Nanomanaging Chronic Wounds with Targeted Exosome Therapeutics
Abstract
:1. Introduction
2. Roads Leading to Wound Chronicity
3. Challenges and Management of Wound Care: The Need to Bridge Socioeconomic Gaps for Better Outcomes
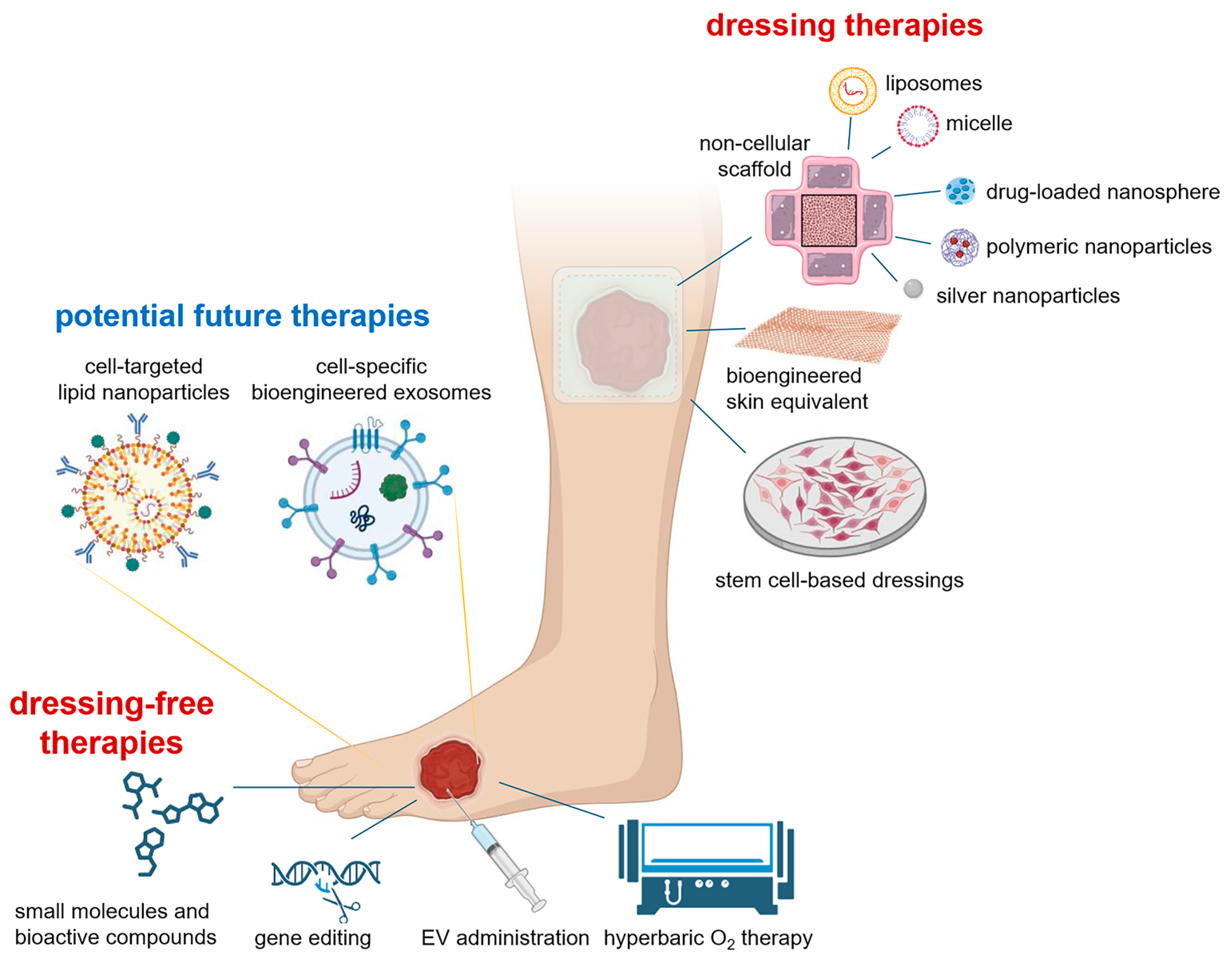
4. Need for New and Integrated Therapies
4.1. Commercial Products and Advanced Biomaterials
4.2. Nanotherapeutic Approaches in Wound Healing
4.3. Exosome-Based Strategies
5. Bioengineered Exosomes Are Emerging as a Novel Tool for Chronic Wound Therapy
6. Targeted Exosome Delivery
- Understanding endocytosis: The endocytosis of exosomes is critical for maximizing exosome uptake and cellular targeting. The endocytic pathways vary depending on the cell type and the source of exosomes. For example, although Joshi et al. has shown that the uptake of vesicles by cells involves endocytosis [175], it has been reported that clathrin-mediated endocytosis and micropinocytosis are predominant process for cellular uptake of PC12 cell-derived exosomes. Decoding the specific endocytic mechanisms will enable the design of exosomes that are more effectively internalized by target cells [153,176,177].
- Preventing MPS internalization: When exosomes encounter physiological fluids like blood or lymph, they can interact with biomolecules such as opsonins, facilitating cellular detection and clearance by the MPS. To improve exosome-based targeted delivery and prevent MPS internalization, the concept of host “bioinvisibility” is crucial. One strategy involves coating the surface of exosomes with self-identifying proteins, such as CD47, which binds to the SIRP-alpha receptor and helps evade the immune system, thereby prolonging circulation time. Recent studies have shown that attaching the active binding sequence of CD47 to exosome surfaces decreases MPS absorption and significantly lengthens circulation durations [178].
| Cell Source | Function | Year | References |
|---|---|---|---|
| Keratinocytes | Enhance macrophage functions by overexpressing MALAT1 | 2023 | [179] |
| Accelerate migration and proliferation of keratinocytes and fibroblasts via MAPK pathways | 2021 | [180] | |
| Modulate number and function of macrophages | 2020 | [141] | |
| Alter VEGF and fibroblast growth factors (FGF) and activate fibroblasts and endothelial cell migration | 2020 | [181] | |
| Macrophages | Promote osteogenesis through microRNA-21a-5p | 2022 | [182] |
| Increase VEGF expression causing proliferation and migration of endothelial cells | 2019 | [145] | |
| Increase expression of VEGF, Wnt3a, and miR-130a to promote angiogenesis, fibroblast proliferation, and re-epithelialization | 2020 | [183] | |
| Promote angiogenesis, proliferation, granulation tissue formation, and collagen accumulation by overexpressing miR-223 | 2022 | [184] | |
| Promote wound closure and re-epithelialization by switching the expression of iNOS to arginase | 2022 | [185] | |
| Fibroblasts | Upregulates the expression of collagen type I and TGFβ | 2019 | [148] |
| Promote re-epithelialization, proliferation, and inhibit inflammation via β-catenin signaling pathway | 2021 | [146] | |
| Transition of fibroblasts to myofibroblasts | 2022 | [147] | |
| Promote fibroblast migration and transformation | 2022 | [147] |
- 3.
- Using host “self” identification signals: Employing host “self” identification signals to lessen complement activation and phagocytic recognition is a promising approach. For example, Factor H, a cofactor of Factor I, deactivates the complement pathway by promoting the dissociation of the Bb complex and cleavage of C3b. Researchers employed sialic acid, a component found on the pathogen surface, to bind Factor H and avoid complement activation and immune detection [186].
- 4.
- Surface energy modifications: Modifying the surface energies of exosomes, such as hydrophilicity/hydrophobicity, can reduce protein adsorption and phagocytic recognition. Hydrophilic poly (ethylene glycol) (PEG) is often immobilized to create a steric barrier, decreasing protein adsorption and extending blood circulation times for nanoparticles [187]. Qie et al. demonstrated that adding PEG to nanoparticles reduces clearance by all macrophage phenotypes while coating nanoparticles with CD47 specifically reduces phagocytic activity by pro-inflammatory macrophages [149,188].
- 5.
- Developing immune-tolerant nanomedicines: Surface modifications of exosomes that enable immune system evasion to offer a rational method for creating immune-tolerant nanomedicines. Further research is needed to develop safe, secure, and efficient methods to deactivate the MPS. One potential goal is to create a highly effective and universal blocker that can avoid dose-related toxicity associated with traditional MPS blocking methods [189].
7. Balancing Boundaries: Navigating Stringency and Innovation in Chronic Wound Healing
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HDF XOs | exosomes derived from three-dimensional spheroids |
| 3D | three-dimensional |
| CD47 | cluster of differentiation 47 |
| CD74 | cluster of differentiation 74 |
| CD206 | cluster of differentiation 206 |
| CTE | chronic traumatic encephalopathy |
| DC | dendritic cells |
| DF-Ex | dermal fibroblasts exosomes |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| sEVs | small extracellular vesicles |
| FDA | food and drug administration |
| FGF | fibroblast growth factors |
| HUVEC | human umbilical vein endothelial cell |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1 beta |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharides |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| MEMS | micro electro mechanical systems |
| MFGE8 | milk fat globule epidermal growth factor 8 |
| miRNA | micro ribonucleic acid |
| MMP-1 | matrix metalloproteinase-1 |
| MPS | mononuclear phagocyte system |
| MOFs | metal-organic frameworks |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSC | mesenchymal stem cell |
| NPWT | negative pressure wound therapy |
| PEG | poly(ethylene glycol) |
| ROS | reactive oxygen species |
| SMAD3 | suppressor of mothers against decapentaplegic 3 |
| TBI | traumatic brain injury |
| TES | traumatic encephalopathy syndrome |
| TGF-β | transforming growth factor-beta |
| TGFβR | transforming growth factor beta receptor |
| TIME | tissue, inflammation/infection, moisture, edge |
| TNF-α | tumor necrosis factor-alpha |
| TWA | triangle of wound assessment |
| UV | ultraviolet |
| VEGF | vascular endothelial growth factor |
| Wnt3a | wingless-type MMTV integration site family, member 3A |
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Gnyawali, S.C.; Sinha, M.; El Masry, M.S.; Wulff, B.; Ghatak, S.; Soto-Gonzalez, F.; Wilgus, T.A.; Roy, S.; Sen, C.K. High resolution ultrasound imaging for repeated measure of wound tissue morphometry, biomechanics and hemodynamics under fetal, adult and diabetic conditions. PLoS ONE 2020, 15, e0241831. [Google Scholar] [CrossRef] [PubMed]
- Gnyawali, S.C.; Blum, K.; Pal, D.; Ghatak, S.; Khanna, S.; Raoy, S.; Sen, C.K. Retooling Laser Speckle Contrast Analysis Algorithm to Enhance Non-Invasive High Resolution Laser Speckle Functional Imaging of Cutaneous Microcirculation. Sci. Rep. 2017, 7, 41048. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Klein, T.M.; Andrees, V.; Kirsten, N.; Protz, K.; Augustin, M.; Blome, C. Social participation of people with chronic wounds: A systematic review. Int. Wound J. 2021, 18, 287–311. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2021, 8, 791899. [Google Scholar] [CrossRef]
- Sharma, A.; Shankar, R.; Yadav, A.; Pratap, A.; Ansari, M.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low. Extrem. Wounds 2024. [Google Scholar] [CrossRef]
- Ffrench, C.; Finn, D.; Velligna, A.; Ivory, J.; Healy, C.; Butler, K.; Sezgin, D.; Carr, P.; Probst, S.; McLoughlin, A.; et al. Systematic review of topical interventions for the management of pain in chronic wounds. Pain Rep. 2023, 8, e1073. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Hoversten, K.P.; Kiemele, L.J.; Stolp, A.M.; Takahashi, P.Y.; Verdoorn, B.P. Prevention, Diagnosis, and Management of Chronic Wounds in Older Adults. Mayo Clin. Proc. 2020, 95, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.; Roy, S.; Gordillo, G. Wound Healing. In Plastic Surgery: Volume One; Neligan, P.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gallego-Perez, D.; Pal, D.; Ghatak, S.; Malkoc, V.; Higuita-Castro, N.; Gnyawali, S.; Chang, L.; Liao, W.C.; Shi, J.; Sinha, M.; et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 2017, 12, 974–979. [Google Scholar] [CrossRef]
- Seth, I.; Lim, B.; Cevik, J.; Gracias, D.; Chua, M.; Kenney, P.S.; Rozen, W.M.; Cuomo, R. Impact of nutrition on skin wound healing and aesthetic outcomes: A comprehensive narrative review. JPRAS Open 2024, 39, 291–302. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvarajah, S.; Mathioudakis, N.; Sherman, R.E.; Hines, K.F.; Black, J.H., 3rd; Abularrage, C.J. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann. Vasc. Surg. 2016, 33, 149–158. [Google Scholar] [CrossRef]
- Namgoong, S.; Baik, S.; Han, S.K.; Son, J.W.; Kim, J.Y. Developing and Establishing a Wound Dressing Team: Experience and Recommendations. J. Korean Med. Sci. 2023, 38, e168. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Durant, F.; Whited, J.L. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv. Sci. 2021, 8, e2100407. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Gieldon, L.; Mysore, V.; Nippe, N.; Taxman, D.J.; Duncan, J.A.; Broglie, P.M.; Marketon, K.; Austermann, J.; Vogl, T.; et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 2012, 92, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef]
- Bluestein, D.; Javaheri, A. Pressure ulcers: Prevention, evaluation, and management. Am. Fam. Physician 2008, 78, 1186–1194. [Google Scholar]
- Mieczkowski, M.; Mrozikiewicz-Rakowska, B.; Kowara, M.; Kleibert, M.; Czupryniak, L. The Problem of Wound Healing in Diabetes-From Molecular Pathways to the Design of an Animal Model. Int. J. Mol. Sci. 2022, 23, 7930. [Google Scholar] [CrossRef]
- Yachmaneni, A., Jr.; Jajoo, S.; Mahakalkar, C.; Kshirsagar, S.; Dhole, S. A Comprehensive Review of the Vascular Consequences of Diabetes in the Lower Extremities: Current Approaches to Management and Evaluation of Clinical Outcomes. Cureus 2023, 15, e47525. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Bootun, R. Effects of immunosuppressive therapy on wound healing. Int. Wound J. 2013, 10, 98–104. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Jacobson, L.K.; Johnson, M.B.; Dedhia, R.D.; Niknam-Bienia, S.; Wong, A.K. Impaired wound healing after radiation therapy: A systematic review of pathogenesis and treatment. JPRAS Open 2017, 13, 92–105. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Bouten, R.M.; Young, E.F.; Selwyn, R.; Iacono, D.; Rittase, W.B.; Day, R.M. Chapter Two—Effects of radiation on endothelial barrier and vascular integrity. In Tissue Barriers in Disease, Injury and Regeneration; Gorbunov, N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 43–94. [Google Scholar] [CrossRef]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Sen, C.K.; Ghatak, S.; Gnyawali, S.C.; Roy, S.; Gordillo, G.M. Cutaneous Imaging Technologies in Acute Burn and Chronic Wound Care. Plast. Reconstr. Surg. 2016, 138, 119s–128s. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Pastar, I.; Vukelic, S.; Mahoney, M.G.; Brennan, D.; Krzyzanowska, A.; Golinko, M.; Brem, H.; Tomic-Canic, M. Deregulation of keratinocyte differentiation and activation: A hallmark of venous ulcers. J. Cell Mol. Med. 2008, 12, 2675–2690. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Models Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625, quiz 625–606. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Roy, S.; Elgharably, H.; Sinha, M.; Ganesh, K.; Chaney, S.; Mann, E.; Miller, C.; Khanna, S.; Bergdall, V.K.; Powell, H.M.; et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J. Pathol. 2014, 233, 331–343. [Google Scholar] [CrossRef]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S.; et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Haesler, E.; Swanson, T.; Ousey, K.; Carville, K. Clinical indicators of wound infection and biofilm: Reaching international consensus. J. Wound Care 2019, 28, s4–s12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Ganesh, K.; Sinha, M.; Mathew-Steiner, S.S.; Das, A.; Roy, S.; Sen, C.K. Chronic Wound Biofilm Model. Adv. Wound Care 2015, 4, 382–388. [Google Scholar] [CrossRef]
- Sen, C.K.; Ghatak, S. miRNA control of tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2629–2640. [Google Scholar] [CrossRef]
- Ghatak, S.; Chan, Y.C.; Khanna, S.; Banerjee, J.; Weist, J.; Roy, S.; Sen, C.K. Barrier Function of the Repaired Skin Is Disrupted Following Arrest of Dicer in Keratinocytes. Mol. Ther. 2015, 23, 1201–1210. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Ghatak, S.; Hemann, C.; Boslett, J.; Singh, K.; Sharma, A.; El Masry, M.S.; Abouhashem, A.S.; Ghosh, N.; Mathew-Steiner, S.S.; Roy, S.; et al. Bacterial Pyocyanin Inducible Keratin 6A Accelerates Closure of Epithelial Defect under Conditions of Mitochondrial Dysfunction. J. Investig. Dermatol. 2023, 143, 2052–2064.e5. [Google Scholar] [CrossRef]
- Singh, K.; Rustagi, Y.; Abouhashem, A.S.; Tabasum, S.; Verma, P.; Hernandez, E.; Pal, D.; Khona, D.K.; Mohanty, S.K.; Kumar, M.; et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J. Clin. Investig. 2022, 132, e157279. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Das, A.; El Masry, M.S.; Gnyawali, S.C.; Ghatak, S.; Singh, K.; Stewart, R.; Lewis, M.; Saha, A.; Gordillo, G.; Khanna, S. Skin Transcriptome of Middle-Aged Women Supplemented with Natural Herbo-mineral Shilajit Shows Induction of Microvascular and Extracellular Matrix Mechanisms. J. Am. Coll. Nutr. 2019, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; Lev-Tov, H.A.; Tomic-Canic, M.; Lee, W.D.; Williams, R.; Strasfeld, D.; Kirsner, R.S.; Herman, I.M. Advanced Wound Diagnostics: Toward Transforming Wound Care into Precision Medicine. Adv. Wound Care 2022, 11, 330–359. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.B. Implementing the Triangle of Wound Assessment framework to transform the care pathway for diabetic foot ulcers. J. Wound Care 2020, 29, 363–369. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Hess, C.T. Comprehensive Patient and Wound Assessments. Adv. Ski. Wound Care 2019, 32, 287–288. [Google Scholar] [CrossRef]
- Roy, S.; Sen, C.K.; Ghatak, S.; Higuita-Castro, N.; Palakurti, R.; Nalluri, N.; Clark, A.; Stewart, R.; Gallego-Perez, D.; Prater, D.N.; et al. Neurogenic tissue nanotransfection in the management of cutaneous diabetic polyneuropathy. Nanomedicine 2020, 28, 102220. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Queen, D.; Harding, K. What’s the true costs of wounds faced by different healthcare systems around the world? Int. Wound J. 2023, 20, 3935–3938. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. Sociogenomic Approach to Wound Care: A New Patient-Centered Paradigm. Adv. Wound Care 2019, 8, 523–526. [Google Scholar] [CrossRef]
- Espaulella-Ferrer, M.; Espaulella-Panicot, J.; Noell-Boix, R.; Casals-Zorita, M.; Ferrer-Sola, M.; Puigoriol-Juvanteny, E.; Cullell-Dalmau, M.; Otero-Viñas, M. Assessment of frailty in elderly patients attending a multidisciplinary wound care centre: A cohort study. BMC Geriatr. 2021, 21, 727. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Allen, L.; Wickramasinghe, K.; Mikkelsen, B.; Roberts, N.; Townsend, N. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J. Glob. Health 2018, 8, 020409. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Conti, G.; Arevalo, J.M.; Ruggiero, A.M.; Heckman, J.J.; Suomi, S.J. Transcriptional modulation of the developing immune system by early life social adversity. Proc. Natl. Acad. Sci. USA 2012, 109, 20578–20583. [Google Scholar] [CrossRef] [PubMed]
- Idaghdour, Y.; Czika, W.; Shianna, K.V.; Lee, S.H.; Visscher, P.M.; Martin, H.C.; Miclaus, K.; Jadallah, S.J.; Goldstein, D.B.; Wolfinger, R.D. Geographical genomics of human leukocyte gene expression variation in southern Morocco. Nat. Genet. 2010, 42, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage—A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205–217. [Google Scholar] [CrossRef]
- Veličković, V.; Janković, D. Challenges around quantifying uncertainty in a holistic approach to hard-to-heal wound management: Health economic perspective. Int. Wound J. 2023, 20, 792–798. [Google Scholar] [CrossRef]
- Sen, C.K. Standardized Wound Care: Patchwork Practices? Adv. Wound Care 2024, 13, 10. [Google Scholar] [CrossRef]
- Sutherland, B.L.; Pecanac, K.; Bartels, C.M.; Brennan, M.B. Expect delays: Poor connections between rural and urban health systems challenge multidisciplinary care for rural Americans with diabetic foot ulcers. J. Foot Ankle Res. 2020, 13, 32. [Google Scholar] [CrossRef]
- Fayne, R.A.; Borda, L.J.; Egger, A.N.; Tomic-Canic, M. The Potential Impact of Social Genomics on Wound Healing. Adv. Wound Care 2020, 9, 325–331. [Google Scholar] [CrossRef]
- Garima; Sharma, D.; Kumar, A.; Mostafavi, E. Extracellular vesicle-based biovectors in chronic wound healing: Biogenesis and delivery approaches. Mol. Ther. Nucleic Acids 2023, 32, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Katayama, S.; Vuola, J.; Kankuri, E. Human Wound-Healing Research: Issues and Perspectives for Studies Using Wide-Scale Analytic Platforms. Adv. Wound Care 2014, 3, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Song, Y.; You, Y.; Xu, X.; Lu, J.; Huang, X.; Zhang, J.; Zhu, L.; Hu, J.; Wu, X.; Xu, X.; et al. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Biopotentiated Extracellular Matrix Hydrogels Accelerate Diabetic Wound Healing and Skin Regeneration. Adv. Sci. 2023, 10, e2304023. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent advances in nanomedicines for regulation of macrophages in wound healing. J. Nanobiotechnol. 2022, 20, 407. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Norman, G.; Shi, C.; Goh, E.L.; Murphy, E.M.; Reid, A.; Chiverton, L.; Stankiewicz, M.; Dumville, J.C. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst. Rev. 2022, 4, CD009261. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair. Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, P.; Yadav, A.; Mishra, A.K.; Hazari, P.P.; Sharma, R.K. Sustained Activity of Stimuli-Responsive Curcumin and Acemannan Based Hydrogel Patches in Wound Healing. ACS Appl. Bio Mater. 2022, 5, 598–609. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Shi, L.; Song, D.; Meng, C.; Cheng, Y.; Wang, B.; Yang, Z. Opportunities and challenges of engineered exosomes for diabetic wound healing. Giant 2024, 18, 100251. [Google Scholar] [CrossRef]
- Zhong, Y.; Wei, E.-t.; Wu, L.; Wang, Y.; Lin, Q.; Wu, N.; Chen, H.; Tang, N. Novel Biomaterials for Wound Healing and Tissue Regeneration. ACS Omega 2024, 9, 32268–32286. [Google Scholar] [CrossRef]
- Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials 2023, 16, 4267. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Nanomaterials for wound healing: Current status and futuristic frontier. Biomed. Technol. 2024, 6, 26–45. [Google Scholar] [CrossRef]
- Afshar, M.; Rezaei, A.; Eghbali, S.; Nasirizadeh, S.; Alemzadeh, E.; Alemzadeh, E.; Shadi, M.; Sedighi, M. Nanomaterial strategies in wound healing: A comprehensive review of nanoparticles, nanofibres and nanosheets. Int. Wound J. 2024, 21, e14953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Copper@ZIF-8 Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2021, 31, 2008054. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Keleştemur, S.; Kilic, E.; Uslu, Ü.; Cumbul, A.; Ugur, M.; Akman, S.; Culha, M. Wound healing properties of modified silver nanoparticles and their distribution in mouse organs after topical application. Nano Biomed. Eng. 2012, 4, 170. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper metal–organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Y.; Wang, J.; Lv, F.; Yi, Z.; Ke, Q.; Xu, H. Space-oriented nanofibrous scaffold with silicon-doped amorphous calcium phosphate nanocoating for diabetic wound healing. ACS Appl. Bio Mater. 2019, 2, 787–795. [Google Scholar] [CrossRef]
- Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 747–765. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Raj, M.; Biswas, R.; Hiroshi, T.; Nair, S.V.; Jayakumar, R. Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm. Res. 2013, 30, 523–537. [Google Scholar] [CrossRef]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.A.; Chen, H.M.; Yao, Y.D.; Hung, C.F.; Tu, C.S.; Liang, Y.J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef]
- Zhou, Z.; Joslin, S.; Dellinger, A.; Ehrich, M.; Brooks, B.; Ren, Q.; Rodeck, U.; Lenk, R.; Kepley, C.L. A novel class of compounds with cutaneous wound healing properties. J. Biomed. Nanotechnol. 2010, 6, 605–611. [Google Scholar] [CrossRef]
- Shahnawaz Khan, M.; Abdelhamid, H.N.; Wu, H.F. Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloids Surf. B Biointerfaces 2015, 127, 281–291. [Google Scholar] [CrossRef]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef]
- Ma, K.; Liao, S.; He, L.; Lu, J.; Ramakrishna, S.; Chan, C.K. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng. Part A 2011, 17, 1413–1424. [Google Scholar] [CrossRef]
- Mao, C.; Chen, X.; Miao, G.; Lin, C. Angiogenesis stimulated by novel nanoscale bioactive glasses. Biomed. Mater. 2015, 10, 025005. [Google Scholar] [CrossRef]
- Kwon, M.J.; An, S.; Choi, S.; Nam, K.; Jung, H.S.; Yoon, C.S.; Ko, J.H.; Jun, H.J.; Kim, T.K.; Jung, S.J.; et al. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J. Gene Med. 2012, 14, 272–278. [Google Scholar] [CrossRef]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Venkata Rao, J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yu, D.; Nie, F.; Wang, Y.; Chong, Y. Iron Nanoparticles Open Up New Directions for Promoting Healing in Chronic Wounds in the Context of Bacterial Infection. Pharmaceutics 2023, 15, 2327. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, J.; Shi, W.; Zhang, J.; Tao, C. Recent advances in metal–organic frameworks and their composites for the phototherapy of skin wounds. J. Mater. Chem. B 2022, 10, 4695–4713. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Q.; Lu, L.; Qiu, X.; Knoedler, S.; Panayi, A.C.; Jiang, D.; Rinkevich, Y.; Lin, Z.; Mi, B.; et al. Metal-Organic Frameworks and Their Composites for Chronic Wound Healing: From Bench to Bedside. Adv. Mater. 2024, 36, e2302587. [Google Scholar] [CrossRef]
- Nascimento, E.G.d.; Sampaio, T.B.M.; Medeiros, A.C.; Azevedo, E.P.d. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir. Bras. 2009, 24, 460–465. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Samaha, R.; Othman, A.; El-Sherbiny, I.; Amer, M.; Elhusseini, F.; ElMissiry, M. Topical Nitric oxide in nanoformulation enhanced wound healing in experimental diabetes in mice. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 499–514. [Google Scholar]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Li, C.; Sharma, R.K.; Swihart, M.T. Microencapsulated UV filter@ZIF-8 based sunscreens for broad spectrum UV protection. RSC Adv. 2020, 10, 34254–34260. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, A.; Sharma, R.K. Mesoporous iron gallate nanocomplex for adsorption and degradation of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123694. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, A.; Cwiklinski, K.; Quaye, E.; Aalinkeel, R.; Mahajan, S.D.; Schwartz, S.A.; Sharma, R.K. In-vitro studies of curcumin encapsulated mesoporous Fe-Phenanthroline nanocluster for reduction of amyloid β plaque. J. Drug Deliv. Sci. Technol. 2019, 54, 101314. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Khanna, S.; Roy, S.; Thirunavukkarasu, M.; Pradeep, S.R.; Wulff, B.C.; El Masry, M.S.; Sharma, A.; Palakurti, R.; Ghosh, N.; et al. Driving adult tissue repair via re-engagement of a pathway required for fetal healing. Mol. Ther. 2023, 31, 454–470. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142, 662–678.e8. [Google Scholar] [CrossRef]
- Dai, W.; Dong, Y.; Han, T.; Wang, J.; Gao, B.; Guo, H.; Xu, F.; Li, J.; Ma, Y. Microenvironmental cue-regulated exosomes as therapeutic strategies for improving chronic wound healing. NPG Asia Mater. 2022, 14, 75. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Wang, Y.; Zhou, K.; Li, H.; Bi, S.; Wang, Y.; Wu, W.; Huang, Y.; Peng, B.; et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 2023, 11, 1029671. [Google Scholar] [CrossRef]
- Riha, S.M.; Maarof, M.; Fauzi, M.B. Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers 2021, 13, 1546. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Rational Design of Multifunctional Hydrogels for Wound Repair. J. Funct. Biomater. 2023, 14, 553. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Long, L.; He, S.; Wang, Z.; Liu, Y.; Yang, L.; Chen, N.; Hu, C.; Wang, Y. Responsive multifunctional hydrogels emulating the chronic wounds healing cascade for skin repair. J. Control. Release 2023, 354, 821–834. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.-L.; Lu, S.-T.; Zhang, N.-Y.; Zhang, H.-J.; Zhang, J.; Zhang, J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res. Ther. 2022, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Swindell, H.S.; Ramasubramanian, L.; Liu, R.; Lam, K.S.; Farmer, D.L.; Wang, A. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified with Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front. Bioeng. Biotechnol. 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Farabi, B.; Roster, K.; Hirani, R.; Tepper, K.; Atak, M.F.; Safai, B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3006. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Ghatak, S.; Singh, K.; Abouhashem, A.S.; Kumar, M.; El Masry, M.S.; Mohanty, S.K.; Palakurti, R.; Rustagi, Y.; Tabasum, S.; et al. Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat. Commun. 2023, 14, 1129. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, K.; Abouhashem, A.S.; Kumar, M.; Kacar, S.; Verma, S.S.; Mohanty, S.K.; Sinha, M.; Ghatak, S.; Xuan, Y.; et al. Human fetal dermal fibroblast-myeloid cell diversity is characterized by dominance of pro-healing Annexin1-FPR1 signaling. iScience 2023, 26, 107533. [Google Scholar] [CrossRef]
- Long, C.; Wang, J.; Gan, W.; Qin, X.; Yang, R.; Chen, X. Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing. Front. Surg. 2022, 9, 1030288. [Google Scholar] [CrossRef]
- Guda, P.R.; Sharma, A.; Anthony, A.J.; ElMasry, M.S.; Couse, A.D.; Ghatak, P.D.; Das, A.; Timsina, L.; Trinidad, J.C.; Roy, S.; et al. Nanoscopic and Functional Characterization of Keratinocyte-Originating Exosomes in the Wound Fluid of Non-Diabetic and Diabetic Chronic Wound Patients. Nano Today 2023, 52, 101954. [Google Scholar] [CrossRef]
- Brown, B.A.; Guda, P.R.; Zeng, X.; Anthony, A.; Couse, A.; Barnes, L.F.; Sharon, E.M.; Trinidad, J.C.; Sen, C.K.; Jarrold, M.F.; et al. Analysis of Keratinocytic Exosomes from Diabetic and Nondiabetic Mice by Charge Detection Mass Spectrometry. Anal. Chem. 2022, 94, 8909–8918. [Google Scholar] [CrossRef]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Pi, L.; Yang, L.; Fang, B.R.; Meng, X.X.; Qian, L. LncRNA MALAT1 from human adipose-derived stem cell exosomes accelerates wound healing via miR-378a/FGF2 axis. Regen. Med. 2022, 17, 627–641. [Google Scholar] [CrossRef]
- Tan, K.; Mo, H.; Guo, L.; Wang, B. MALAT1 accelerates proliferation and inflammation and suppresses apoptosis of endometrial stromal cells via the microRNA-142-3p/CXCR7 axis. Reprod. Biol. 2022, 22, 100675. [Google Scholar] [CrossRef] [PubMed]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef]
- Han, X.; Wu, P.; Li, L.; Sahal, H.M.; Ji, C.; Zhang, J.; Wang, Y.; Wang, Q.; Qian, H.; Shi, H.; et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell Cycle 2021, 20, 616–629. [Google Scholar] [CrossRef]
- Xia, W.; Li, M.; Jiang, X.; Huang, X.; Gu, S.; Ye, J.; Zhu, L.; Hou, M.; Zan, T. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J. Nanobiotechnol. 2022, 20, 144. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef]
- Qie, Y.; Yuan, H.; von Roemeling, C.A.; Chen, Y.; Liu, X.; Shih, K.D.; Knight, J.A.; Tun, H.W.; Wharen, R.E.; Jiang, W.; et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 2016, 6, 26269. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: A review. Crit. Rev. Biotechnol. 2020, 40, 804–820. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.-F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gao, X.; Wang, S.; He, Y.; Ma, X.; Zhang, T.; Liu, X. Advanced strategies to evade the mononuclear phagocyte system clearance of nanomaterials. Exploration 2023, 3, 20220045. [Google Scholar] [CrossRef] [PubMed]
- Khona, D.K.; Roy, S.; Ghatak, S.; Huang, K.; Jagdale, G.; Baker, L.A.; Sen, C.K. Ketoconazole resistant Candida albicans is sensitive to a wireless electroceutical wound care dressing. Bioelectrochemistry 2021, 142, 107921. [Google Scholar] [CrossRef]
- Sousa, P.; Lopes, B.; Sousa, A.C.; Moreira, A.; Coelho, A.; Alvites, R.; Alves, N.; Geuna, S.; Maurício, A.C. Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023). Biomedicines 2023, 11, 2099. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef]
- Yadav, A.; Xuan, Y.; Sen, C.K.; Ghatak, S. Standardized Reporting of Research on Exosomes to Ensure Rigor and Reproducibility. Adv. Wound Care 2024, 13, 584–599. [Google Scholar] [CrossRef]
- Li, J.; Ghatak, S.; El Masry, M.S.; Das, A.; Liu, Y.; Roy, S.; Lee, R.J.; Sen, C.K. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound. Mol. Ther. 2018, 26, 2178–2188. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.J.; Kang, S.W.; Yoo, T.H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Ye, H.; Wang, F.; Xu, G.; Shu, F.; Fan, K.; Wang, D. Advancements in engineered exosomes for wound repair: Current research and future perspectives. Front. Bioeng. Biotechnol. 2023, 11, 1301362. [Google Scholar] [CrossRef]
- Li, Z.; Xuan, Y.; Ghatak, S.; Guda, P.R.; Roy, S.; Sen, C.K. Modeling the gene delivery process of the needle array-based tissue nanotransfection. Nano Res. 2022, 15, 3409–3421. [Google Scholar] [CrossRef]
- Xuan, Y.; Wang, C.; Ghatak, S.; Sen, C.K. Tissue Nanotransfection Silicon Chip and Related Electroporation-Based Technologies for In Vivo Tissue Reprogramming. Nanomaterials 2024, 14, 217. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- Parada, N.; Romero-Trujillo, A.; Georges, N.; Alcayaga-Miranda, F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 2021, 31, 61–74. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro Dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef]
- Si, C.; Gao, J.; Ma, X. Engineered exosomes in emerging cell-free therapy. Front. Oncol. 2024, 14, 1382398. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Han, G.; Liang, M.; Yang, Z.; Zhang, C.; Huang, S.; Tai, S.; Yu, S. The Therapeutic Potential and Clinical Significance of Exosomes as Carriers of Drug Delivery System. Pharmaceutics 2022, 15, 21. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Sharma, V.; Mukhopadhyay, C.D. Exosome as drug delivery system: Current advancements. Extracell. Vesicle 2024, 3, 100032. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, J.N.; Rodrigues, L.R. New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol. Hematol. 2022, 172, 103628. [Google Scholar] [CrossRef]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef]
- Kuang, L.; Zhang, C.; Li, B.; Deng, H.; Chen, R.; Li, G. Human Keratinocyte-Derived Exosomal MALAT1 Promotes Diabetic Wound Healing by Upregulating MFGE8 via microRNA-1914-3p. Int. J. Nanomed. 2023, 18, 949–970. [Google Scholar] [CrossRef]
- Glady, A.; Vandebroek, A.; Yasui, M. Human keratinocyte-derived extracellular vesicles activate the MAPKinase pathway and promote cell migration and proliferation in vitro. Inflamm. Regen. 2021, 41, 4. [Google Scholar] [CrossRef]
- Belvedere, R.; Pessolano, E.; Porta, A.; Tosco, A.; Parente, L.; Petrella, F.; Perretti, M.; Petrella, A. Mesoglycan induces the secretion of microvesicles by keratinocytes able to activate human fibroblasts and endothelial cells: A novel mechanism in skin wound healing. Eur. J. Pharmacol. 2020, 869, 172894. [Google Scholar] [CrossRef]
- Liu, K.; Luo, X.; Lv, Z.-Y.; Zhang, Y.-J.; Meng, Z.; Li, J.; Meng, C.-X.; Qiang, H.-F.; Hou, C.-Y.; Hou, L.; et al. Macrophage-Derived Exosomes Promote Bone Mesenchymal Stem Cells Towards Osteoblastic Fate Through microRNA-21a-5p. Front. Bioeng. Biotechnol. 2022, 9, 801432. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp. Cell Res. 2020, 394, 112146. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef] [PubMed]
- Kwak, G.; Cheng, J.; Kim, H.; Song, S.; Lee, S.J.; Yang, Y.; Jeong, J.H.; Lee, J.E.; Messersmith, P.B.; Kim, S.H. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small 2022, 18, e2200060. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Grady, C. Institutional Review Boards: Purpose and Challenges. Chest 2015, 148, 1148–1155. [Google Scholar] [CrossRef]

| Types of Nanomaterials | Biomolecules Loaded | Role in Wound Healing | References |
|---|---|---|---|
| Polymeric nanoparticles | Drugs, nitric oxide, curcumin, siRNA | Hemostasis, proliferation, inflammation, remodeling | [101] |
| Zinc Oxide nanoparticles | Hemostasis | [102] | |
| Nanoceria | Hemostasis, inflammation, remodeling | [103] | |
| Gold nanoparticles | Drugs, siRNA | Proliferation, inflammation | [104] |
| Fullerene, Graphene Oxide, Carbon nanotubes | Proliferation, inflammation | [105,106] | |
| Zinc Oxide nanoflowers | Proliferation | [107] | |
| Polymeric nanofibers | Plasmid DNA | Proliferation | [108] |
| Polymeric nanoscaffolds | Stem cells | Proliferation, remodeling | [109] |
| Bioactive glass particles | Proliferation | [110] | |
| Dendrimers | Plasmid DNA | Proliferation | [111] |
| Liposomes | Growth factor, drugs | Proliferation, inflammation | [112] |
| Copper nanoparticles | Inflammation | [113] | |
| Silver nanoparticles | Drugs, oligonucleotide | Inflammation | [114] |
| Ceramic nanoparticles | Nitric oxide, curcumin | Inflammation | [103] |
| Iron Oxide nanoparticles | Nitric oxide | Remodeling | [115] |
| Metal–Organic Frameworks (M- Zn, Cu, Fe, Mg, Ag, and others) | Drugs | Hemostasis, proliferation, inflammation, remodeling | [116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.; Sharma, A.; Moulick, M.; Ghatak, S. Nanomanaging Chronic Wounds with Targeted Exosome Therapeutics. Pharmaceutics 2025, 17, 366. https://doi.org/10.3390/pharmaceutics17030366
Yadav A, Sharma A, Moulick M, Ghatak S. Nanomanaging Chronic Wounds with Targeted Exosome Therapeutics. Pharmaceutics. 2025; 17(3):366. https://doi.org/10.3390/pharmaceutics17030366
Chicago/Turabian StyleYadav, Anita, Anu Sharma, Mohini Moulick, and Subhadip Ghatak. 2025. "Nanomanaging Chronic Wounds with Targeted Exosome Therapeutics" Pharmaceutics 17, no. 3: 366. https://doi.org/10.3390/pharmaceutics17030366
APA StyleYadav, A., Sharma, A., Moulick, M., & Ghatak, S. (2025). Nanomanaging Chronic Wounds with Targeted Exosome Therapeutics. Pharmaceutics, 17(3), 366. https://doi.org/10.3390/pharmaceutics17030366






