The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals
Abstract
:1. Introduction
2. Pros and Cons of 3D Pharmaceutical Printing
3. Three-Dimensional Printing Techniques for Pharmaceuticals
3.1. Fused Deposition Modelling (FDM)
3.2. Direct Powder Extrusion (DPE)
3.3. Semi-Solid Extrusion (SSE)
3.4. Selective Laser Sintering (SLS)
3.5. Binder Jetting (BJ)
3.6. Stereolithography (SLA)
3.7. Additional 3D Printing Techniques
3.7.1. Digital Light Processing (DLP)
3.7.2. Inkjet Printing (IP)
3.7.3. Direct Writing (DW)
4. Bioinks, New Tablet Shapes, and Different Layers of Drug Substances
5. Innovative Substances for 3D-Printed Pharmaceutical Forms in Geriatric and Pediatric Applications
6. Personalized Treatment and Complex Drug Regimens for Pediatric and Geriatric Patients
7. Three-Dimensional Pharmaceutical Printing for Patients with Chronic Diseases
8. Novel Dosage Forms Developed Using 3D Printing: Future and Innovation
9. Overview of the Specifications and Requirements of 3D Printing in the Pharmaceutical Industry
10. Production of Customized 3D-Printed Pharmaceutical Dosage Forms
11. Artificial Intelligence (AI) in 3D Pharmaceutical Printing
12. Future Directions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Garg, A.; Mustafa, G.; Mohammed, A.A.; Ahmad, M.Z. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics 2023, 15, 1448. [Google Scholar] [CrossRef]
- Mohammed, A.; Algahtani, M.; Ahmad, M.; Ahmad, J.; Kotta, S. 3D Printing in Medicine: Technology Overview and Drug Delivery Applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Milliken, R.L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. Application of 3D printing in early phase development of pharmaceutical solid dosage forms. Int. J. Pharm. 2024, 653, 123902. [Google Scholar] [CrossRef]
- Pravin, S.; Sudhir, A. Integration of 3D printing with dosage forms: A new perspective for modern healthcare. Biomed. Pharmacother. 2018, 107, 146–154. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. 3D printing applications for healthcare research and development. Glob. Health J. 2022, 6, 217–226. [Google Scholar] [CrossRef]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D printing: Innovative solutions for patients and pharmaceutical industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Parhi, R.; Jena, G.K. An updated review on application of 3D printing in fabricating pharmaceutical dosage forms. Drug Deliv. Transl. Res. 2022, 12, 2428–2462. [Google Scholar] [CrossRef] [PubMed]
- Bácskay, I.; Ujhelyi, Z.; Fehér, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Desu, P.K.; Maddiboyina, B.; Vanitha, K.; Rao Gudhanti, S.N.K.; Anusha, R.; Jhawat, V. 3D Printing Technology in Pharmaceutical Dosage Forms: Advantages and Challenges. Curr. Drug Targets 2021, 22, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kar, R.K.; Biswal, P.K.; Bindhani, S. Approaches of 3D printing in current drug delivery. Sens. Int. 2022, 3, 100146. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef]
- Beer, N.; Kaae, S.; Genina, N.; Sporrong, S.K.; Alves, T.L.; Hoebert, J.; De Bruin, M.L.; Hegger, I. Magistral Compounding with 3D Printing: A Promising Way to Achieve Personalized Medicine. Ther. Innov. Regul. Sci. 2023, 57, 26–36. [Google Scholar] [CrossRef]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Tranová, T.; Pyteraf, J.; Kurek, M.; Jamróz, W.; Brniak, W.; Spálovská, D.; Loskot, J.; Jurkiewicz, K.; Grelska, J.; Kramarczyk, D.; et al. Fused Deposition Modeling as a Possible Approach for the Preparation of Orodispersible Tablets. Pharmaceuticals 2022, 15, 69. [Google Scholar] [CrossRef]
- Wang, H.; Vemula, S.K.; Bandari, S.; Repka, M.A. Preparation of core-shell controlled release tablets using direct powder extrusion 3D printing techniques. J. Drug Deliv. Sci. Technol. 2023, 88, 104896. [Google Scholar] [CrossRef]
- Jeong, H.M.; Weon, K.Y.; Shin, B.S.; Shin, S. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules 2020, 25, 2330. [Google Scholar] [CrossRef]
- Awad, A.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed infliximab suppositories for rectal biologic delivery. Int. J. Pharm. X 2023, 5, 100176. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Müller, L.; Sarwinska, D.; Seidlitz, A.; Sznitowska, M.; Weitschies, W. 3D Printing of Mini Tablets for Pediatric Use. Pharmaceuticals 2021, 14, 143. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Raje, V.; Palekar, S.; Banella, S.; Patel, K. Tunable Drug Release from Fused Deposition Modelling (FDM) 3D-Printed Tablets Fabricated Using a Novel Extrudable Polymer. Pharmaceutics 2022, 14, 2192. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Casas, M.; Ferrero, C.; Linares, V.; Caraballo, I. 3D Printing Direct Powder Extrusion in the Production of Drug Delivery Systems: State of the Art and Future Perspectives. Pharmaceutics 2024, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Kuofie, H.; Scoble, J.; Boulton, S.; Douroumis, D. Selective Laser Sintering for printing pharmaceutical dosage forms. J. Drug Deliv. Sci. Technol. 2023, 86, 104699. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Peng, X.; Kuang, X.; Roach, D.J.; Wang, Y.; Hamel, C.M.; Lu, C.; Qi, H.J. Integrating digital light processing with direct ink writing for hybrid 3D printing of functional structures and devices. Addit. Manuf. 2021, 40, 101911. [Google Scholar] [CrossRef]
- Balani, S.B.; Ghaffar, S.H.; Chougan, M.; Pei, E.; Şahin, E. Processes and materials used for direct writing technologies: A review. Results Eng. 2021, 11, 100257. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Mogan, J.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Foudzi, F.M.; Sulong, A.B.; Tarlochan, F.; Ahmad, F. Fused Deposition Modelling of Polymer Composite: A Progress. Polymers 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Araújo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The Digital Pharmacies Era: How 3D Printing Technology Using Fused Deposition Modeling Can Become a Reality. Pharmaceutics 2019, 11, 128. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Kluska, E.; Gruda, P.; Majca-Nowak, N. The Accuracy and the Printing Resolution Comparison of Different 3D Printing Technologies. Trans. Aerosp. Res. 2018, 2018, 75–92. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics 2022, 14, 2411. [Google Scholar] [CrossRef]
- Hussain, A.; Mahmood, F.; Arshad, M.S.; Abbas, N.; Qamar, N.; Mudassir, J.; Farhaj, S.; Nirwan, J.S.; Ghori, M.U. Personalised 3D Printed Fast-Dissolving Tablets for Managing Hypertensive Crisis: In-Vitro/In-Vivo Studies. Polymers 2020, 12, 3057. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, X.; Tena, G.; Duque, A.; Uranga, N.; Campanero, M.Á.; Alonso, J. Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing. Pharmaceutics 2021, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef]
- Rosch, M.; Gutowski, T.; Baehr, M.; Eggert, J.; Gottfried, K.; Gundler, C.; Nürnberg, S.; Langebrake, C.; Dadkhah, A. Development of an immediate release excipient composition for 3D printing via direct powder extrusion in a hospital. Int. J. Pharm. 2023, 643, 123218. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Lindfors, L.; Wang, X.; Ojala, S.; Sandler, N. Semi-solid extrusion 3D printing of tailored ChewTs for veterinary use—A focus on spectrophotometric quantification of gabapentin. Eur. J. Pharm. Sci. 2022, 174, 106190. [Google Scholar] [CrossRef]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed Oral Dosage Forms: Mechanical Properties, Computational Approaches and Applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Zhu, X.; Chen, F.; Han, X. Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering 2023, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced printable hydrogels from pre-crosslinked alginate as a new tool in semi solid extrusion 3D printing process. Carbohydr. Polym. 2022, 276, 118746. [Google Scholar] [CrossRef] [PubMed]
- Balasankar, A.; Anbazhakan, K.; Arul, V.; Mutharaian, V.N.; Sriram, G.; Aruchamy, K.; Oh, T.H.; Ramasundaram, S. Recent Advances in the Production of Pharmaceuticals Using Selective Laser Sintering. Biomimetics 2023, 8, 330. [Google Scholar] [CrossRef]
- Nagarajan, B.; Hu, Z.; Song, X.; Zhai, W.; Wei, J. Development of Micro Selective Laser Melting: The State of the Art and Future Perspectives. Engineering 2019, 5, 702–720. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Gonot-Munck, Q.; Baudoux, A.; Garg, V.; Farnish, R.; Katsamenis, O.L.; Hui, H.W.; Boersen, N.; Roberts, S.; Jones, J.; et al. 3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering. Pharmaceutics 2023, 15, 2230. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Bataille, B.; Aubert, A.; Leclercq, L.; Rossi, J.C.; Soulairol, I. Selective Laser Sintering of Solid Oral Dosage Forms with Copovidone and Paracetamol Using a CO2 Laser. Pharmaceutics 2021, 13, 160. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Wu, J.; Duan, S.; Wang, X.; Hong, X.; Han, X.; Li, C.; Kang, D.; Wang, Z.; et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics 2022, 14, 2589. [Google Scholar] [CrossRef]
- Yu, D.G.; Branford-White, C.; Ma, Z.H.; Zhu, L.M.; Li, X.Y.; Yang, X.L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 2009, 370, 160–166. [Google Scholar] [CrossRef]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef]
- Triacca, A.; Pitzanti, G.; Mathew, E.; Conti, B.; Dorati, R.; Lamprou, D.A. Stereolithography 3D printed implants: A preliminary investigation as potential local drug delivery systems to the ear. Int. J. Pharm. 2022, 616, 121529. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111773. [Google Scholar] [CrossRef]
- Curti, C.; Kirby, D.J.; Russell, C.A. Current formulation approaches in design and development of solid oral dosage forms through three-dimensional printing. Prog. Addit. Manuf. 2020, 5, 111–123. [Google Scholar] [CrossRef]
- Joji George, A.; Saju, F. 3D Printing of medicines: A review. Int. J. Pharm. Drug Anal. 2022, 10, 6–8. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-dimensional Printing for Medical Applications. Int. J. Bioprint. 2020, 6, 242. [Google Scholar] [CrossRef]
- Kundu, A.; Arnett, P.; Bagde, A.; Azim, N.; Kouagou, E.; Singh, M.; Rajaraman, S. DLP 3D Printed “Intelligent” Microneedle Array (iμNA) for Stimuli Responsive Release of Drugs and Its in Vitro and ex Vivo Characterization. J. Microelectromech. Syst. 2020, 29, 685–691. [Google Scholar] [CrossRef]
- Krkobabić, M.; Medarević, D.; Cvijić, S.; Grujić, B.; Ibrić, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Hyun, J. Silk fibroin microneedles fabricated by digital light processing 3D printing. J. Ind. Eng. Chem. 2021, 95, 126–133. [Google Scholar] [CrossRef]
- Carou-Senra, P.; Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Inkjet Printing of Pharmaceuticals. Adv. Mater. 2024, 36, e2309164. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-printed electrochemical sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Winkless, L. Inkjet printing for drug delivery. Mater. Today 2014, 17, 261–262. [Google Scholar] [CrossRef]
- Kholghi Eshkalak, S.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. A review on inkjet printing of CNT composites for smart applications. Appl. Mater. Today 2017, 9, 372–386. [Google Scholar] [CrossRef]
- Buanz, A.B.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Hon, K.K.B.; Li, L.; Hutchings, I.M. Direct writing technology—Advances and developments. CIRP Ann. 2008, 57, 601–620. [Google Scholar] [CrossRef]
- Desai, S.; Harrison, B. Direct-Writing of Biomedia for Drug Delivery and Tissue Regeneration. In Printed Biomaterials; Springer: New York, NY, USA, 2009; pp. 71–89. [Google Scholar]
- Xu, C.; Yu, S.; Wu, W.; Liu, Q.; Ren, L. Direct ink writing of Fe bone implants with independently adjustable structural porosity and mechanical properties. Addit. Manuf. 2022, 51, 102589. [Google Scholar] [CrossRef]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D bioprinting in tissue engineering and regenerative medicine: Current progress and challenges. Int. J. Bioprint. 2023, 9, 207–238. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, D.S.; Gaur, K.; Arya, N.; Choubey, V.K.; Khan, R. 3D bioprinting for drug development and screening: Recent trends towards personalized medicine. Hybrid. Adv. 2024, 7, 100320. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. 3D Bioprinting: Challenges in Commercialization and Clinical Translation. J. 3D Print. Med. 2023, 7, 3DP8. [Google Scholar] [CrossRef]
- Saylam, E.; Akkaya, Y.; Ilhan, E.; Cesur, S.; Guler, E.; Sahin, A.; Cam, M.E.; Ekren, N.; Oktar, F.N.; Gunduz, O.; et al. Levodopa-Loaded 3D-Printed Poly (Lactic) Acid/Chitosan Neural Tissue Scaffold as a Promising Drug Delivery System for the Treatment of Parkinson’s Disease. Appl. Sci. 2021, 11, 10727. [Google Scholar] [CrossRef]
- Kutlehria, S.; D’Souza, A.; Bleier, B.S.; Amiji, M.M. Role of 3D Printing in the Development of Biodegradable Implants for Central Nervous System Drug Delivery. Mol. Pharm. 2022, 19, 4411–4427. [Google Scholar] [CrossRef]
- King, J.L.; Maturavongsadit, P.; Hingtgen, S.D.; Benhabbour, S.R. Injectable pH Thermo-Responsive Hydrogel Scaffold for Tumoricidal Neural Stem Cell Therapy for Glioblastoma Multiforme. Pharmaceutics 2022, 14, 2243. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Mahya, S.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Berberine loaded chitosan nanoparticles encapsulated in polysaccharide-based hydrogel for the repair of spinal cord. Int. J. Biol. Macromol. 2021, 182, 82–90. [Google Scholar] [CrossRef]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2020, 30, 1906237. [Google Scholar] [CrossRef]
- Liao, W.; Shi, Y.; Li, Z.; Yin, X. Advances in 3D printing combined with tissue engineering for nerve regeneration and repair. J. Nanobiotechnol. 2025, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/bioinks and extrusion bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3D Bioprinting with Live Cells. Eng. Regen. 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.; El-Sheikh, M.; Seleem, A. Antimicrobial Wound Dressing and Anti-inflammatory Efficacy of Silver Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’Connell, C.D. The Regulatory Challenge of 3D Bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef]
- Freeman, S.; Calabro, S.; Williams, R.; Jin, S.; Ye, K. Bioink Formulation and Machine Learning-Empowered Bioprinting Optimization. Front. Bioeng. Biotechnol. 2022, 10, 913579. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Fang, Y.; Xiong, Z.; Zhang, T. AI-driven 3D bioprinting for regenerative medicine: From bench to bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef]
- Auel, T.; Mentrup, A.F.C.; Oldfield, L.R.; Seidlitz, A. 3D printing of pharmaceutical dosage forms: Recent advances and applications. Adv. Drug Deliv. Rev. 2025, 217, 115504. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Kshirsagar, S.J. Review on Tablet in Tablet techniques. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Molavi, F.; Hamishehkar, H.; Nokhodchi, A. Impact of Tablet Shape on Drug Dissolution Rate Through Immediate Released Tablets. Adv. Pharm. Bull. 2020, 10, 656–661. [Google Scholar] [CrossRef]
- Kalmer, R.R.; Haddadan, M.M.; Azizi, M.; Ghanbari, M.; Samandarian, D.; Sadjadinia, A.; Ramezanalizadeh, H.; Karimi, A.; Golizadeh, M. Industrial Manufacture of Enteric Hard Capsules Using Novel Formulations Based on Hypromellose Phthalate/Gelatin and Investigation of Pantoprazole Release. ACS Omega 2023, 8, 11293–11303. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Breitkreutz, J.; Quodbach, J. 3D Printed Mini-Floating-Polypill for Parkinson’s Disease: Combination of Levodopa, Benserazide, and Pramipexole in Various Dosing for Personalized Therapy. Pharmaceutics 2022, 14, 931. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, J.; Wang, M.; Wang, L.; Yang, J. 3D Printed Polyvinyl Alcohol Tablets with Multiple Release Profiles. Sci. Rep. 2019, 9, 12487. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, Y.-R.; Kang, J.-H.; Park, Y.-S.; Kim, D.-W.; Park, C.-W. Geometry-Driven Fabrication of Mini-Tablets via 3D Printing: Correlating Release Kinetics with Polyhedral Shapes. Pharmaceutics 2024, 16, 783. [Google Scholar] [CrossRef] [PubMed]
- Parulski, C.; Bya, L.-A.; Goebel, J.; Servais, A.-C.; Lechanteur, A.; Evrard, B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicine. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef] [PubMed]
- Shyr, Z.A.; Cheng, Y.S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.K.; Son, K.H.; Lee, J.W. PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels 2022, 8, 163. [Google Scholar] [CrossRef]
- McDonagh, T.; Belton, P.; Qi, S. Manipulating drug release from 3D printed dual-drug loaded polypills using challenging polymer compositions. Int. J. Pharm. 2023, 637, 122895. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Vieta, E.; Siamouli, M.; Valenti, M.; Magiria, S.; Oral, T.; Fresno, D.; Giannakopoulos, P.; Kaprinis, G.S. Treatment of bipolar disorder: A complex treatment for a multi-faceted disorder. Ann. Gen. Psychiatry 2007, 6, 27. [Google Scholar] [CrossRef]
- Andrews, G.P.; Li, S.; Almajaan, A.; Yu, T.; Martini, L.; Healy, A.; Jones, D.S. Fixed Dose Combination Formulations: Multilayered Platforms Designed for the Management of Cardiovascular Disease. Mol. Pharm. 2019, 16, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh Tabriz, A.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 120147. [Google Scholar] [CrossRef]
- Hu, J.; Wan, J.; Xi, J.; Shi, W.; Qian, H. AI-driven design of customized 3D-printed multi-layer capsules with controlled drug release profiles for personalized medicine. Int. J. Pharm. 2024, 656, 124114. [Google Scholar] [CrossRef]
- Basit, A.; Trenfield, S. 3D printing of pharmaceuticals and the role of pharmacy. Pharm. J. 2022, 308, 7959. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?-A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Dolores Bóveda, M.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. Int. J. Pharm. 2024, 657, 124140. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Garg, A.; Deshmukh, R. A Snapshot of Current Updates and Future Prospects of 3D Printing in Medical and Pharmaceutical Science. Curr. Pharm. Des. 2023, 29, 604–619. [Google Scholar] [CrossRef]
- Chakka, L.R.J.; Chede, S. 3D printing of pharmaceuticals for disease treatment. Front. Med. Technol. 2022, 4, 1040052. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef]
- González, K.; Larraza, I.; Berra, G.; Eceiza, A.; Gabilondo, N. 3D printing of customized all-starch tablets with combined release kinetics. Int. J. Pharm. 2022, 622, 121872. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Renu; Dahiya, J.; Jalwal, P.; Singh, B. Chewable Tablets: A Comprehensive Review. Pharma Innov. J. 2015, 4, 100–105. [Google Scholar]
- Bendicho-Lavilla, C.; Rodríguez-Pombo, L.; Januskaite, P.; Rial, C.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Ensuring the quality of 3D printed medicines: Integrating a balance into a pharmaceutical printer for in-line uniformity of mass testing. J. Drug Deliv. Sci. Technol. 2024, 92, 105337. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Z.; Li, X.; Wang, S.; Han, X.; Chen, D.; Zheng, A. 3D Printing Technology Based on Versatile Gelatin-Carrageenan Gel System for Drug Formulations. Pharmaceutics 2023, 15, 1218. [Google Scholar] [CrossRef]
- Ochoa, E.; Morelli, L.; Salvioni, L.; Giustra, M.; De Santes, B.; Spena, F.; Barbieri, L.; Garbujo, S.; Viganò, M.; Novati, B.; et al. Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology. Eur. J. Pharm. Biopharm. 2024, 194, 85–94. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Hong, X.; Han, X.; Liu, B.; Li, X.; Zhang, H.; Gao, J.; Liu, N.; Gao, X.; et al. Taste Masking Study Based on an Electronic Tongue: The Formulation Design of 3D Printed Levetiracetam Instant-Dissolving Tablets. Pharm. Res. 2021, 38, 831–842. [Google Scholar] [CrossRef]
- Maheshwari, S.; Singh, A.; Varshney, A.P.; Sharma, A. Advancing oral drug delivery: The science of fast dissolving tablets (FDTs). Intell. Pharm. 2024, 2, 580–585. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Pérez-Ramos, T.; Liu, J.; Januskaite, P.; Guerra-Baamonde, E.; González-Ramírez, J.; Vázquez-Caruncho, M.; Basit, A.W.; Goyanes, A. Visualizing disintegration of 3D printed tablets in humans using MRI and comparison with in vitro data. J. Control. Release 2024, 365, 348–357. [Google Scholar] [CrossRef]
- Kimbell, G.; Azad, M.A. Chapter FIFTEEN—3D printing: Bioinspired materials for drug delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Nurunnabi, M., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 295–318. [Google Scholar]
- Prajapati, S.T.; Patel, P.B.; Patel, C.N. Formulation and evaluation of sublingual tablets containing Sumatriptan succinate. Int. J. Pharm. Investig. 2012, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vidal, L.; Paredes, A.J.; Palma, S.D.; Real, J.P. Design and Development of Sublingual Printlets Containing Domperidone Nanocrystals Using 3D Melting Solidification Printing Process (MESO-PP). Pharmaceutics 2023, 15, 1459. [Google Scholar] [CrossRef] [PubMed]
- Suárez-González, J.; Magariños-Triviño, M.; Díaz-Torres, E.; Cáceres-Pérez, A.R.; Santoveña-Estévez, A.; Fariña, J.B. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: A comparative study for hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Farto-Vaamonde, X.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Sousa Lobo, J.M.; Alvarez-Lorenzo, C. Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.A.; Ahmed, M.M.; Mohammed, A.A.; Ahmad, J. 3D Printed Pharmaceutical Systems for Personalized Treatment in Metabolic Syndrome. Pharmaceutics 2023, 15, 1152. [Google Scholar] [CrossRef]
- Bg, P.K.; Mehrotra, S.; Marques, S.M.; Kumar, L.; Verma, R. 3D printing in personalized medicines: A focus on applications of the technology. Mater. Today Commun. 2023, 35, 105875. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Abdullah, M.M.; Saleh, E. 3D Printing of Dapagliflozin Containing Self-Nanoemulsifying Tablets: Formulation Design and In Vitro Characterization. Pharmaceutics 2021, 13, 993. [Google Scholar] [CrossRef]
- Englezos, K.; Wang, L.; Tan, E.C.K.; Kang, L. 3D printing for personalised medicines: Implications for policy and practice. Int. J. Pharm. 2023, 635, 122785. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Raje, V.; Maczko, P.; Patel, K. Application of 3D printing technology for the development of dose adjustable geriatric and pediatric formulation of celecoxib. Int. J. Pharm. 2024, 655, 123941. [Google Scholar] [CrossRef]
- Hejduk, A.; Lulek, J. Dispensing of minitablets—Has the problem been resolved? Int. J. Pharm. 2022, 619, 121666. [Google Scholar] [CrossRef]
- Protopapa, C.; Siamidi, A.; Kolipaka, S.S.; Junqueira, L.A.; Douroumis, D.; Vlachou, M. In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets. Pharmaceutics 2024, 16, 385. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Bracken, L.; Vasey, N.; Ehtezazi, T. 3D printing—An alternative strategy for pediatric medicines. Expert. Rev. Clin. Pharmacol. 2023, 16, 613–616. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. 3D printed medicine for the management of chronic diseases: The road less travelled. Ann. 3D Print. Med. 2022, 5, 100043. [Google Scholar] [CrossRef]
- Domsta, V.; Krause, J.; Weitschies, W.; Seidlitz, A. 3D Printing of Paracetamol Suppositories: An Automated Manufacturing Technique for Individualized Therapy. Pharmaceutics 2022, 14, 2676. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.W.; Kumar, S.; Douroumis, D. Personalised paediatric chewable Ibuprofen tablets fabricated using 3D micro-extrusion printing technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef]
- Biglino, G.; Hopfner, C.; Lindhardt, J.; Moscato, F.; Munuera, J.; Oberoi, G.; Tel, A.; Esteve, A. Perspectives on medical 3D printing at the point-of-care from the new European 3D Printing Special Interest Group. 3D Print. Med. 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- İnci, H. Evaluation of multiple drug use in patients with type 2 diabetes mellitus. Diabetol. Int. 2021, 12, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sathisaran, I. 3D printing and bioprinting in the battle against diabetes and its chronic complications. Front. Bioeng. Biotechnol. 2024, 12, 1363483. [Google Scholar] [CrossRef]
- Polamaplly, P.; Cheng, Y.; Shi, X.; Deivanai, K.M.; Kremer, G.; Qin, H. 3D Printing and Characterization of Hydroxypropyl Methylcellulose and Methylcellulose for Biodegradable Support Structures. Procedia Manuf. 2019, 34, 552–559. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid. Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadian, S.Z.; Varshosaz, J.; Minaiyan, M.; Taheri, A. 3D printed tablets containing oxaliplatin loaded alginate nanoparticles for colon cancer targeted delivery. An in vitro/in vivo study. Int. J. Biol. Macromol. 2022, 205, 90–109. [Google Scholar] [CrossRef]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-On-Powder 3D Printing of Tablets with an Anti-Cancer Drug, 5-Fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef]
- Zhang, B.; Nasereddin, J.; McDonagh, T.; von Zeppelin, D.; Gleadall, A.; Alqahtani, F.; Bibb, R.; Belton, P.; Qi, S. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int. J. Pharm. 2021, 604, 120626. [Google Scholar] [CrossRef] [PubMed]
- Maurizii, G.; Moroni, S.; Khorshid, S.; Aluigi, A.; Tiboni, M.; Casettari, L. 3D-printed EVA-based patches manufactured by direct powder extrusion for personalized transdermal therapies. Int. J. Pharm. 2023, 635, 122720. [Google Scholar] [CrossRef]
- Villota, I.; Calvo, P.C.; Campo, O.I.; Villarreal-Gómez, L.J.; Fonthal, F. Manufacturing of a Transdermal Patch in 3D Printing. Micromachines 2022, 13, 2190. [Google Scholar] [CrossRef] [PubMed]
- Nadda, R.; Singh, P.K.; Das, D.B. Revolutionizing microneedle array fabrication using additive manufacturing technologies: Potential applications and clinical translation. J. Drug Deliv. Sci. Technol. 2024, 101, 106288. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Parupelli, S.K.; Andar, A.; Desai, S. 3D Printing of Biodegradable Polymeric Microneedles for Transdermal Drug Delivery Applications. Pharmaceutics 2024, 16, 237. [Google Scholar] [CrossRef]
- Razzaghi, M.; Alexander Ninan, J.; Akbari, M. Advancements in Materials for 3D-Printed Microneedle Arrays: Enhancing Performance and Biocompatibility. Micromachines 2024, 15, 1433. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Jiang, X.; Li, L.; Wu, S.; Yuan, X.; Cheng, H.; Jiang, X.; Gou, M. 3D-printed microneedle arrays for drug delivery. J. Control. Release 2022, 350, 933–948. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, N.; Rai, A.K.; Kathuria, H. Recent advances in microneedles for drug delivery and theranostic application. Eur. Polym. J. 2025, 228, 113773. [Google Scholar] [CrossRef]
- Liu, H.; Nail, A.; Meng, D.; Zhu, L.; Guo, X.; Li, C.; Li, H.-J. Recent progress in the 3D printing of microneedle patches for biomedical applications. Int. J. Pharm. 2025, 668, 124995. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef]
- Monou, P.-K.; Andriotis, E.G.; Saropoulou, E.; Tzimtzimis, E.; Tzetzis, D.; Komis, G.; Bekiari, C.; Bouropoulos, N.; Demiri, E.; Vizirianakis, I.S.; et al. Fabrication of Hybrid Coated Microneedles with Donepezil Utilizing Digital Light Processing and Semisolid Extrusion Printing for the Management of Alzheimer’s Disease. Mol. Pharm. 2024, 21, 4450–4464. [Google Scholar] [CrossRef]
- Razzaghi, M.; Ninan, J.A.; Azimzadeh, M.; Askari, E.; Najafabadi, A.H.; Khademhosseini, A.; Akbari, M. Remote-Controlled Sensing and Drug Delivery via 3D-Printed Hollow Microneedles. Adv. Healthc. Mater. 2024, 13, 2400881. [Google Scholar] [CrossRef] [PubMed]
- Pünnel, L.C.; Palmtag, M.; Lunter, D.J.; Perry, J.L. Development of 3D printed microneedles of varied needle geometries and lengths, designed to improve the dermal delivery of topically applied psoriasis treatments. Eur. J. Pharm. Biopharm. 2024, 204, 114523. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printed pellets (miniprintlets): A novel, multi-drug, controlled release platform technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- First 3D-printed pill. Nat. Biotechnol. 2015, 33, 1014. [CrossRef]
- Elbl, J.; Veselý, M.; Blaháčková, D.; Ondruš, J.; Kulich, P.; Mašková, E.; Mašek, J.; Gajdziok, J. Development of 3D Printed Multi-Layered Orodispersible Films with Porous Structure Applicable as a Substrate for Inkjet Printing. Pharmaceutics 2023, 15, 714. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef]
- Al-Litani, K.; Ali, T.; Robles Martinez, P.; Buanz, A. 3D printed implantable drug delivery devices for women’s health: Formulation challenges and regulatory perspective. Adv. Drug Deliv. Rev. 2023, 198, 114859. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-De-la-Cruz, J.; Rodriguez-Salvador, M.; An, J.; Chua, C.K. Three-Dimensional Printing Technologies for Drug Delivery Applications: Processes, Materials, and Effects. Int. J. Bioprint 2022, 8, 622. [Google Scholar] [CrossRef]
- Picco, C.J.; Domínguez-Robles, J.; Utomo, E.; Paredes, A.J.; Volpe-Zanutto, F.; Malinova, D.; Donnelly, R.F.; Larrañeta, E. 3D-printed implantable devices with biodegradable rate-controlling membrane for sustained delivery of hydrophobic drugs. Drug Deliv. 2022, 29, 1038–1048. [Google Scholar] [CrossRef]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef]
- Zgouro, P.; Katsamenis, O.L.; Moschakis, T.; Eleftheriadis, G.K.; Kyriakidis, A.S.; Chachlioutaki, K.; Kyriaki Monou, P.; Ntorkou, M.; Zacharis, C.K.; Bouropoulos, N.; et al. A floating 3D printed polypill formulation for the coadministration and sustained release of antihypertensive drugs. Int. J. Pharm. 2024, 655, 124058. [Google Scholar] [CrossRef]
- Manoj, A.; Bhuyan, M.; Raj Banik, S.; Ravi Sankar, M. 3D printing of nasopharyngeal swabs for COVID-19 diagnose: Past and current trends. Mater. Today Proc. 2021, 44, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Kumar Gupta, D.; Ali, M.H.; Ali, A.; Jain, P.; Anwer, M.K.; Iqbal, Z.; Mirza, M.A. 3D printing technology in healthcare: Applications, regulatory understanding, IP repository and clinical trial status. J. Drug Target. 2022, 30, 131–150. [Google Scholar] [CrossRef]
- Beitler, B.G.; Abraham, P.F.; Glennon, A.R.; Tommasini, S.M.; Lattanza, L.L.; Morris, J.M.; Wiznia, D.H. Interpretation of regulatory factors for 3D printing at hospitals and medical centers, or at the point of care. 3D Print. Med. 2022, 8, 7. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Mirza, M.; Iqbal, Z. 3D printing in pharmaceuticals: Regulatory perspective. Curr. Pharm. Des. 2018, 24, 5081–5083. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou-Kaparia, M. The Evaluation of Directive 85/374/EEC on Liability for Defective Products and Directive 2006/42/EC on Machinery; European Stakeholder Forum—Workshop on Regulatory Challenges for a Digitizing Industry: Essen, Germany, 2019; Available online: https://ec.europa.eu/futurium/en/system/files/ged/b3-spiliopoulou-liability.pdf (accessed on 25 February 2025).
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Kaushik, B.; Subramaniyan, A.K.; Pareek, M.; Sharma, S.; Velu, R. 3D Printing of Pharmaceutical Products Using AI Technology. In Digital Design and Manufacturing of Medical Devices and Systems; Velu, R., Subburaj, K., Subramaniyan, A.K., Eds.; Springer Nature: Singapore, 2023; pp. 233–248. [Google Scholar]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef]
- Grof, Z.; Štěpánek, F. Artificial intelligence based design of 3D-printed tablets for personalised medicine. Comput. Chem. Eng. 2021, 154, 107492. [Google Scholar] [CrossRef]
- Elbadawi, M.; Li, H.; Sun, S.; Alkahtani, M.E.; Basit, A.W.; Gaisford, S. Artificial intelligence generates novel 3D printing formulations. Appl. Mater. Today 2024, 36, 102061. [Google Scholar] [CrossRef]
- Meshram, S.; Hatwar, P.; Bakal; Raut, P. Artificial Intelligence-Assisted Fabrication of 3D Printed Technology in Pharmaceutical Development and Its Application. J. Drug Deliv. Ther. 2024, 14, 214–222. [Google Scholar] [CrossRef]
- Biswas, A.A.; Dhondale, M.R.; Agrawal, A.K.; Serrano, D.R.; Mishra, B.; Kumar, D. Advancements in microneedle fabrication techniques: Artificial intelligence assisted 3D-printing technology. Drug Deliv. Transl. Res. 2024, 14, 1458–1479. [Google Scholar] [CrossRef]
- FDA. 2025. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-use-artificial-intelligence-support-regulatory-decision-making-drug-and-biological (accessed on 25 February 2025).
- EMA. 2023. Available online: https://www.ema.europa.eu/en/news/artificial-intelligence-workplan-guide-use-ai-medicines-regulation (accessed on 25 February 2025).
- EMA. 2024. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-use-artificial-intelligence-ai-medicinal-product-lifecycle_en.pdf (accessed on 25 February 2025).
- Varghese, R.; Sood, P.; Salvi, S.; Karsiya, J.; Kumar, D. 3D printing in the pharmaceutical sector: Advances and evidences. Sens. Int. 2022, 3, 100177. [Google Scholar] [CrossRef]
- Nori, L.; Mani Kiran, S.S. An outlook on regulatory aspects of 3D printing in pharmaceutical and medical sectors. Curr. Trends Pharm. Pharm. Chem. 2022, 4, 98–108. [Google Scholar] [CrossRef]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Ngo, T.; Kashani, A.; Imbalzano, G.; Nguyen, K.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Gao, G.; Ahn, M.; Cho, W.W.; Kim, B.S.; Cho, D.W. 3D Printing of Pharmaceutical Application: Drug Screening and Drug Delivery. Pharmaceutics 2021, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication 2024, 16, 032001. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Fang, H.; Wang, Y.; Zeng, Q.; Hu, G.; Bao, G.; Wan, Y. Academic Insights and Perspectives in 3D Printing: A Bibliometric Review. Appl. Sci. 2021, 11, 8298. [Google Scholar] [CrossRef]


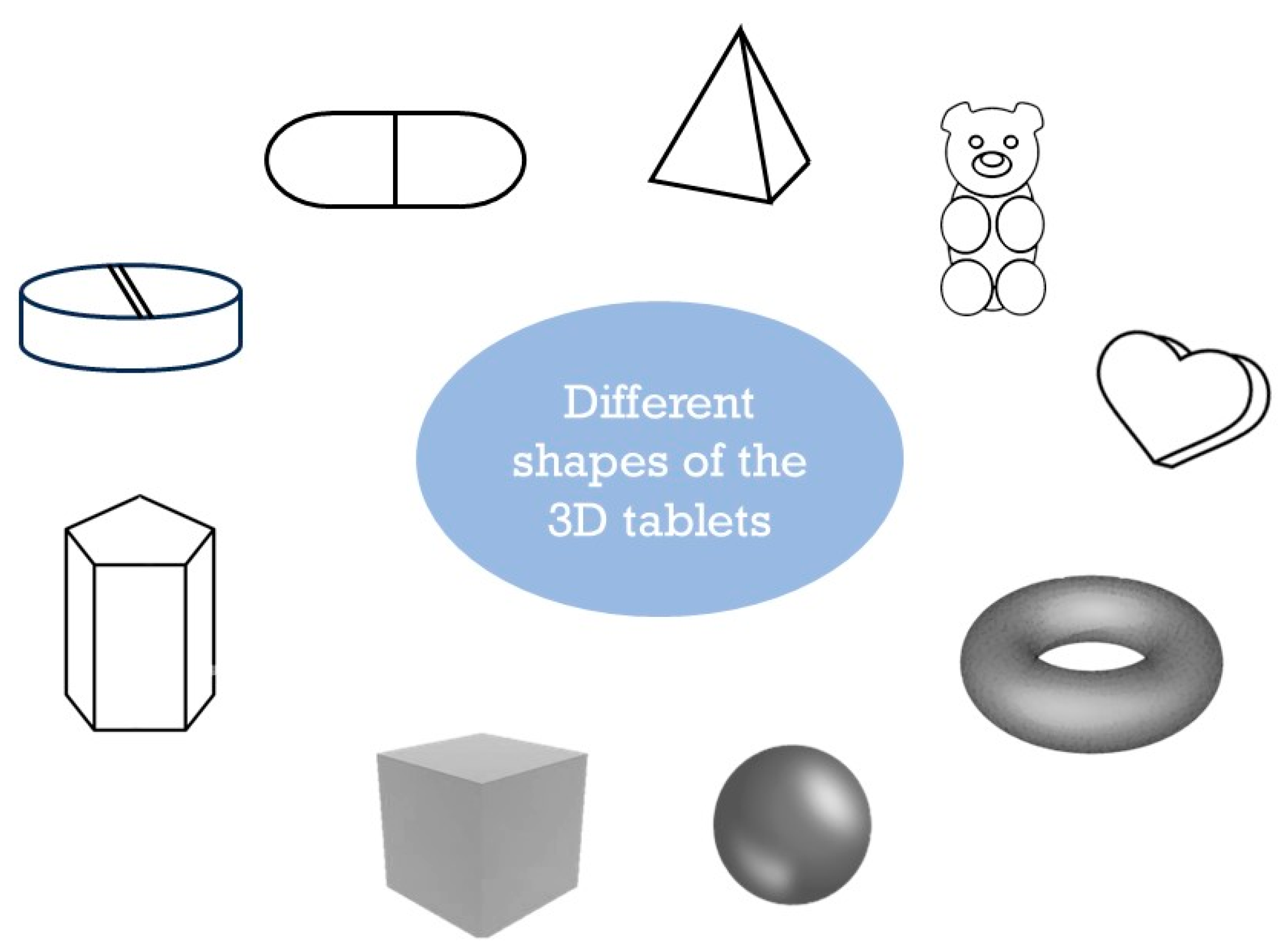

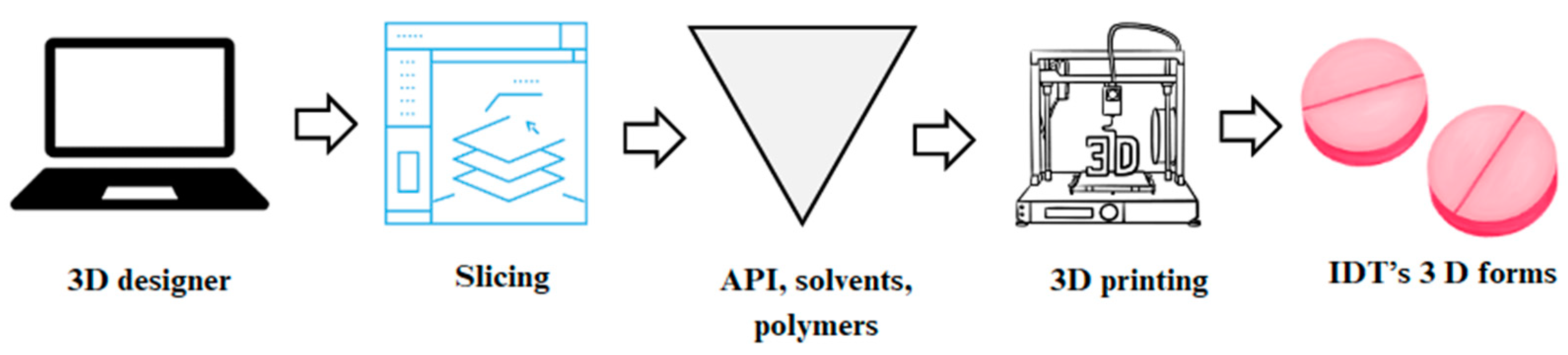

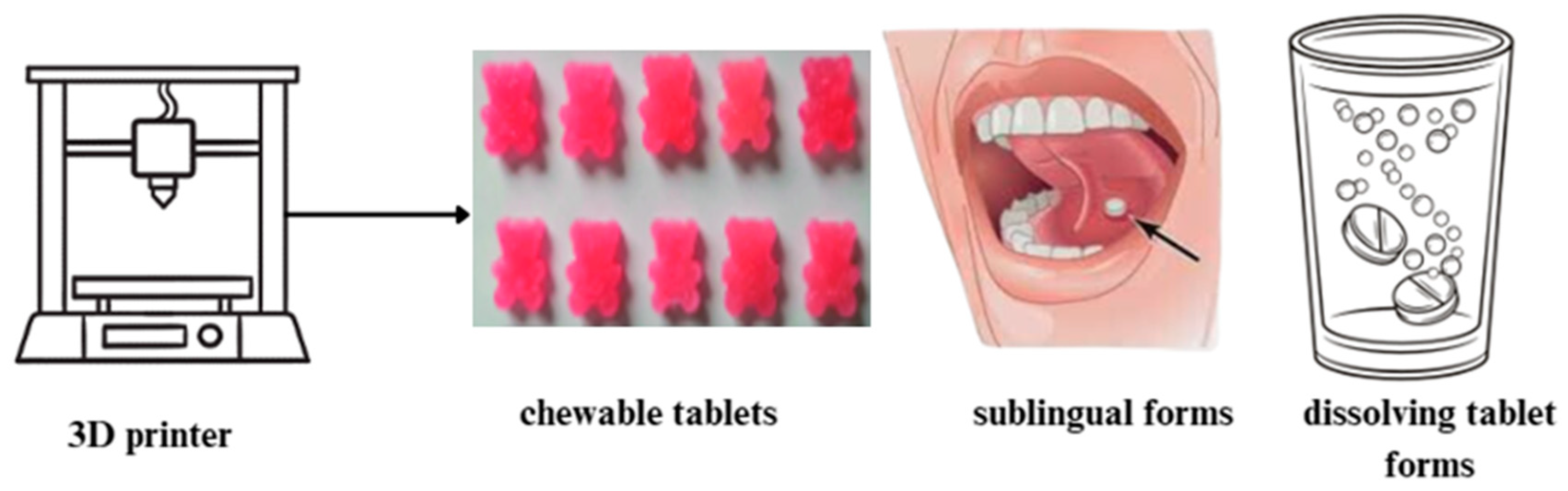
| Technology | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| FDM (fused deposition model) | FDM operates by heating a drug-infused polymer filament and extruding it through a nozzle that follows computer-controlled paths, depositing the material layer by layer onto a build platform where it solidifies upon contact. This process allows for the creation complex dosage forms through precise, layer-by-layer fabrication [30]. | Simple setup, economical, wide range of materials, ability to create complex dosage forms [45,46]. | Potential drug degradation due to high temperatures, lower resolution, coarser surface finish, and structural weaknesses at layer junctions [78,79]. |
| DPE (direct powder extrusion) | DPE combines drug powders with binders and extrudes and fuses them into solid forms without solvent [55]. | Without solvent, high drug load, reduced post-processing [55]. | Powder flow issues, limited excipient compatibility [55]. |
| SSE (semi-solid extrusion) | SSE involves extruding semi-solid materials, such as gels or pastes, from a syringe-like system in successive layers to form a 3D object. The materials are carefully blended to achieve the ideal viscosity for printing, allowing for the creation of complex drug formulations at lower temperatures to preserve the integrity of the active ingredients [59]. | Versatile, suitable for temperature-sensitive drugs, low waste generation, and efficient [61]. | Requires careful selection of excipients, the potential for shrinkage and deformation during post-processing, and lower resolution prints [61]. |
| SLS (selective laser sintering) | SLS uses a laser to selectively fuse powder particles on a powder bed according to a predetermined pattern. After each layer is fused, a new layer of powder is spread over the bed, and the process is repeated layer by layer to construct the final 3D object [63]. | High precision, no need for support structures, suitable for complex drug delivery systems [64,65]. | High equipment costs and intense laser energy can cause drug degradation and challenge scaling up large production [64,65]. |
| BJ (binder jetting) | Binder jet printing involves a nozzle that dispenses a binding liquid onto a flat powder bed, moving along the x–y-axis. This liquid binds the powder particles together, solidifying each layer. The build plate then lowers along the z-axis, and a new powder layer is applied, with the process repeating layer by layer to create the final 3D-printed structure [69]. | High-resolution parts are versatile and suitable for complex and multi-drug formulations [69,70]. | It requires post-processing for strength, a less smooth surface finish, and is limited by binder droplet size and powder granularity [1]. |
| SLA (stereolithography) | SLA involves exposing a photopolymer resin to a high-energy light source, such as UV light, which induces polymerization and solidification of the resin. The platform moves down vertically along the z-axis after each layer is solidified, and the process repeats with a new layer of resin being applied and cured, building the 3D object layer by layer [73,74]. | High precision, cost-effective, suitable for complex drug delivery systems, and minimal local heating preserves heat-sensitive drugs [34,75]. | Limited photocrosslinkable polymers are approved for medical use, there are potential safety concerns with some resins, and the stability of drug formulations is compromised [34,75]. |
| Materials | Functions | References | 3D Printing Techniques |
|---|---|---|---|
| Gelucire 48/16 Klucel ELF | Excipients | [19] | FDM, SSE |
| Xanthan gum | Excipients | [20,21] | SSE |
| Carrageenan-gelatin | Excipients | [22] | SSE |
| Gelatin | Excipients | [21,23,24] | SSE |
| Bitter chocolate | Excipients | [25] | SSE |
| Corn (glucose) syrup Potato starches | Excipients | [14] | SSE |
| Materials | Functions | References | 3D Printing Techniques |
|---|---|---|---|
| Copovidone VA 64, Mannitol | Excipients | [146,147] | SLS |
| HPβCD (72.1%) HPMC F4M (1.4%) NaCCS (2.5%) | Excipients | [148] | SSE |
| HPMC E5 | Excipients | [149] | SLS |
| Polyvinylpyrrolidone (PVP) PEO and PVA | Excipients | [145] | SSE |
| Maltodextrin, HEC Cellosize®, Sorbitol (plasticizer) | Excipients | [150] | FDM |
| HPMC and glycerol | Excipients | [151] | IJ |
| HPMC E5 and Kollidon® VA 64 | Excipients | [152] | SLS |
| MCC PH 101 Peorlitol 50C Aerosil 200 | Excipients | [139] | BJ-3DP |
| PEG 6000 HPC Sodium starch glycolate Croscarmellose | Excipients | [54] | FDM |
| Kollidon VA 64 Kollicoat IR Mannogem XL Compressol SM | Excipients | [153] | FDM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatoniene, J.; Stabrauskiene, J.; Kazlauskaite, J.A.; Bernatonyte, U.; Kopustinskiene, D.M. The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics 2025, 17, 390. https://doi.org/10.3390/pharmaceutics17030390
Bernatoniene J, Stabrauskiene J, Kazlauskaite JA, Bernatonyte U, Kopustinskiene DM. The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics. 2025; 17(3):390. https://doi.org/10.3390/pharmaceutics17030390
Chicago/Turabian StyleBernatoniene, Jurga, Jolita Stabrauskiene, Jurga Andreja Kazlauskaite, Urte Bernatonyte, and Dalia Marija Kopustinskiene. 2025. "The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals" Pharmaceutics 17, no. 3: 390. https://doi.org/10.3390/pharmaceutics17030390
APA StyleBernatoniene, J., Stabrauskiene, J., Kazlauskaite, J. A., Bernatonyte, U., & Kopustinskiene, D. M. (2025). The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics, 17(3), 390. https://doi.org/10.3390/pharmaceutics17030390












