The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Selection of Studies
- Inclusion criteria are shown as follows:
- Investigations utilizing natural photosensitizers in photodynamic therapy for the treatment of peri-implantitis, encompassing both in vitro and animal studies.
- Randomized controlled trials where natural photosensitizers serve as the primary photosensitizer in PDT for managing peri-implantitis.
- Research evaluating the combined effects of natural photosensitizers in PDT with other antimicrobial or anti-inflammatory treatments.
- Studies incorporating control groups that compare aPDT mediated by natural photosensitisers against standard mechanical debridement, alternative non-surgical therapies, or no treatment.
- Studies directly comparing the effectiveness of aPDT mediated by natural photosensitisers with other non-surgical treatment modalities for peri-implantitis.
- Research featuring extended follow-up periods to assess the sustained impact of naturally mediated aPDT on peri-implantitis management.
- Articles that meet predefined quality standards and specifically address the reduction or management of peri-implantitis symptoms using naturally mediated aPDT.
- Exclusion criteria are shown as follows:
- Unpublished theses, conference abstracts, dissertations, and other non-peer-reviewed materials.
- Articles published in languages other than English.
- No full text available.
- Multiple reports of the same study or those sharing identical ethical approval numbers.
- Research focused on dental or medical issues unrelated to peri-implantitis.
- Studies utilizing synthetic photosensitizing agents.
- Laboratory studies that do not replicate oral conditions pertinent to peri-implantitis or fail to address relevant microbial strains.
- Case reports, case series, narrative reviews, systematic reviews, editorials, books, and other formats that do not provide original research data.
- Studies that do not include a control or comparison group.
- Applications of PDT not intended as a therapeutic method for peri-implantitis.
2.4. Data Extraction
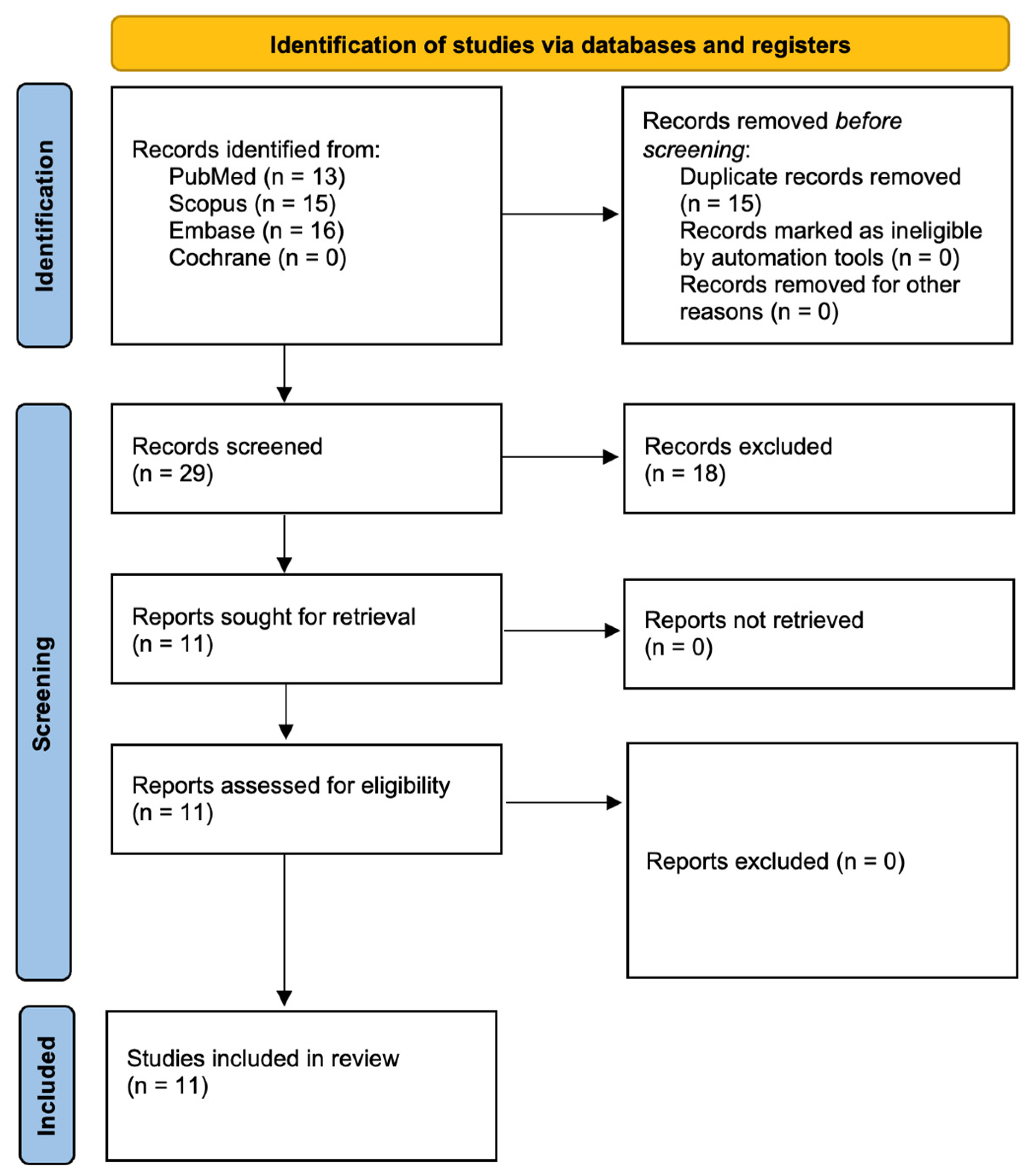
3. Results
3.1. Study Selection
3.2. Quality Assessment Presentation and Risk of Bias in Individual Studies
3.3. Data Presentation
3.4. General Characteristics of the Included Studies
3.5. Characteristics of Light Sources Used in aPDT
4. Discussion
4.1. Results in the Context of Other Evidence
4.2. Other Natural Photosensitisers Potentially Appropriate for aPDT Against Periimplantitis
4.3. Limitations of the Evidence
4.4. Limitations of the Review Process
4.5. Implications for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heitz-Mayfield, L.J.A. Peri-implant mucositis and peri-implantitis: Key features and differences. Br. Dent. J. 2024, 236, 791–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misch, C.M. (Ed.) Peri-implantitis. In Misch’s Avoiding Complications in Oral Implantology; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and risk indicators for peri-implant diseases: A literature review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head. Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monje, A.; Salvi, G.E. Diagnostic methods/parameters to monitor peri-implant conditions. Periodontology 2000 2024, 95, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V. An insight into peri-implantitis: A systematic literature review. Prim. Dent. J. 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89, S304–S312. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Periodontol. 2018, 89, S257–S266. [Google Scholar] [CrossRef]
- Berglundh, T.; Mombelli, A.; Schwarz, F.; Derks, J. Etiology, pathogenesis and treatment of peri-implantitis: A European perspective. Periodontology 2000 2024, 00, 1–36. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-implant infections of oral biofilm etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Thomsen, P.; Kunrath, M.F. The Role of Titanium Particles and Ions in the Pathogenesis of Peri-Implantitis. J. Bone Metab. 2022, 29, 145–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kheder, W.; Al Kawas, S.; Khalaf, K.; Samsudin, A.R. Impact of tribocorrosion and titanium particles release on dental implant complications—A narrative review. Jpn. Dent. Sci. Rev. 2021, 57, 182–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaur, S.; Agnihotri, R.; Albin, S. Bio-tribocorrosion of titanium dental implants and its toxicological implications: A scoping review. Sci. World J. 2022, 2022, 4498613. [Google Scholar] [CrossRef]
- Soler, M.D.; Hsu, S.-M.; Fares, C.; Ren, F.; Jenkins, R.J.; Gonzaga, L.; Clark, A.E.; O’Neill, E.; Neal, D.; Esquivel-Upshaw, J.F. Titanium Corrosion in Peri-Implantitis. Materials 2020, 13, 5488. [Google Scholar] [CrossRef]
- Rana, M.F.; Bukhari, J.; Idrees, E.; Akram, A.; Yousaf, L.; Anwar, A. Tribocorrosion of dental implants: A systematic review. Pak. J. Med. Health Sci. 2022, 16, 1289. [Google Scholar] [CrossRef]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Serra, P.; Francesco, I.; Di Carmine, M.; Tari, S.R.; Leo, L.; Lorusso, F. Current Status of Peri-Implant Diseases: A Clinical Review for Evidence-Based Decision Making. J. Funct. Biomater. 2023, 14, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef]
- Alghamdi, H.; Leventis, M.; Deliberador, T. Management of Infected Tissues Around Dental Implants: A Short Narrative Review. Braz. Dent. J. 2024, 35, e246160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suresh, N.; Joseph, B.; Sathyan, P.; Sweety, V.K.; Waltimo, T.; Anil, S. Photodynamic therapy: An emerging therapeutic modality in dentistry. Bioorg Med. Chem. 2024, 114, 117962. [Google Scholar] [CrossRef]
- Al Hafez, A.S.S.; Ingle, N.; Alshayeb, A.A.; Tashery, H.M.; Alqarni, A.A.M.; Alshamrani, S.H. Effectiveness of mechanical debridement with and without adjunct antimicrobial photodynamic for treating peri-implant mucositis among prediabetic cigarette-smokers and non-smokers. Photodiagn. Photodyn. Ther. 2020, 31, 101912. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and hypericin-mediated antimicrobial photodynamic therapy as alternative treatments for oral candidiasis: A systematic review. Pharmaceutics 2025, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aragão, M.Â.; Pires, L.; Santos-Buelga, C.; Barros, L.; Calhelha, R.C. Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Foods 2024, 13, 2255. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Avdeev, O.; Shanaida, Y.; Bjørklund, G. Metabolic conditions and peri-implantitis. Antibiotics 2023, 12, 65. [Google Scholar] [CrossRef]
- Tavares, L.J.; Pavarina, A.C.; Vergani, C.E.; de Avila, E.D. The impact of antimicrobial photodynamic therapy on peri-implant disease: What mechanisms are involved in this novel treatment? Photodiagn. Photodyn. Ther. 2017, 17, 236–244. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nejadghaderi, S.A.; Balibegloo, M.; Rezaei, N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci. Rep. 2024, 7, e2165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qamar, Z.; Abdul, N.S.; Soman, C.; Shenoy, M.; Bamousa, B.; Rabea, S.; Albahkaly, H.S. Clinical and radiographic peri-implant outcomes with riboflavin loaded Poly-L-glycolic acid nanoparticles incorporated in aloe-vera gel treating peri-implantitis in chronic hyperglycemic patients. Photodiagn. Photodyn. Ther. 2023, 44, 103752. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- Etemadi, A.; Hashemi, S.S.; Chiniforush, N. Evaluation of the effect of photodynamic therapy with Curcumin and Riboflavin on implant surface contaminated with Aggregatibacter actinomycetemcomitans. Photodiagn. Photodyn. Ther. 2023, 44, 103833. [Google Scholar] [CrossRef]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagn. Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef]
- Mahdizade-ari, M.; Pourhajibagher, M.; Bahador, A. Changes of microbial cell survival, metabolic activity, efflux capacity, and quorum sensing ability of Aggregatibacter actinomycetemcomitans due to antimicrobial photodynamic therapy-induced bystander effects. Photodiagn. Photodyn. Ther. 2019, 26, 287–294. [Google Scholar] [CrossRef]
- Morelato, L.; Budimir, A.; Smojver, I.; Katalinić, I.; Vuletić, M.; Ajanović, M.; Gabrić, D. A novel technique for disinfection treatment of contaminated dental implant surface using 0.1% riboflavin and 445 nm diode laser—An in vitro study. Bioengineering 2022, 9, 308. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor Graphene Quantum Dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms. Photodiagn. Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tonon, C.C.; Panariello, B.; Chorilli, M.; Spolidorio, D.M.P.; Duarte, S. Effect of curcumin-loaded photoactivatable polymeric nanoparticle on peri-implantitis-related biofilm. Photodiagn. Photodyn. Ther. 2022, 40, 103150. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.-S.; Wurzel, S.C.; Falk, W.; Böcher, S.; Wurzel, P.P.; Braun, A. Bactericidal effect of different photochemical-based therapy options on implant surfaces—An in vitro study. J. Clin. Med. 2024, 13, 4212. [Google Scholar] [CrossRef]
- Rossi, R.; Rispoli, L.; Lopez, M.A.; Netti, A.; Petrini, M.; Piattelli, A. Photodynamic therapy by mean of 5-aminolevulinic acid for the management of periodontitis and peri-implantitis: A retrospective analysis of 20 patients. Antibiotics 2022, 11, 1267. [Google Scholar] [CrossRef]
- Petrini, M.; Di Lodovico, S.; Iezzi, G.; Cellini, L.; Tripodi, D.; Piattelli, A.; D’Ercole, S. Photodynamic antibiofilm and antibacterial activity of a new gel with 5-aminolevulinic acid on infected titanium surfaces. Biomedicines 2022, 10, 572. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a novel gel containing 5-aminolevulinic acid and red LED against bacteria involved in peri-implantitis and other oral infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef] [PubMed]
- Rahman, B.; Acharya, A.B.; Siddiqui, R.; Verron, E.; Badran, Z. Photodynamic Therapy for Peri-Implant Diseases. Antibiotics 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fraga, R.S.; Antunes, L.A.A.; Fontes, K.B.F.D.C.; Küchler, E.C.; Iorio, N.L.P.P.; Antunes, L.S. Is Antimicrobial Photodynamic Therapy Effective for Microbial Load Reduction in Peri-implantitis Treatment? A Systematic Review and Meta-Analysis. Photochem. Photobiol. 2018, 94, 752–759. [Google Scholar] [CrossRef] [PubMed]
- A Lopez, M.; Passarelli, P.C.; Marra, M.; Lopez, A.; Moffa, A.; Casale, M.; D’Addona, A. Antimicrobial efficacy of photodynamic therapy (PDT) in periodontitis and peri-implantitis: A systematic review. J. Biol. Regul. Homeost. Agents 2020, 34, 59. [Google Scholar]
- Wychowański, P.; Starzyńska, A.; Adamska, P.; Słupecka-Ziemilska, M.; Sobocki, B.K.; Chmielewska, A.; Wysocki, B.; Alterio, D.; Marvaso, G.; Jereczek-Fossa, B.A.; et al. Methods of Topical Administration of Drugs and Biological Active Substances for Dental Implants-A Narrative Review. Antibiotics 2021, 10, 919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tancredi, S.; De Angelis, P.; Marra, M.; Lopez, M.A.; Manicone, P.F.; Passarelli, P.C.; Romeo, A.; Grassi, R.; D’Addona, A. Clinical Comparison of Diode Laser Assisted “v-Shape Frenectomy” and Conventional Surgical Method as Treatment of Ankyloglossia. Healthcare 2022, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef]
- Jervøe-Storm, P.M.; Bunke, J.; Worthington, H.V.; Needleman, I.; Cosgarea, R.; MacDonald, L.; Walsh, T.; Lewis, S.R.; Jepsen, S. Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases. Cochrane Database Syst. Rev. 2024, 7, CD011778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, T.; Song, J.; Ping, Y.; Li, M. The Application of Antimicrobial Photodynamic Therapy (aPDT) in the Treatment of Peri-Implantitis. Comput. Math. Methods Med. 2022, 2022, 3547398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, A.; Gaikwad, A.; Padhye, A.; Nadgere, J. Overview of Systematic Reviews and Meta-analyses Investigating the Efficacy of Different Nonsurgical Therapies for the Treatment of Peri-implant Diseases. Int. J. Oral. Maxillofac. Implant. 2022, 37, e13–e27. [Google Scholar] [CrossRef]
- Farsai, P.S. Overview of Systematic Reviews Suggests That Various Nonsurgical Therapies (NSTS) May be Effective for the Treatment of Peri-Implant Mucositis. J. Evid. Based Dent. Pract. 2023, 23, 101893. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr.; Chambrone, L.; Listl, S.; Tu, Y.K. Network meta-analysis for evaluating interventions in implant dentistry: The case of peri-implantitis treatment. Clin. Implant. Dent. Relat. Res. 2013, 15, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.A.; Diaz, K.T.; Aranda, L.; Cafferata, E.A.; Faggion, C.M., Jr.; Monje, A. Use of Biologic Agents to Promote Bone Formation in Implant Dentistry: A Critical Assessment of Systematic Reviews. Int. J. Oral. Maxillofac. Implants 2017, 32, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bombeccari, G.P.; Guzzi, G.; Gualini, F.; Gualini, S.; Santoro, F.; Spadari, F. Photodynamic therapy to treat periimplantitis. Implant. Dent. 2013, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, P.; Wang, X.; Wang, Z.; Ma, W.; Guo, J.; Chen, J.; Yu, Z.; Li, J.; Zhou, D. Light-activatable prodrug and AIEgen copolymer nanoparticle for dual-drug monitoring and combination therapy. ACS Appl. Mater. Interfaces 2019, 11, 18691–18700. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, X.; He, C.; Chen, L.; Chen, X. Dual pH-responsive mesoporous silica nanoparticles for efficient combination of chemotherapy and photodynamic therapy. J. Mater. Chem. B 2015, 3, 4707–4714. [Google Scholar] [CrossRef] [PubMed]
- Schär, D.; Ramseier, C.A.; Eick, S.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: Six-month outcomes of a prospective randomized clinical trial. Clin. Oral. Implants Res. 2013, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Li, W.; Zhang, D.; Li, W.; Wang, Z. Adjunctive photodynamic therapy improves the outcomes of peri-implantitis: A randomized controlled trial. Aust. Dent. J. 2019, 64, 256–262. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashef, N.; Borghei, Y.S.; Djavid, G.E. Photodynamic effect of hypericin on the microorganisms and primary human fibroblasts. Photodiagn. Photodyn. Ther. 2013, 10, 150–155. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef]
- Theodorakopoulou, A.; Pylarinou, I.; Anastasiou, I.A.; Tentolouris, N. The Putative Antidiabetic Effect of Hypericum perforatum on Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 354. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Egner, P.A.; Kensler, T.W.; Talalay, P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis 2005, 26, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S.; Palitti, F.; Natarajan, A.T. Chemopreventive potential of chlorophyllin: A review of the mechanisms of action and molecular targets. Nutr. Cancer 2015, 67, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.; Ul Haq, I.; Ali, I.; Rehman, A.; Almehmadi, M.; Alsuwat, M.A.; Zaman, T.; Qasim, M. Antibacterial and Antibiofilm Potential of Chlorophyllin Against Streptococcus mutans In Vitro and In Silico. Antibiotics 2024, 13, 899. [Google Scholar] [CrossRef]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Karaiyagowder Govindarajan, D.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 1992, 14, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wong, R.C.H.; Wang, Y.; Guo, X.; Yang, Z.; Lo, P.C. Shifting the absorption to the near-infrared region and inducing a strong photothermal effect by encapsulating zinc(II) phthalocyanine in poly(lactic-co-glycolic acid)-hyaluronic acid nanoparticles. Acta Biomater. 2020, 116, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Awaji, A.I.; Köksoy, B.; Durmuş, M.; Aljuhani, A.; Alraqa, S.Y. Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties. Molecules 2019, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, G.; Mokrzyński, K. Concentration-Dependent Photoproduction of Singlet Oxygen by Common Photosensitizers. Molecules 2025, 30, 1130. [Google Scholar] [CrossRef]
- Payne, D.T.; Webre, W.A.; Gobeze, H.B.; Seetharaman, S.; Matsushita, Y.; Karr, P.A.; Chahal, M.K.; Labuta, J.; Jevasuwan, W.; Fukata, N.; et al. Nanomolecular singlet oxygen photosensitizers based on hemiquinonoid-resorcinarenes, the fuchsonarenes. Chem. Sci. 2020, 11, 2614–2620. [Google Scholar] [CrossRef]
| Source | Search Term | Number of Results |
|---|---|---|
| Medline via PubMed | ((“Curcumin”[MeSH] OR “Curcumin”) OR (“Riboflavin”[MeSH] OR “Riboflavin” OR “Vitamin B2”) OR (“Hypericin”[MeSH] OR “Hypericin”) OR (“Chlorophyll”[MeSH] OR “Chlorophyll” OR “Chlorophyllin”) OR (“Carotenoids”[MeSH] OR “Carotenoids” OR “Lycopene”) OR (“Hematoporphyrins”[MeSH] OR “Hematoporphyrin Derivatives”) OR (“5-Aminolevulinic Acid”[MeSH] OR “5-ALA” OR “Aminolevulinic Acid”) OR (“Phthalocyanines”[MeSH] OR “Phthalocyanines” OR “ZnPc” OR “AlPc” OR “AlClPc”) OR (“Protoporphyrins”[MeSH] OR “Protoporphyrin IX” OR “Natural porphyrins”)) AND ((“Photodynamic Therapy”[MeSH] OR “Photodynamic Therapy” OR “PDT”)) AND ((“Peri-implantitis”[MeSH] OR “Peri-implantitis”)) | 13 |
| Scopus | (TITLE-ABS-KEY(“Curcumin”) OR TITLE-ABS-KEY(“Riboflavin”) OR TITLE-ABS-KEY(“Vitamin B2”) OR TITLE-ABS-KEY(“Hypericin”) OR TITLE-ABS-KEY(“Chlorophyll”) OR TITLE-ABS-KEY(“Chlorophyllin”) OR TITLE-ABS-KEY(“Carotenoids”) OR TITLE-ABS-KEY(“Lycopene”) OR TITLE-ABS-KEY(“Hematoporphyrin Derivatives”) OR TITLE-ABS-KEY(“5-Aminolevulinic Acid”) OR TITLE-ABS-KEY(“5-ALA”) OR TITLE-ABS-KEY(“Aminolevulinic Acid”) OR TITLE-ABS-KEY(“Phthalocyanines”) OR TITLE-ABS-KEY(“ZnPc”) OR TITLE-ABS-KEY(“AlPc”) OR TITLE-ABS-KEY(“AlClPc”) OR TITLE-ABS-KEY(“Protoporphyrin IX”) OR TITLE-ABS-KEY(“Natural porphyrins”)) AND (TITLE-ABS-KEY(“Photodynamic Therapy”) OR TITLE-ABS-KEY(“PDT”)) AND (TITLE-ABS-KEY(“Peri-implantitis”)) | 15 |
| Embase | ((“curcumin”/exp OR “curcumin”) OR (“riboflavin”/exp OR “riboflavin” OR “vitamin B2”) OR (“hypericin”/exp OR “hypericin”) OR (“chlorophyll”/exp OR “chlorophyllin” OR “chlorophyll”) OR (“carotenoid”/exp OR “carotenoids” OR “lycopene”) OR (“hematoporphyrin derivative”/exp OR “hematoporphyrin derivatives”) OR (“5-aminolevulinic acid”/exp OR “5-ALA” OR “aminolevulinic acid”) OR (“phthalocyanine”/exp OR “phthalocyanines” OR “ZnPc” OR “AlPc” OR “AlClPc”) OR (“protoporphyrin”/exp OR “protoporphyrin IX” OR “natural porphyrins”)) AND (“photodynamic therapy”/exp OR “photodynamic therapy” OR “PDT”) AND (“periimplantitis”/exp OR “peri-implantitis” OR “periimplantitis”) | 16 |
| Cochrane data base | ((MH “Curcumin” OR “Curcumin”) OR (MH “Riboflavin” OR “Riboflavin” OR “Vitamin B2”) OR (MH “Hypericin” OR “Hypericin”) OR (MH “Chlorophyll” OR “Chlorophyllin” OR “Chlorophyll”) OR (MH “Carotenoids” OR “Carotenoids” OR “Lycopene”) OR (MH “Hematoporphyrins” OR “Hematoporphyrin Derivatives”) OR (MH “5-Aminolevulinic Acid” OR “5-ALA” OR “Aminolevulinic Acid”) OR (MH “Phthalocyanines” OR “Phthalocyanines” OR “ZnPc” OR “AlPc” OR “AlClPc”) OR (MH “Protoporphyrins” OR “Protoporphyrin IX” OR “Natural porphyrins”)) AND (MH “Photodynamic Therapy” OR “Photodynamic Therapy” OR “PDT”) AND (MH “Peri-implantitis” OR “Peri-implantitis”) | 0 |
| Study | Study Type | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of Outcomes | Selective Reporting | Overall RoB 2 Rating |
|---|---|---|---|---|---|---|---|
| Qamar et al., 2023 [37] | RCT | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Author and Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total Score | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Etemadi et al., 2023 [39] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Leelanarathiwatat et al., 2020 [40] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | Low |
| Mahdizade-Ari et al., 2019 [41] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | Low |
| Morelato et al., 2022 [42] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | Low |
| Pourhajibagher et al., 2019 [43] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Low |
| Tonon et al., 2022 [44] | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | Moderate |
| Wenzler et al., 2024 [45] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | Low |
| Rossi et al., 2022 [46] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Petrini et al., 2022 [47] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Radunović et al., 2020 [48] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Author and Year | Country | Study Design |
|---|---|---|
| Qamar et al., 2023 [37] | Saudi Arabia | Clinical study |
| Etemadi et al., 2023 [39] | Iran and Italy | In vitro |
| Leelanarathiwatat et al., 2020 [40] | Thailand and Japan | In vitro |
| Mahdizade-Ari et al., 2019 [41] | Iran | In vitro |
| Morelato et al., 2022 [42] | Croatia, Bosnia, and Herzegovina | In vitro |
| Pourhajibagher et al., 2019 [43] | Iran and Italy | In vitro |
| Tonon et al., 2022 [44] | Brazil and USA | In vitro |
| Wenzler et al., 2024 [45] | Germany | In vitro |
| Rossi et al., 2022 [46] | Italy | Retrospective Analysis |
| Petrini et al., 2022 [47] | Italy | In vitro |
| Radunović et al., 2020 [48] | Serbia and Italy | In vitro |
| Author and Year | Species | Photosensitizer Concentration | Outcome Measures |
|---|---|---|---|
| Qamar et al., 2023 [37] | Porphyromonas gingivalis and Tannerella forsythia | Riboflavin loaded on poly-L-glycolic acid nanoparticles incorporated in aloe vera gel (PGA/RF/AV) 0.1% riboflavin concentration in the PGA/RF/AV gel | Significant reduction in microbial counts of P. gingivalis and T. forsythia Improvement in clinical parameters: BoP, PD, PI, and MBL; Enhanced treatment outcomes compared to PDT alone. |
| Author and Year | Species | Photosensitizer Concentration | Outcome Measures |
|---|---|---|---|
| Etemadi et al., 2023 [39] | A. actinomycetemcomitans | Curcumin (5 mg/mL); Riboflavin (0.5%) | CFU/mL: quantitative measure of bacterial reduction. Groups were analyzed to determine the efficacy of treatments in reducing A.a biofilm. |
| Leelanarathiwatat et al., 2020 [40] | Staphylococcus aureus | MB: 10 mg/mL FMN: 0.18 mg/mL | Log reduction in CFU/mL (colony-forming units); Biofilm mass reduction measured by crystal violet assay; Direct observation using SEM and fluorescent stereomicroscopy. |
| Mahdizade-Ari et al., 2019 [41] | A. actinomycetemcomitans | Curcumin: 80 μg/mL | Reduction in microbial cell survival; Decrease in metabolic activity; Reduction in quorum sensing; No significant change in efflux capacity. |
| Morelato et al., 2022 [42] | Staphylococcus aureus and Candida albicans | Riboflavin (0.1%) MB (0.1%) | Reduction in CFU of Staphylococcus aureus and Candida albicans. Antimicrobial effect was comparable between 445 nm riboflavin and 660 nm methylene blue photodynamic therapy protocols. SEM analysis showed reduced biofilm, but some microorganisms remained visible. |
| Pourhajibagher et al., 2019 [43] | Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia | GQD-Cur: 100 µg/mL | Reduction in microbial cell viability; Reduction in biofilm biomass; Significant ROS generation in a dose-dependent manner; Downregulation of biofilm-related genes: rcpA (8.1-fold), fimA (9.6-fold), and inpA (11.8-fold). |
| Tonon et al., 2022 [44] | Porphyromonas gingivalis, Fusobacterium nucleatum, and Streptococcus oralis | Curcumin-loaded polymeric nanoparticles (Curcumin-NP) Concentration: 500 µg/mL | Reduction in bacterial viability in planktonic cultures (log reduction values); Limited antibiofilm activity on multispecies biofilms; Significant antimicrobial effects when photoactivated with blue light (420 nm); Non-cytotoxic to human periodontal ligament fibroblast cells. |
| Wenzler et al., 2024 [45] | Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, Parvimonas micra, Treponema denticola, and Tannerella forsythia | PDT: HELBO® Blue Photosensitizer; PTT: EmunDo® dye; Curcumin/DMSO solution: curcumin: 100 mg/L with 0.5% DMSO. | PDT: bacterial reduction; PTT: bacterial reduction; Curcumin/DMSO + laser: bacterial reduction. |
| Petrini et al., 2022 [47] | Streptococcus oralis | ALAD 5% | Reduction in bacterial biofilm on infected titanium surfaces |
| Radunović et al., 2020 [48] | Enterococcus faecalis, Escherichia coli, Staphylococcus aureus, Veillonella parvula, and Porphyromonas gingivalis | ALAD 10%, 25%, and 50% | Reductions in CFU |
| Author and Year | Species | Photosensitizer Concentration | Outcome Measures |
|---|---|---|---|
| Rossi et al., 2022 [46] | Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Tannerella forsythia, Streptococcus spp., Staphylococcus spp., and Enterococcus faecalis | ALAD 5% | PPD, BOP, MOB, REC, and VAS |
| Author/Year | Study Groups | Main Results |
| Qamar et al., 2023 [37] | Riboflavin-loaded nanoparticles in aloe vera gel (PGA/RF/AV) + MD. Treated with riboflavin-mediated PDT combined with MD. Treated with MD alone. | Riboflavin-loaded nanoparticles incorporated into aloe vera gel (PGA/RF/AV) both showed effectiveness in treating peri-implantitis in diabetic patients, with the PGA/RF/AV complex achieving superior clinical and microbiological outcomes. While both treatment approaches significantly reduced microbial counts of Porphyromonas gingivalis and Tannerella forsythia and improved parameters like probing depth, plaque index, and marginal bone levels, the PGA/RF/AV complex was particularly effective in reducing bleeding on probing and minimizing microbial loads over six months. This highlights the potential of combining riboflavin with aloe vera for enhanced antimicrobial and anti-inflammatory effects. |
| Reference | Author/Year | Study Groups | Main Results |
|---|---|---|---|
| [39] | Etemadi et al., 2023 | Negative control: sterile PBS wash only. Positive Control: 0.12% chlorhexidine. Curcumin alone: moderate antibacterial effect, comparable to PDT with curcumin. Riboflavin alone: lesser antibacterial effect than curcumin. LED alone: minimal effect on CFU/mL reduction. PDT with curcumin + LED: highly effective, second only to chlorhexidine. PDT with riboflavin + LED: moderately effective but less than curcumin-based PDT. | PDT with curcumin + LED was significantly more effective than PDT with riboflavin + LED, showing greater reductions in bacterial colonies (CFU/mL) compared to riboflavin-based PDT, LED alone, and the negative control. Curcumin-based PDT exhibited antimicrobial properties comparable to CHX, the gold standard, highlighting its potential as a non-invasive disinfection method. In contrast, PDT with riboflavin + LED achieved moderate reductions in CFU/mL, though it was less effective than curcumin-based PDT. These results suggest that curcumin is a more promising photosensitizer for PDT due to its superior bactericidal activity and potential as a safe alternative for implant surface decontamination. |
| [40] | Leelanarathiwatat et al., 2020 | Control group Light groups: biofilm exposed to light sources only. Red diode laser: applied for 60 s. Blue LED: applied for 10 s. Photosensitizer groups: biofilm treated with photosensitizers only. Methylene blue: no light activation. FMN: no light activation. Photoactivated PS groups (aPDT): MB with a red diode laser (MB*R): activated for 60 s. FMN with blue LED (FMN*B): activated for 10 s. | Riboflavin-mediated photodynamic therapy using FMN activated by blue LED light demonstrated significant antibacterial effects against Staphylococcus aureus biofilms, achieving a log reduction of 1.23 (approximately 93% reduction in viable bacteria) and a 52–62% decrease in biofilm mass. The blue LED alone showed no antibacterial activity, confirming the essential role of FMN activation. FMN-mediated PDT was as effective as MB-mediated PDT activated by a red diode laser but required only 10 s of irradiation compared to 60 s for MB, offering a faster and more practical treatment option. Additionally, FMN’s light yellow color was noted for cosmetic ease of removal. However, while effective, FMN-PDT did not completely eradicate bacteria, particularly in deeper biofilm layers, suggesting it is best used in combination with other therapies. These findings highlight FMN-PDT as a promising and efficient method for biofilm reduction with potential clinical advantages. |
| [41] | Mahdizade-Ari et al., 2019 | Whole bacterial cell suspension from A. actinomycetemcomitans treated with Cur-aPDT. Whole bacterial cell suspension from untreated A. actinomycetemcomitans. Cell-free supernatant fluid from A. actinomycetemcomitans treated with Cur-aPDT. Cell-free supernatant fluid from untreated A. actinomycetemcomitans. Bacterial cell pellet from A. actinomycetemcomitans treated with Cur-aPDT. Bacterial cell pellet from untreated A. actinomycetemcomitans. | Cur-aPDT significantly reduces microbial cell survival, metabolic activity, and QS abilities in Aggregatibacter actinomycetemcomitans, with bystander effects playing a critical role. The therapy decreased cell survival by up to 82.7%, metabolic activity by 42.6%, and QS mediator production by 83.2%, particularly when using treated whole bacterial cell suspensions and cell-free supernatant fluids. These effects suggest that Cur-aPDT generates metabolites with antimicrobial properties that influence neighboring untreated cells, thereby enhancing the overall efficacy of the treatment. However, the therapy showed no significant impact on bacterial efflux pump activity. These findings indicate that the combined direct and bystander effects of Cur-aPDT could make it a potent adjunct therapy for managing localized infections such as periodontitis and peri-implantitis. |
| [42] | Morelato et al., 2022 | Negative control: no surface treatment was applied to the implants. Positive control: surface treatment with 0.2% CHX using a sterile cotton pellet for 60 s. Photodynamic therapy group 1: treatment using a 660 nm diode laser (red light) with 0.1% methylene blue as the photosensitizer. Photodynamic therapy group 2: Treatment using a 445 nm diode laser (blue light) with 0.1% riboflavin as the photosensitizer. | Riboflavin-mediated photodynamic therapy using a 445 nm diode laser for disinfecting contaminated dental implant surface was evaluated against a conventional PDT protocol involving methylene blue with a 660 nm laser and CHX treatment. Results revealed that both PDT approaches significantly reduced Staphylococcus aureus and Candida albicans biofilms, showing comparable efficacy to CHX. The riboflavin-445 nm combination offered aesthetic advantages over methylene blue, as it did not cause discoloration. However, no approach achieved complete microbial eradication, highlighting PDT as a complementary method to mechanical cleaning for peri-implantitis treatment. This study suggests the potential of riboflavin-based PDT as a safe, effective adjunctive therapy, particularly suitable for the aesthetic zone. Further in vivo studies are needed to validate these findings. |
| [43] | Pourhajibagher et al., 2019 | Biofilms treated with GQD. Biofilms treated with curcumin. Biofilms treated with GQD-Cur. Biofilms exposed to blue LED. Biofilms treated with GQD and exposed to blue LED. Biofilms treated with curcumin and exposed to blue LED. Biofilms treated with GQD-Cur and exposed to blue LED. Control: untreated biofilms. | Cur-aPDT using GQD-Cur effectively suppresses perio-pathogens in both planktonic and biofilm forms. GQD-Cur composites were successfully synthesized and characterized using various techniques, confirming their structural and chemical properties, with minimal cytotoxicity to human gingival fibroblasts. Under blue LED irradiation, photoexcited GQD-Cur significantly reduced bacterial viability (93%) and biofilm formation capacity (76%), showing superior antimicrobial effects compared to other treatment groups. The treatment also induced a concentration-dependent increase in ROS production and markedly downregulated key biofilm-related gene expressions, including rcpA, fimA, and inpA, by 8.1-, 9.6-, and 11.8-fold, respectively. These findings highlight GQD-Cur as a promising, non-cytotoxic, nanoscale platform for enhanced antimicrobial photodynamic therapy in periodontitis management. |
| [44] | Tonon et al., 2022 | Curcumin (free): with light activation (L+); without light activation (L−). Curcumin-loaded nanoparticles (Curcumin-NP): with light activation (L+); without light activation (L−). Controls: positive control: 0.12% CHX; negative control: ultrapure water (L−). Nanoparticles without curcumin (NP L+ and L−). 10% DMSO. | Curcumin-NP were successfully synthesized, showing stability, homogeneity, a size of 189 nm, and a 67.5% encapsulation efficiency. Curcumin-NP demonstrated enhanced antimicrobial effects when activated by blue light, particularly against planktonic cultures of Porphyromonas gingivalis, Fusobacterium nucleatum, and Streptococcus oralis but showed limited efficacy against mature multispecies biofilms on titanium surfaces. Blue-light activation improved the photodynamic effects, with curcumin-NP exhibiting higher activity against Gram-negative bacteria like P. gingivalis and F. nucleatum compared to Gram-positive S. oralis. Importantly, the curcumin-NP and free curcumin were non-cytotoxic to human periodontal ligament fibroblast cells. These findings suggest that while curcumin-NP shows promise in antimicrobial PDT, further improvements in biofilm penetration and activity are needed to optimize its therapeutic potential. |
| [45] | Wenzler et al., 2024 | PDT: HELBO® Blue Photosensitizer + laser application. PDT dye: HELBO® Blue. Photosensitizer without laser application. Curcumin/DMSO + laser. Curcumin/DMSO only. DMSO: DMSO solution only, without laser application. PTT: EmunDo® dye + 810 nm laser irradiation. PTT Dye: EmunDo® dye without laser application. Control: untreated group. | Cur-aPDT using 445 nm laser irradiation did not show significant antibacterial improvement compared to curcumin/DMSO without laser activation, suggesting the antibacterial effect was primarily due to the solvent DMSO. DMSO alone demonstrated significant bacterial reduction, highlighting its inherent antimicrobial properties, though its efficacy was slightly reduced when combined with curcumin, possibly due to dilution effects. Compared to conventional PDT or PTT, curcumin-based PDT with 445 nm laser irradiation was less effective, potentially due to suboptimal absorption at this wavelength, indicating a need for further optimization of laser parameters, solvents, and curcumin concentrations. |
| [46] | Rossi et al., 2022 | Periodontitis group (n = 10) and peri-implantitis group (n = 10) | PDT using 5-ALA demonstrated significant clinical benefits in the treatment of periodontitis and peri-implantitis. A retrospective analysis of 20 patients revealed that adjunctive PDT with 5% 5-ALA gel significantly reduced probing PPD and BOP at 3 and 6 months post-treatment. In periodontal sites, PPD showed a significant reduction (p < 0.001), while BOP also decreased significantly (p = 0.001). Similarly, in peri-implantitis cases, PPD reduction was statistically significant (p < 0.001), though other improvements, such as decreased BOP and slight increases in exposed implant threads, were noted without reaching statistical significance. Patients reported no pain and perceived sustained benefits. These findings support 5-ALA PDT as a promising adjunct to non-surgical therapy for managing periodontal and peri-implant infections |
| [47] | Petrini et al., 2022 | MACHINED (control group), MACHINED + ALAD (experimental group), and DAE (control group) | PDT using 5-ALA demonstrated significant antibacterial and antibiofilm activity against Streptococcus oralis biofilm on titanium implant surfaces. The study showed that applying a 5% 5-ALA gel followed by red LED light irradiation (630 nm) significantly reduced bacterial CFUs and biofilm biomass on both machined and DAE titanium surfaces. CFU counts decreased by 89% on machined surfaces and 77% on DAE surfaces compared to unexposed controls (p < 0.050). Live/dead staining confirmed 100% bacterial cell death in PDT-treated samples, and SEM revealed a significant reduction in biofilm accumulation. These findings support 5-ALA-mediated PDT as an effective strategy for implant decontamination and peri-implant disease management. |
| [48] | Radunović et al., 2020 | Enterococcus faecalis (1-h incubation with 10% and 50% ALAD); Enterococcus faecalis (25 min incubation with 10%, 25%, and 50% ALAD); Escherichia coli (25 min incubation with 10%, 25%, and 50% ALAD); Staphylococcus aureus (25 min incubation with 10%, 25%, and 50% ALAD); Veillonella parvula (25 min incubation with 10%, 25%, and 50% ALAD); Porphyromonas gingivalis (25 min incubation with 10%, 25%, and 50% ALAD). | PDT using ALAD gel and red LED irradiation demonstrated a significant antibacterial effect across all tested bacterial species. Enterococcus faecalis showed total inactivation with 50% ALAD + 7 min LED, while lower ALAD concentrations (25%) combined with 5 min LED were effective in reducing CFUs. Escherichia coli and Porphyromonas gingivalis required 50% ALAD for significant reduction, with LED irradiation enhancing bacterial eradication at lower concentrations. Staphylococcus aureus and Veillonella parvula exhibited intrinsic sensitivity to ALAD alone, but LED irradiation amplified the bactericidal effect. Overall, 25 min of 50% ALAD incubation followed by 5 min LED irradiation was the most effective protocol, achieving substantial bacterial reduction, with implications for treating oral infections and antibiotic-resistant bacteria. |
| Author/Year | Light Source | Operating Mode | Wavelength (nm) | Energy Density (J/cm2) | Power Output (mW) | Powermeter Used | Energy Output (J) | Irradiation Time (s) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Qamar et al., 2023 [37] | Diode laser (670 nm) | Continuous wave | 670 | 1.1 | 280 | Not specified | Not specified | 60 | 1100 |
| Author/Year | Light Source | Operating Mode | Wavelength (nm) | Energy Density (J/cm2) | Power Output (mW) | Powermeter Used | Energy Output (J) | Irradiation Time (s) | Power Density (mW/cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Etemadi et al., 2023 [39] | LED (DY400-4, Denjoy, China) | Continuous wave | 390–480 | 300–420 | 1000 ± 100 | LaserPoint s.r.l, Milano, Italy | 300 | ||
| Leelanarathiwatat et al., 2020 [40] | Blue LED and red diode laser | Pulsed wave | 450–470, 670 | 37–40, 4.24 | 291–314 12 | Not specified | 29.1–31.4 0.2 | 10 60 | 3700–4000 75 |
| Mahdizade-Ari et al., 2019 [41] | Blue LED | Continuous wave | 435 ± 20 | 300–420 | Not specified | Not specified | 300 | 1000–1400 | |
| Morelato et al., 2022 [42] | Diode laser | 660 nm laser: Continuous-wave mode 445 nm laser: Pulsed mode (100 Hz) | 445 660 | 1.24 1240 | 100 | Not specified | 1.24 | 60 | 124.34 |
| Pourhajibagher et al., 2019 [43] | Blue LED | Continuous wave | 60–420 | Not specified | 1000–1400 | LaserPoint s.r.l, Milan | Not specified | 60 | 1000–1400 |
| Tonon et al., 2022 [44] | Blue Light | Fixed output power | 420 | 72 | - | Not specified | Not specified | 720 | 95.5 |
| Wenzler et al., 2024 [45] | HELBO® TheraLite laser SIROLaserBlue FOX Q810plus laser | Continuous wave | 660 445 810 | Not specified | 100 600 200 | Not specified | Not specified | 10 (per point × 6) | 70.74 373.02 565.90 |
| Petrini et al., 2022 [47] | AlGaAs power LED device (TL-01) | Not specified | 630 ± 10 | 100 | 380 | Not specified | Not specified | 420 | 380 |
| Radunović et al., 2020 [48] | AlGaAs power LED (TL-01) | Continuous | 630 ± 10 | 23 | Not specified | Not specified | Not specified | Not specified | 380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. https://doi.org/10.3390/pharmaceutics17040443
Warakomska A, Fiegler-Rudol J, Kubizna M, Skaba D, Wiench R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics. 2025; 17(4):443. https://doi.org/10.3390/pharmaceutics17040443
Chicago/Turabian StyleWarakomska, Aleksandra, Jakub Fiegler-Rudol, Magdalena Kubizna, Dariusz Skaba, and Rafał Wiench. 2025. "The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review" Pharmaceutics 17, no. 4: 443. https://doi.org/10.3390/pharmaceutics17040443
APA StyleWarakomska, A., Fiegler-Rudol, J., Kubizna, M., Skaba, D., & Wiench, R. (2025). The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics, 17(4), 443. https://doi.org/10.3390/pharmaceutics17040443







