochemical sex differences. Biochem. Pharmacol. 2024, 223, 116184. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.; Paiva-Santos, A.C.; Veiga, F.; Pires, P.C. Lipid and Polymeric Nanoparticles: Successful Strategies for Nose-to-Brain Drug Delivery in the Treatment of Depression and Anxiety Disorders. Pharmaceutics 2022, 14, 2742. [Google Scholar] [CrossRef] [PubMed]
- Pyrak, B.; Rogacka-Pyrak, K.; Gubica, T.; Szeleszczuk, Ł. Exploring Cyclodextrin-Based Nanosponges as Drug Delivery Systems: Understanding the Physicochemical Factors Influencing Drug Loading and Release Kinetics. Int. J. Mol. Sci. 2024, 25, 3527. [Google Scholar] [CrossRef]
- Young, L.T. Neuroprotective effects of antidepressant and mood stabilizing drugs. J. Psychiatry Neurosci. 2002, 27, 8–9. [Google Scholar]
- Morales, J.O.; Fathe, K.; Brunaugh, A.; Kim, N.; Zahner, D.; Ishihara, K.; Han, T.; Gupta, P.; Jha, A.; Smyth, H.D.C. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Osman, D.A.; Mohamed, O.S. In vitro and in vivo evaluation of taste-masked orodispersible tablets of fluoxetine hydrochloride for the treatment of depression. Drug Dev. Ind. Pharm. 2021, 47, 645–653. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Celik, S.; Sezgin-Bayindir, Z.; Bravo-Fernández, S.; Bravo-Díaz, C. Carrier systems for advanced drug delivery: Improving drug solubility/bioavailability and administration routes. Pharmaceutics 2024, 16, 852. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Marian, E.; Ganea, M.; Frenț, O.D.; Maghiar, P.B.; Bodea, F.I.; Dejeu, G.E. Innovative approaches to enhancing the biomedical properties of liposomes. Pharmaceutics 2024, 16, 1525. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Resolving a paradox: Antidepressants, neuroinflammation, and neurodegeneration. Explor. Neuroprot. Ther. 2024, 4, 11–37. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ahmed, F.; Mohammed, A.M.; Alsharidah, M.; Al-Subaiyel, A.; Samman, W.A.; Alhaddad, A.A.; Al-Mijalli, S.H.; Amin, M.A.; Barakat, H.; et al. Recent advances in the pharmaceutical and biomedical applications of cyclodextrin-capped gold nanoparticles. Int. J. Nanomed. 2023, 18, 3247–3281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depressive Disorder (Depression); WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 18 January 2025).
- Ruiz, N.A.L.; Del Ángel, D.S.; Brizuela, N.O.; Peraza, A.V.; Olguín, H.J.; Soto, M.P.; Guzmán, D.C. Inflammatory process and immune system in major depressive disorder. Int. J. Neuropsychopharmacol. 2022, 25, 46–53. [Google Scholar] [CrossRef]
- Radell, M.L.; Hamza, E.G.A.; Daghustani, W.H.; Perveen, A.; Moustafa, A.A. The impact of different types of abuse on depression. Depress. Res. Treat. 2021, 2021, 6654503. [Google Scholar] [CrossRef]
- Ge, L.; Yap, C.W.; Ong, R.; Heng, B.H. Social isolation, loneliness, and their relationships with depressive symptoms: A population-based study. PLoS ONE 2017, 12, e0182145. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, H.; Zhang, Y.; Cao, Z.; Li, D.; Sun, L.; Zhang, X.; Wang, Y. Association of time spent in outdoor light and genetic risk with the incidence of depression. Transl. Psychiatry 2023, 13, 40. [Google Scholar] [CrossRef]
- Hirschmann, R.; Gomes, A.P.; Gonçalves, H. Sintomatologia depressiva entre moradores da zona rural de uma cidade no Sul do Brasil. Rev. Saude Publica 2018, 52 (Suppl. S1), 11. [Google Scholar] [CrossRef]
- de Paula, G.M.R.; Silva, V.I.A.; Tenorio, M.S.D.P.; Pinto, D.Q.; de Vasconcelos, C.C.; Barbosa, A.S.L. Sintomas depressivos em estudantes de medicina e sua relação com variáveis hormonais e socioeconômicas. Rev. Bras. Educ. Médica 2020, 44, e133. [Google Scholar] [CrossRef]
- Ettman, C.K.; Fan, A.Y.; Philips, A.P.; Adam, G.P.; Ringlein, G.; Clark, M.A.; Wilson, I.B.; Vivier, P.M.; Galea, S. Financial strain and depression in the U.S.: A scoping review. Transl. Psychiatry 2023, 13, 168. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 depressive symptoms: Epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef]
- Liu, W.; Yu, F.; Geldsetzer, P.; Yang, J.; Wang, Z.; Golden, T.; Jiao, L.; Chen, Q.; Liu, H.; Wu, P.J.B. Prevalence of depression in China during the early stage of the COVID-19 pandemic: A cross-sectional study in an online survey sample. J. Public Health 2022, 12, e056667. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Bhattad, D.J. Immediate and short-term prevalence of depression in COVID-19 patients and its correlation with continued symptoms experience. Indian J. Psychiatry 2022, 64, 301–306. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors, and clinical correlates of post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Renaud-Charest, O.; Lui, L.M.W.; Eskander, S.; Ceban, F.; Ho, R.; Di Vincenzo, J.D.; Rosenblat, J.D.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 2021, 144, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, T.; Yoshikawa, Y.; Matsuo, K.; Kurahara, K.; Uehara, Y.; Nakao, T.; Ishiguro, H.; Kumazaki, H.; Kato, T.A. Development of depression assessment tools using humanoid robots: Can tele-operated robots talk with depressive persons like humans? J. Psychiatr. Res. 2023, 170, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Baul, K.; Roy, N.; Deb, S.; Ghosh, B.; Roy, D.; Choudhury, S.; Rahaman, H.; Dakua, V.K.; Roy, M.N. Exploring the inclusion complex of an antidepressant drug (AXP) with ɣ-CD to reduce the risky effect of AXP by experimental and computational studies. J. Mol. Struct. 2024, 1306, 8. [Google Scholar] [CrossRef]
- Aree, T. β-Cyclodextrin encapsulation of nortriptyline HCl and amitriptyline HCl: Molecular insights from single-crystal X-ray diffraction and DFT calculation. Int. J. Pharm. 2020, 575, 118899. [Google Scholar] [CrossRef]
- Aree, T. Distinctive supramolecular features of beta-cyclodextrin inclusion complexes with antidepressants protriptyline and maprotiline: A comprehensive structural investigation. Pharmaceuticals 2021, 14, 812. [Google Scholar] [CrossRef] [PubMed]
- Kola-Mustapha, A.T. Polymers used in the preparation of nano-conjugates. In Novel Biomimetic Polymeric Nanoconjugates as Drug Delivery Carriers for Poorly Soluble Drugs; De Montfort University: Leicester, UK, 2013; p. 37. [Google Scholar]
- Misiuk, W. Study on the inclusion complex formation of desipramine with B-cyclodextrin and its pharmaceutical application. World J. Pharm. Pharm. Sci. 2015, 4, 18–33. [Google Scholar]
- Jalali, F.; Ezzati, N. Spectrofluorimetric study and determination of desipramine in the presence of beta-cyclodextrin. J. Anal. Chem. 2014, 69, 367–370. [Google Scholar] [CrossRef]
- Viswalingam, M.; Prabu, S.; Sivakumar, K.; Rajamohan, R. Spectral characteristics of desipramine in beta-cyclodextrin cavity through inclusion complex. J. Macromol. Sci. Part A 2016, 53, 781–790. [Google Scholar] [CrossRef]
- Junquera, E.; Romero, J.C.; Aicart, E. Behavior of tricyclic antidepressants in aqueous solution: Self-aggregation and association with beta-cyclodextrin. Langmuir 2001, 17, 1826–1832. [Google Scholar] [CrossRef]
- Piperaki, S.; Parissi-Poulou, M.; Koupparis, M. A separation study of tricyclic antidepressant drugs by HPLC with beta-cyclodextrin bonded stationary phase. J. Liq. Chromatogr. Relat. Technol. 1993, 16, 3487–3508. [Google Scholar] [CrossRef]
- Kundu, M.; Roy, M.N. Preparation, interaction and spectroscopic characterization of inclusion complex of a cyclic oligosaccharide with an antidepressant drug. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 177–187. [Google Scholar] [CrossRef]
- Valsami, G.; Koupparis, M.A.; Macheras, P.E. Complexation studies of cyclodextrins with tricyclic antidepressants using ion-selective electrodes. Pharm. Res. 1992, 9, 94–100. [Google Scholar] [CrossRef]
- Carcu-Dobrin, M.; Buda, M.; Hancu, G.; Gagyi, L.; Rusu, A.; Kelemen, H. Enantioselective analysis of fluoxetine in pharmaceutical formulations by capillary zone electrophoresis. Saudi Pharm. J. 2017, 25, 397–403. [Google Scholar] [CrossRef]
- Aree, T. Advancing insights on β-cyclodextrin inclusion complexes with SSRIs through the lens of X-ray diffraction and DFT calculation. Int. J. Pharm. 2021, 609, 121113. [Google Scholar] [CrossRef]
- Belica-Pacha, S.; Daśko, M.; Buko, V.; Zavodnik, I.; Miłowska, K.; Bryszewska, M. Thermodynamic studies of interactions between sertraline hydrochloride and randomly methylated β-cyclodextrin molecules supported by circular dichroism spectroscopy and molecular docking results. Int. J. Mol. Sci. 2021, 22, 12357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; He, B.; Zhang, Q.; Tu, Y. Chiral separation of sertraline with microemulsion electrokinetic chromatography on a polymer/β-cyclodextrin assembling molecular film modified capillary. Anal. Sci. 2010, 26, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Unveiling the Thermodynamic Aspects of Drug-Cyclodextrin Interactions Through Isothermal Titration Calorimetry. In Supramolecules in Drug Discovery and Drug Delivery. Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2207, pp. 187–198. [Google Scholar]
- Aree, T. Inclusion scenarios and conformational flexibility of the SSRI paroxetine as perceived from polymorphism of β-cyclodextrin–paroxetine complex. Pharmaceuticals 2022, 15, 98. [Google Scholar] [CrossRef]
- Ignaczak, A.; Orszański, Ł. In search of the most stable molecular configuration of heptakis(2,6 O dimethyl)-β-cyclodextrin and its complex with mianserin: A comparison of the B3LYP-GD2 and M062X-GD3 results. J. Phys. Chem. B 2021, 125, 13077–13087. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, W.-T.; Cao, W.-Q.; Li, J.; Gao, F.-Y.; Yang, N.; Fan, G.-R. Enantioselective separation of mirtazapine and its metabolites by capillary electrophoresis with acetonitrile field-amplified sample stacking and its application. Molecules 2014, 19, 4907–4923. [Google Scholar] [CrossRef]
- Alrabiah, H.; Aljohar, H.I.; Bakheit, A.H.; Homoda, A.M.A.; Mostafa, G.A.H. Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-tert-butylcalix [8]arene ionophores as electroactive materials for the construction of new sensors for trazodone based on host-guest recognition. Drug Des. Devel. Ther. 2019, 13, 2283–2293. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant effects of Clinopodium mexicanum Benth. Govaerts (Lamiaceae) on the central nervous system. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef]
- Cassani, J.; Escalona Araujo, A.G.; Martínez-Vázquez, M.; Manjarrez, N.; Moreno, J.; Estrada-Reyes, R. Anxiolytic-like and antinociceptive effects of 2(S)-Neoponcirin in mice. Molecules 2013, 18, 7584–7599. [Google Scholar] [CrossRef]
- Martínez-Mota, L.; Cassani, J.; Mayagoitia-Novales, L.; Benítez-King, G.; Becerril-Villanueva, L.E.; Dorantes-Barrón, A.M.; Jurado-Hernández, N.; Estrada-Reyes, R. Antidepressant-like and beneficial effects of a neoponcirin-beta-cyclodextrin inclusion complex in mice exposed to prolonged stress. Int. J. Mol. Sci. 2024, 25, 8289. [Google Scholar] [CrossRef]
- Aquib, M.; Najmi, A.K.; Akhtar, M. Antidepressant effect of thymoquinone in animal models of depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef]
- Bruijniks, S.J.E.; Meeter, M.; Lemmens, L.H.J.M.; Peeters, F.; Cuijpers, P.; Huibers, M.J.H. Temporal and specific pathways of change in cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT) for depression. Behav. Res. Ther. 2022, 151, E104010. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. SSRI antidepressant medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Shariatifar, A.; Riazi, M.; Ebnolelm, M.; Jahromy, M.H. Effects of Nigella sativa L. seed extract on fatigue, blood biochemical parameters and thyroid function in male mice. Chin. Med. 2014, 5, 16–21. [Google Scholar] [CrossRef]
- Nag, K.; Solanki, D.; Peddha, M.S.; Mehdi, S.; Logesh, R.; Roohi, T.F.; Kinattingal, N.; Shakeel, F. Effect of cyclodextrin-complexed lyophilized nanosuspension of Nigella sativa seeds oleoresin on chronic unpredictable mild stress (CUMS)-induced depression in mice. Neurosci. Lett. 2024, 834, 137844. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming challenges in small-molecule drug bioavailability: A review of key factors and approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Bonasser, L.S.S.; Silva, C.M.d.S.; Fratelli, C.F.; Gontijo, B.R.; Seixas, J.M.A.; Barreto, L.C.L.d.S.; Silva, I.C.R.d. CYP2C19 Genetic Variants and Major Depressive Disorder: A Systematic Review. Pharmaceuticals 2024, 17, 1461. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Svob Strac, D.; Jancic, J.; Samardzic, J. The Role of Pharmacogenetics in Personalizing the Antidepressant and Anxiolytic Therapy. Genes 2023, 14, 1095. [Google Scholar] [CrossRef]
- O’Brien, F.E.; Clarke, G.; Dinan, T.; Cryan, J.F.; Griffin, B.T. Human P-glycoprotein differentially affects antidepressant drug transport: Relevance to blood-brain barrier permeability. Int. J. Neuropsychopharmacol. 2013, 16, 2259–2272. [Google Scholar] [CrossRef]
- Fan, P.; Zeng, L.; Ding, Y.; Kofler, J.; Silverstein, J.; Krivinko, J.; Sweet, R.A.; Wang, L. Combination of antidepressants and antipsychotics as a novel treatment option for psychosis in Alzheimer’s disease. CPT Pharmacometrics Syst. Pharmacol. 2023, 12, 1119–1131. [Google Scholar] [CrossRef]
- Serrano-Martínez, A.; Victoria-Montesinos, D.; García-Muñoz, A.M.; Hernández-Sánchez, P.; Lucas-Abellán, C.; González-Louzao, R. A Systematic Review of Clinical Trials on the Efficacy and Safety of CRLX101 Cyclodextrin-Based Nanomedicine for Cancer Treatment. Pharmaceutics 2023, 15, 1824. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Cyclodextrin Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Caira, M.R.; De Vries, E.; Nassimbeni, L.R.; Jacewicz, V.W. Inclusion of the antidepressant paroxetine in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2003, 46, 37–42. [Google Scholar] [CrossRef]
| Class | Drug |
|---|---|
| SSRIs | Fluvoxamine, Paroxetine, Escitalopram, Citalopram, Sertraline, Fluoxetine |
| SNRIs | Venlafaxine, Desvenlafaxine, Duloxetine, Levomilnacipran, Milnacipran |
| TCAs | Imipramine, Nortriptyline, Amitriptyline, Clomipramine, Desimipramine, Doxepin, Amoxapine, Protryptiline |
| MAOIs | Phenelzine, Tranylcypromine, Isocarboxazid, Selegiline |
| Atypical Antidepressants | Bupropion, Mirtazapine, Trazodone, Nefazodone, Vortioxetine, Vilazodone, Mianserine |
| NMDA Receptor Antagonists | Esketamine, Ketamine |
| Serotonin Modulators | Trazodone, Nefazodone, Vortioxetine, Vilazodone |
| Study (Year) | Drug (Guest) | Cyclodextrin Type | Study Findings | Study Limitations |
|---|---|---|---|---|
| 2020 [107] | Desipramine | β-CD (1:1) | X-ray crystallography showed the drug’s aromatic rings fit into the β-CD cavity, stabilized by C–H···π and N–H···O interactions, indicating a stable 1:1 complex and suggesting enhanced aqueous solubility | Structural analysis only; no in vivo data to confirm improved bioavailability (study was purely in vitro) |
| 2020 [104] | Imipramine | β-CD (1:1) | β-CD encapsulation yielded a similar inclusion complex as with desipramine, improving drug stability. The complex formation reinforces β-CD’s role in stabilizing TCAs and potentially enhancing delivery | In vitro characterization without in vivo validation; primarily a structural study without direct pharmacokinetic data |
| 2015 [108] | Desipramine | β-CD (1:1) | Confirmed formation of a 1:1 inclusion complex that significantly increased desipramine’s aqueous solubility and was explored for improved pharmaceutical formulation | Experiments were laboratory-based; lacked any in vivo study to demonstrate actual improvement in drug absorption or efficacy |
| 2014 [109] | Desipramine | β-CD (2:1) | Spectrofluorimetric analysis showed enhanced fluorescence of desipramine with β-CD, indicating complex formation. A 2:1 drug:CD ratio improved complex stability but altered the drug release profile. | Analytical study focusing on detection; did not evaluate therapeutic effects or long-term stability in biological systems (no in vivo component) |
| 2016 [110] | Desipramine | β-CD (1:1) | Spectroscopic characterization confirmed 1:1 inclusion complexation of desipramine with β-CD, supporting improved stability of the drug in solution | Findings are based on solution-phase spectroscopic data; no evaluation of how the complex behaves in vivo or in a full dosage form |
| 2001 [104] | Desipramine | β-CD (1:1) | Observed that desipramine tends to self-aggregate in aqueous solution, and β-CD inclusion disrupts these aggregates by forming stable 1:1 complexes | Study in aqueous solution only; did not address drug behavior in vivo or in complex biological fluids (focus was on physico-chemical interactions) |
| 2020 [111] | Nortriptyline | β-CD (1:1) | Single-crystal XRD and DFT demonstrated a stable 1:1 β-CD–nortriptyline complex. Inclusion reduced the drug conformational flexibility (“butterfly” angle) and improved its stability, supporting the idea that CD encapsulation could reduce nortriptyline’s side effects | Structural and theoretical study only; no direct measurement of pharmacological outcomes or drug release in vivo was performed |
| 1993 [112] | Nortriptyline | β-CD (1:1) | Using a β-CD-bonded HPLC column, this study achieved separation of nortriptyline, indicating formation of an inclusion complex during chromatography. It provided early evidence of specific nortriptyline–β-CD interactions in solution. | Focused on chromatographic behavior rather than therapeutic application; results are method-specific and not translated to actual drug delivery or bioavailability improvements |
| 1993 [109] | Maprotiline | β-CD (1:1) | Reported that maprotiline can form a 1:1 complex with β-CD, similarly to other TCAs, which is expected to enhance its water solubility and stability in solution (by analogy with other drugs in the study) | The investigation was limited in scope (in vitro); no direct data on how the complex affects maprotiline’s pharmacokinetics or efficacy in vivo in vivo was provided |
| 2017 [113] | Nortriptyline | β-CD (1:1) | Prepared and characterized a nortriptyline–β-CD inclusion complex. The authors noted enhanced solubility and suggested that complexation may mitigate dose-related side effects and improve patient compliance by more controlled drug release. | Results are based on in vitro analyses; the study did not include in vivo tests to confirm reduced side effects or improved therapeutic outcomes in practice |
| 1992 [114] | Nortriptyline | β-CD (1:1) | Ion-selective electrode studies quantified the binding of nortriptyline with β-CD, confirming 1:1 complex formation. The presence of β-CD reduced nortriptyline’s tendency to partition into a non-aqueous phase, indicating improved aqueous retention. | Entirely in vitro measurement of binding affinity; did not examine the complex in biological systems or assess actual improvements in drug absorption |
| 1992 [104] | Nortriptyline | α-CD (1:1) | Showed that nortriptyline also forms a 1:1 inclusion complex with α-cyclodextrin, though the smaller α-CD cavity may lead to a different binding affinity. Complexation efficacy differed by cyclodextrin type, underscoring the influence of CD size on stability. | In vitro chemical study; the relative benefit of using α-CD over β-CD was not confirmed in a biological context, and the smaller CD efficacy remains theoretical without in vivo data |
| 1992 [104] | Maprotiline | β-CD (1:1) | Found that maprotiline forms a stable 1:1 complex with β-CD, which likely increases its solubility in water. This result is in line with other TCAs forming inclusion complexes with β-CD, hinting at improved delivery potential for maprotiline as well. | Based on in vitro binding studies; no follow-up in vivo research was conducted to verify any improvement in maprotiline’s pharmacological profile or reduction in side effects |
| 1992 [104] | Maprotiline | α-CD (1:1) | Demonstrated that maprotiline can also complex with α-CD in a 1:1 ratio, although the smaller cavity may not accommodate the drug as effectively as β-CD. This suggests that cyclodextrin cavity size is critical for optimal inclusion. | Only investigated under laboratory conditions; it remains unclear how well the α-CD complex of maprotiline would perform in vivo or if it offers any therapeutic advantage over the β-CD complex |
| 1992 [104] | Protriptyline | β-CD (1:1) | Early evidence | Initial findings were preliminary and in vitro; the study did not provide in vivo data, and robust structural confirmation came only in subsequent research years later |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Văruț, R.M.; Popescu, A.I.S.; Gaman, S.; Niculescu, C.E.; Niculescu, A.Ș.; Dop, D.; Stepan, M.D.; Ionovici, N.; Singer, C.E.; Popescu, C. Cyclodextrin-Based Drug Delivery Systems for Depression: Improving Antidepressant Bioavailability and Targeted Central Nervous System Delivery. Pharmaceutics 2025, 17, 355. https://doi.org/10.3390/pharmaceutics17030355
Văruț RM, Popescu AIS, Gaman S, Niculescu CE, Niculescu AȘ, Dop D, Stepan MD, Ionovici N, Singer CE, Popescu C. Cyclodextrin-Based Drug Delivery Systems for Depression: Improving Antidepressant Bioavailability and Targeted Central Nervous System Delivery. Pharmaceutics. 2025; 17(3):355. https://doi.org/10.3390/pharmaceutics17030355
Chicago/Turabian StyleVăruț, Renata Maria, Alin Iulian Silviu Popescu, Simina Gaman, Carmen Elena Niculescu, Adrian Ștefan Niculescu, Dalia Dop, Mioara Desdemona Stepan, Nina Ionovici, Cristina Elena Singer, and Cristina Popescu. 2025. "Cyclodextrin-Based Drug Delivery Systems for Depression: Improving Antidepressant Bioavailability and Targeted Central Nervous System Delivery" Pharmaceutics 17, no. 3: 355. https://doi.org/10.3390/pharmaceutics17030355
APA StyleVăruț, R. M., Popescu, A. I. S., Gaman, S., Niculescu, C. E., Niculescu, A. Ș., Dop, D., Stepan, M. D., Ionovici, N., Singer, C. E., & Popescu, C. (2025). Cyclodextrin-Based Drug Delivery Systems for Depression: Improving Antidepressant Bioavailability and Targeted Central Nervous System Delivery. Pharmaceutics, 17(3), 355. https://doi.org/10.3390/pharmaceutics17030355








