Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases
Abstract
1. Introduction
2. Relationship Between MMP-9 and Tumors and Vascular Diseases
2.1. Relationship Between MMP-9 and Tumors
2.1.1. Relationship Between MMP-9 and Tumor Epithelial–Mesenchymal Transition
2.1.2. Relationship Between MMP-9 and Tumor Angiogenesis and Metastasis
2.2. Relationship Between MMP-9 and Vascular Diseases
2.2.1. Relationship Between MMP-9 and Macrovascular Diseases
2.2.2. Relationship Between MMP-9 and Cerebrovascular Diseases
2.2.3. Relationship Between MMP-9 and Ocular Vascular Diseases
3. Signal Pathways Regulating MMP-9 Expression
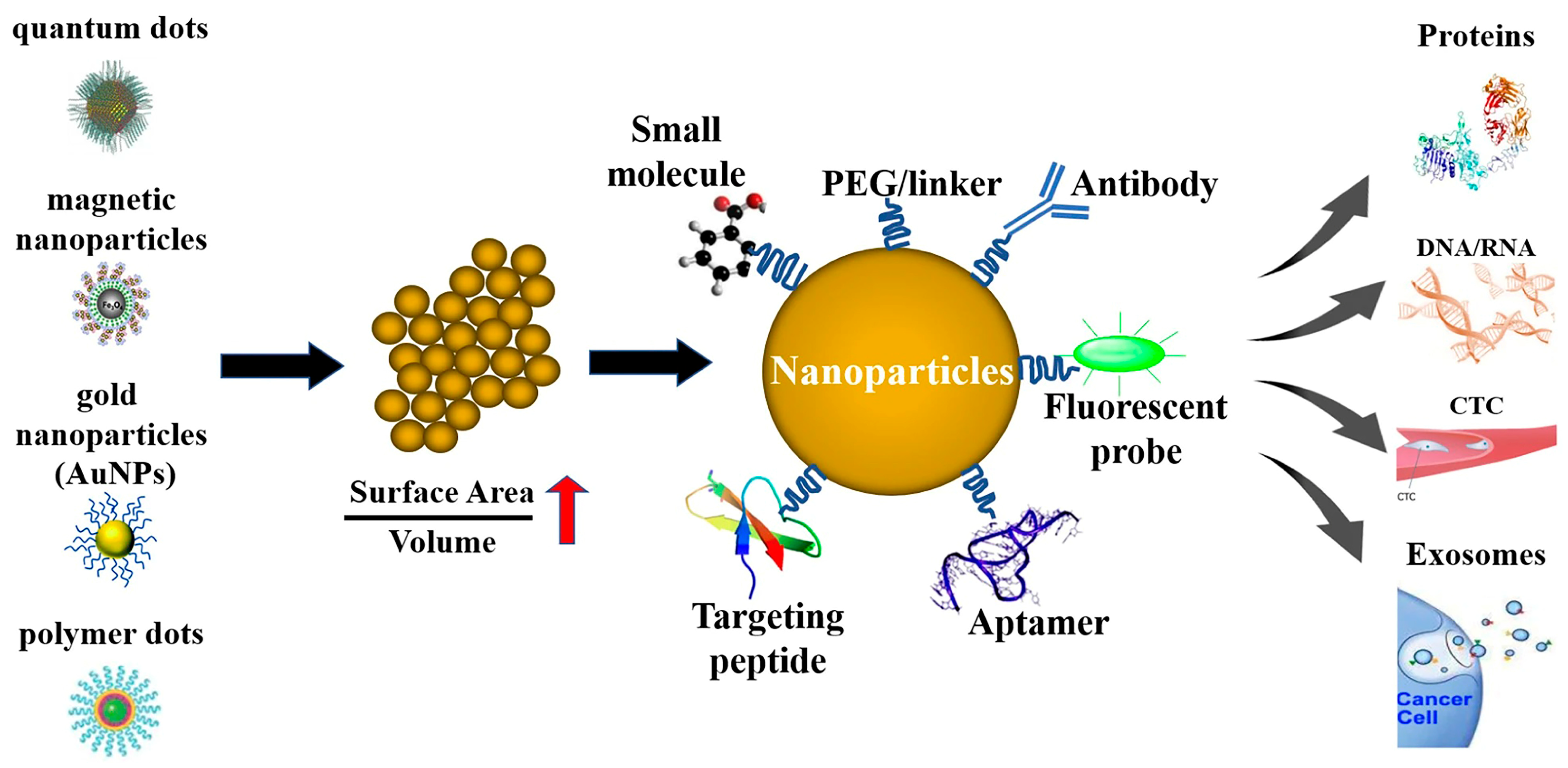
4. Nanomedicine and MMP-9-Targeted Nanoparticles
4.1. Comparison of MMP-9-Targeted and Non-Targeted Nanomedicines
4.2. MMP-9 Overexpression and MMP-9-Responsive Nanoparticles
4.3. Mechanisms of Action of MMP-9-Responsive Nanoparticles
4.3.1. Degradation
- (a)
- (b)
- (c)
- Matrix degradation: Nanoparticles embedded within the ECM are released upon MMP-9-mediated matrix degradation [142].
4.3.2. Drug Release
- (a)
- Degradation-triggered release: MMP-9 activity degrades the nanoparticles, altering their composition, size, and cross-linking density, thereby modulating drug release rates [139].
- (b)
4.3.3. Targeting Specificity
- (a)
- (b)
- Passive targeting: Nanoparticles passively accumulate in tumors due to the EPR effect, exploiting leaky tumor vasculature and impaired lymphatic drainage [147].
4.4. Heterogeneity of MMP-9 Expression and Targeted Therapy Efficacy
5. MMP-9-Related Nanomedicines in Tumor Angiogenesis and Metastasis
5.1. MMP-9-Targeted Nanomedicine in Anti-Tumor Angiogenesis and Metastasis Therapy
5.2. MMP-9-Targeted Assays for Tumor Cells
5.3. Effect of Tumor Heterogeneity on MMP-9 Targeting Efficacy
6. MMP-9-Related Nanomedicines in Vascular Diseases
6.1. MMP-9-Related Nanomedicines in Macrovascular Diseases
6.1.1. Nanomedicines Regulating MMP-9 Expression in Macrovascular Diseases
6.1.2. MMP-9-Responsive Nanocarriers in Macrovascular Diseases
6.2. MMP-9-Related Nanomedicines in Cerebrovascular and Ocular Vascular Diseases
6.2.1. MMP-9-Related Nanomedicines in Cerebrovascular Diseases
6.2.2. MMP-9-Related Nanomedicines in Ocular Vascular Diseases
6.2.3. Challenges of Blood–Brain Barrier and Blood–Retinal Barrier to Nanodrug Delivery
7. Effects of the Immune System on MMP-9-Targeted Nanoparticles
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4T1 | mouse mammary tumor cell line |
| 6-MCH | 6-hydroxy-1-hexanethiol |
| Akt | protein kinase B |
| AP-1 | activator protein 1 |
| bFGF | basic fibroblast growth factor |
| CDKN1B | cycling-dependent kinase inhibitor 1B |
| CNV | choroidal neovascularization |
| CpG | 5′-C-phosphate-G-3′ |
| CQDs | carbon quantum dots |
| DOPE, 1 | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| EGCG | epigallocatechin-3-gallate |
| ELNs | exosome-like nanoparticles |
| EMMPRIN | extracellular matrix metalloproteinase inducer |
| EMT | epithelial–mesenchymal transition |
| ERK | extracellular regulated protein kinases |
| GFLG | Gly-Phe-Leu-Gly |
| G-MDSC | granulocyte-like myeloid derived suppressor cells |
| GOQD | graphene oxide quantum dots |
| GPRC5B | G protein-coupled receptor 5B |
| GST | glutathione-S-transferase |
| HIV | human immunodeficiency virus |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| HSP70 | heat shock 70 kda protein |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL-10 | interleukin 10 |
| IP-10 | interferon–γ–inducible protein 10 |
| JAK2/STAT3 | janus kinase 2/signal transduction and activator of transcription 3 |
| JNK | C-jun N-terminal kinase |
| LDL | low-density lipoprotein |
| LRP | low density lipoprotein receptor-related protein |
| MAPK | mitogen-activated protein kinase |
| MCF7 | a breast cancer cell line |
| MMP | matrix metalloproteinase |
| mPEG | methoxypolyethylene glycol |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NKCT1 | purified naja kaouthia protein toxin |
| NMDAR | N-methyl-D-aspartate receptor |
| PAF | platelet-activating factor |
| PAR-1 | protease-activated receptor 1 |
| PARP-1 | poly [adp-ribose] polymerase 1 |
| PDI | protein disulfide isomerase |
| PDMS–PMOXA | poly(dimethylsiloxane)-poly-b-(methyloxazoline) |
| PEG | polyethylene glycol |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PLGA | poly(lactic-co-glycolic acid) |
| PP-HA/NPs | hyaluronic acid/poly(lactic acid)-glycolic acid-poly(ethyleneimine) nanoparticles |
| PTK | poly (5,5-dimethyl-4,6-dithio-propylene glycol azelate) |
| PVP-b-PCL | poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) |
| RGD | L-Arginyl-Glycyl-L-Aspartic acid |
| ROCK | rho-associated protein kinase |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SERS | surface-enhanced Raman scattering |
| Shh | sonic hedgehog protein |
| SKOV3 SMCs | an ovarian cancer cell line smooth muscle cells |
| SNAs | spherical nucleic acid |
| SPECT | single-photon emission computed tomography |
| TAK1 | TGF-β-activated kinase 1 |
| TGF-β | transforming growth factor β |
| TGK | protein-glutamine gamma-glutamyltransferase K |
| TNF-α | tumor necrosis factor α |
| TOS | tocopheryl succinate |
| tPA | tissue plasminogen activator |
| TRPV4 | transient receptor potential cation channel subfamily V member 4 |
| VEGF | vascular endothelial growth factor |
| VEGF-R1 VSM | vascular endothelial growth factor receptor 1 vascular smooth muscle |
| ZO | zonula occludens |
| α-TPGS | α-tocopherol polyethylene glycol succinate |
References
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical-Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The Cysteine Switch: A Principle of Regulation of Metalloproteinase Activity with Potential Applicability to the Entire Matrix Metalloproteinase Gene Family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar]
- Bode, W. A Helping Hand for Collagenases: The Haemopexin-like Domain. Structure 1995, 3, 527–530. [Google Scholar] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [PubMed]
- Buttacavoli, M.; Di Cara, G.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Integrated Multi-Omics Investigations of Metalloproteinases in Colon Cancer: Focus on MMP2 and MMP9. Int. J. Mol. Sci. 2021, 22, 12389. [Google Scholar] [CrossRef]
- Barillari, G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. Int. J. Mol. Sci. 2020, 21, 4526. [Google Scholar] [CrossRef]
- Kanugula, A.K.; Adapala, R.K.; Jamaiyar, A.; Lenkey, N.; Guarino, B.D.; Liedtke, W.; Yin, L.; Paruchuri, S.; Thodeti, C.K. Endothelial TRPV4 Channels Prevent Tumor Growth and Metastasis via Modulation of Tumor Angiogenesis and Vascular Integrity. Angiogenesis 2021, 24, 647–656. [Google Scholar]
- Lindsey, M.L. Assigning Matrix Metalloproteinase Roles in Ischaemic Cardiac Remodelling. Nat. Rev. Cardiol. 2018, 15, 471–479. [Google Scholar] [PubMed]
- Sharifi, M.A.; Wierer, M.; Dang, T.A.; Milic, J.; Moggio, A.; Sachs, N.; von Scheidt, M.; Hinterdobler, J.; Müller, P.; Werner, J.; et al. ADAMTS-7 Modulates Atherosclerotic Plaque Formation by Degradation of TIMP-1. Circ. Res. 2023, 133, 674–686. [Google Scholar] [CrossRef]
- Liu, X.; Gu, J.; Wang, J.; Zhang, W.; Wang, Y.; Xu, Z. Cell Membrane-Anchored SERS Biosensor for the Monitoring of Cell-Secreted MMP-9 during Cell-Cell Communication. ACS Sens. 2023, 8, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hou, Y.; Zeng, J.; Liu, C.; Zhang, P.; Jing, L.; Shangguan, D.; Gao, M. Dual-Ratiometric Target-Triggered Fluorescent Probe for Simultaneous Quantitative Visualization of Tumor Microenvironment Protease Activity and pH in Vivo. J. Am. Chem. Soc. 2018, 140, 211–218. [Google Scholar] [CrossRef]
- Rainu, S.; Parameswaran, S.; Krishnakumar, S.; Singh, N. Dual-Sensitive Fluorescent Nanoprobes for Detection of Matrix Metalloproteinases and Low pH in a 3D Tumor Microenvironment. J. Mater. Chem. B 2022, 10, 5388–5401. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhou, J.; Gao, Z.; Sun, X.; Liu, C.; Shangguan, D.; Yang, W.; Gao, M. Protease-Activated Ratiometric Fluorescent Probe for pH Mapping of Malignant Tumors. ACS Nano 2015, 9, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Cancers. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer/ (accessed on 4 April 2025).
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 23 March 2025).
- World Stroke Day 2022. Available online: https://www.who.int/srilanka/news/detail/29-10-2022-world-stroke-day-2022 (accessed on 23 March 2025).
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Sun, R.; Del Piccolo, N.; Stevens, M.M. Microneedle-Mediated Nanomedicine to Enhance Therapeutic and Diagnostic Efficacy. Nano Converg. 2024, 11, 15. [Google Scholar] [CrossRef]
- Ren, S.; Xu, Y.; Dong, X.; Mu, Q.; Chen, X.; Yu, Y.; Su, G. Nanotechnology-Empowered Combination Therapy for Rheumatoid Arthritis: Principles, Strategies, and Challenges. J. Nanobiotechnol. 2024, 22, 431. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in Cancer Diagnosis: Progress, Challenges and Opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Gonnelli, A.; Gerbé de Thoré, M.; Ermini, M.L.; Frusca, V.; Zamborlin, A.; Signolle, N.; Bawa, O.; Clémenson, C.; Meziani, L.; Bergeron, P.; et al. Nonpersistent Nanoarchitectures Enhance Concurrent Chemoradiotherapy in an Immunocompetent Orthotopic Model of HPV+ Head/Neck Carcinoma. Adv. Mater. 2024, 36, e2400949. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, J.; Liu, Y.; Zhao, Y.; Li, Z.; Xu, B.; Chen, D.; Wang, B.; Wang, Q.; Shen, Y. Hybrid Nanopotentiators with Dual Cascade Amplification for Glioma Combined Interventional Therapy. J. Control Release 2024, 372, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Cai, W.; Tan, Y.; Zhao, Z.; Liu, J. Highly Controllable Nanoassemblies of Luminescent Gold Nanoparticles with Abnormal Disassembly-Induced Emission Enhancement for In Vivo Imaging Applications. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212214. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Pitarresi, G.; Palumbo, F.S.; Catania, V.; Schillaci, D.; Mauro, N.; Fiorica, C.; Giammona, G. Fabrication of Silver Nanoparticles by a Diethylene Triamine-Hyaluronic Acid Derivative and Use as Antibacterial Coating. Carbohydr. Polym. 2022, 295, 119861. [Google Scholar] [PubMed]
- Chen, X.; Li, H.; Qiao, X.; Jiang, T.; Fu, X.; He, Y.; Zhao, X. Agarose Oligosaccharide- Silver Nanoparticle- Antimicrobial Peptide- Composite for Wound Dressing. Carbohydr. Polym. 2021, 269, 118258. [Google Scholar] [PubMed]
- Yu, B.; Wang, Y.; Bing, T.; Tang, Y.; Huang, J.; Xiao, H.; Liu, C.; Yu, Y. Platinum Prodrug Nanoparticles with COX-2 Inhibition Amplify Pyroptosis for Enhanced Chemotherapy and Immune Activation of Pancreatic Cancer. Adv. Mater. 2024, 36, e2310456. [Google Scholar] [CrossRef]
- Lv, K.; Hou, M.; Kou, Y.; Yu, H.; Liu, M.; Zhao, T.; Shen, J.; Huang, X.; Zhang, J.; Mady, M.F.; et al. Black Titania Janus Mesoporous Nanomotor for Enhanced Tumor Penetration and Near-Infrared Light-Triggered Photodynamic Therapy. ACS Nano 2024, 18, 13910–13923. [Google Scholar] [CrossRef]
- Guo, S.; Shu, G.; Luo, H.; Kuang, X.; Zheng, L.; Wang, C.; Zhou, C.-A.; Song, L.; Ma, K.; Yue, H. Low-Cytotoxic Core-Sheath ZnO NWs@TiO2-xNy Triggered Piezo-Photocatalytic Antibacterial Activity. ACS Appl. Mater. Interfaces 2024, 16, 24410–24420. [Google Scholar] [CrossRef] [PubMed]
- Jacqmarcq, C.; Picot, A.; Flon, J.; Lebrun, F.; Martinez de Lizarrondo, S.; Naveau, M.; Bernay, B.; Goux, D.; Rubio, M.; Malzert-Fréon, A.; et al. MRI-Based Microthrombi Detection in Stroke with Polydopamine Iron Oxide. Nat. Commun. 2024, 15, 5070. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lian, W.; Yu, N.; Meng, J.; Zeng, H.; Wang, Y.; Wang, X.; Wen, M.; Chen, Z. Erythrocyte-Membrane-Camouflaged Magnetic Nanocapsules with Photothermal/Magnetothermal Effects for Thrombolysis. Adv. Healthc. Mater. 2024, 13, e2400127. [Google Scholar] [CrossRef]
- Xiao, H.; Meng, X.; Li, S.; Li, Z.; Fang, S.; Wang, Y.; Li, J.; Tang, J.; Ma, L. Combined Drug Anti-Deep Vein Thrombosis Therapy Based on Platelet Membrane Biomimetic Targeting Nanotechnology. Biomaterials 2024, 311, 122670. [Google Scholar]
- Pan, P.; Yue, Q.; Yang, X.; Ren, Y.; Alharthi, F.A.; Alghamdi, A.; Su, J.; Deng, Y. Structure Engineering of Yolk-Shell Magnetic Mesoporous Silica Microspheres with Broccoli-Like Morphology for Efficient Catalysis and Enhanced Cellular Uptake. Small 2021, 17, e2006925. [Google Scholar] [PubMed]
- Tanabe, M.; Ando, K.; Komatsu, R.; Morigaki, K. Nanofluidic Biosensor Created by Bonding Patterned Model Cell Membrane and Silicone Elastomer with Silica Nanoparticles. Small 2018, 14, e1802804. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, T.M.; Dassanayake, A.C.; Abeydeera, N.; Pant, B.D.; Jaroniec, M.; Kim, M.-H.; Huang, S.D. An Aluminum Lining to the Dark Cloud of Silver Resistance: Harnessing the Power of Potent Antimicrobial Activity of γ-Alumina Nanoparticles. Biomater. Sci. 2021, 9, 7996–8006. [Google Scholar] [PubMed]
- Yan, Y.; Liu, Z.; Pang, W.; Huang, S.; Deng, M.; Yao, J.; Huang, Q.; Jin, M.; Shui, L. Integrated Biosensor Array for Multiplex Biomarkers Cancer Diagnosis via In-Situ Self-Assembly Carbon Nanotubes with an Ordered Inverse-Opal Structure. Biosens. Bioelectron. 2024, 262, 116528. [Google Scholar]
- Sui, N.; Ji, Y.; Li, M.; Zheng, F.; Shao, S.; Li, J.; Liu, Z.; Wu, J.; Zhao, J.; Li, L.-J. Photoprogrammed Multifunctional Optoelectronic Synaptic Transistor Arrays Based on Photosensitive Polymer-Sorted Semiconducting Single-Walled Carbon Nanotubes for Image Recognition. Adv. Sci. 2024, 11, e2401794. [Google Scholar] [CrossRef] [PubMed]
- Behrouznejad, B.; Sadat, S.B.; Masaeli, E. The Orchestration of Sustained Drug Delivery by Bacterial Cellulose/Gelatin Nanocomposites Reinforced with Carboxylic Carbon Nanotubes. Carbohydr. Polym. 2024, 333, 121917. [Google Scholar] [CrossRef]
- Sun, J.; Jia, W.; Qi, H.; Huo, J.; Liao, X.; Xu, Y.; Wang, J.; Sun, Z.; Liu, Y.; Liu, J.; et al. An Antioxidative and Active Shrinkage Hydrogel Integratedly Promotes Re-Epithelization and Skin Constriction for Enhancing Wound Closure. Adv. Mater. 2024, 36, e2312440. [Google Scholar] [CrossRef]
- Zaręba, N.; Więcławik, K.; Kizek, R.; Hosnedlova, B.; Kepinska, M. The Impact of Fullerenes as Doxorubicin Nano-Transporters on Metallothionein and Superoxide Dismutase Status in MCF-10A Cells. Pharmaceutics 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, Z.; Guo, B.; Lu, Y.; Lu, X.; Ren, Q.; Lv, A.; Nie, J.; Ji, D.; Rotenberg, M.Y.; et al. Robust, Stretchable Bioelectronic Interfaces for Cardiac Pacing Enabled by Interfacial Transfer of Laser-Induced Graphene via Water-Response, Nonswellable PVA Gels. Biosens. Bioelectron. 2024, 261, 116453. [Google Scholar]
- Lu, J.; Chen, X.; Ding, X.; Jia, Z.; Li, M.; Zhang, M.; Liu, F.; Tang, K.; Yu, X.; Li, G. Droplet Micro-Sensor and Detection of Respiratory Droplet Transmission. Adv. Sci. 2024, 11, e2401940. [Google Scholar]
- Toro-González, M.; Akingbesote, N.; Bible, A.; Pal, D.; Sanders, B.; Ivanov, A.S.; Jansone-Popova, S.; Popovs, I.; Benny, P.; Perry, R.; et al. Development of 225Ac-Doped Biocompatible Nanoparticles for Targeted Alpha Therapy. J. Nanobiotechnol. 2024, 22, 306. [Google Scholar] [CrossRef]
- Baruah, N.; Ahamad, N.; Halder, P.; Koley, H.; Katti, D.S. Facile Synthesis of Multi-Faceted, Biomimetic and Cross-Protective Nanoparticle-Based Vaccines for Drug-Resistant Shigella: A Flexible Platform Technology. J. Nanobiotechnol. 2023, 21, 34. [Google Scholar]
- Blaya-Cánovas, J.L.; Griñán-Lisón, C.; Blancas, I.; Marchal, J.A.; Ramírez-Tortosa, C.; López-Tejada, A.; Benabdellah, K.; Cortijo-Gutiérrez, M.; Cano-Cortés, M.V.; Graván, P.; et al. Autologous Patient-Derived Exhausted Nano T-Cells Exploit Tumor Immune Evasion to Engage an Effective Cancer Therapy. Mol. Cancer 2024, 23, 83. [Google Scholar] [CrossRef]
- Pathan, S.; Jayakannan, M. Zwitterionic Strategy to Stabilize Self-Immolative Polymer Nanoarchitecture under Physiological pH for Drug Delivery In Vitro and In Vivo. Adv. Healthc. Mater. 2024, 13, e2304599. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F.F.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-Loaded Coaxial PGS/PCL Aligned Nanofibers for Cartilage Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110291. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Y.; Wu, S.; Zhang, J.; Zhu, M.; Chen, X.; Fu, X.; Duan, X.; Men, K. Dual-mRNA Delivery Using Tumor Cell Lysate-Based Multifunctional Nanoparticles as an Efficient Colon Cancer Immunogene Therapy. Int. J. Nanomed. 2024, 19, 4779–4801. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yan, S.; Chen, G.; Wang, Y.; Zhang, X.; Lan, J.; Chen, J.; Yao, X. A Cavity Induced Mode Hybridization Plasmonic Sensor for Portable Detection of Exosomes. Biosens. Bioelectron. 2024, 261, 116492. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, Y.; Cohen, O.T.; Wald, O.; Bavli, D.; Kaplan, T.; Benny, O. Particle Uptake in Cancer Cells Can Predict Malignancy and Drug Resistance Using Machine Learning. Sci. Adv. 2024, 10, eadj4370. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhu, L.; Ye, X.; Ke, Q.; Zhang, Q.; Xie, X.; Piao, J.-G.; Wei, Y. Integrated Oral Microgel System Ameliorates Renal Fibrosis by Hitchhiking Co-Delivery and Targeted Gut Flora Modulation. J. Nanobiotechnol. 2024, 22, 305. [Google Scholar] [CrossRef]
- Li, B.; Niu, H.; Zhao, X.; Huang, X.; Ding, Y.; Dang, K.; Yang, T.; Chen, Y.; Ma, J.; Liu, X.; et al. Targeted Anti-Cancer Therapy: Co-Delivery of VEGF siRNA and Phenethyl Isothiocyanate (PEITC) via cRGD-Modified Lipid Nanoparticles for Enhanced Anti-Angiogenic Efficacy. Asian J. Pharm. Sci. 2024, 19, 100891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Peng, J.; Jiang, N.; Zhang, W.; Liu, S.; Li, J.; Duan, D.; Li, Y.; Peng, C.; Yan, Y.; et al. Chitosan Phytate Nanoparticles: A Synergistic Strategy for Effective Dental Caries Prevention. ACS Nano 2024, 18, 13528–13537. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wu, X.; Wang, Y.; Wang, J.; Zhao, Y. Cuttlefish-Inspired Photo-Responsive Antibacterial Microparticles with Natural Melanin Nanoparticles Spray. Small 2024, 20, e2310444. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Zhao, D.; Liu, X.; Wang, Y.; Chen, Y.; Ma, X. Enzyme-Powered Hollow Nanorobots for Active Microsampling Enabled by Thermoresponsive Polymer Gating. ACS Nano 2022, 16, 10354–10363. [Google Scholar] [CrossRef]
- Zaritski, A.; Castillo-Ecija, H.; Kumarasamy, M.; Peled, E.; Sverdlov Arzi, R.; Carcaboso, Á.M.; Sosnik, A. Selective Accumulation of Galactomannan Amphiphilic Nanomaterials in Pediatric Solid Tumor Xenografts Correlates with GLUT1 Gene Expression. ACS Appl. Mater. Interfaces 2019, 11, 38483–38496. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Liang, B.; Zheng, Y.; Exner, A.; Kolios, M.; Xu, T.; Guo, D.; Cai, X.; Wang, Z.; Ran, H.; et al. PMMA-Fe3O4 for Internal Mechanical Support and Magnetic Thermal Ablation of Bone Tumors. Theranostics 2019, 9, 4192–4207. [Google Scholar] [CrossRef]
- Dosta, P.; Dion, M.Z.; Prado, M.; Hurtado, P.; Riojas-Javelly, C.J.; Cryer, A.M.; Soria, Y.; Andrews Interiano, N.; Muñoz-Taboada, G.; Artzi, N. Matrix Metalloproteinase- and pH-Sensitive Nanoparticle System Enhances Drug Retention and Penetration in Glioblastoma. ACS Nano 2024, 18, 14145–14160. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Z.; Cao, W.; Tang, J.; Xia, Y.; Peng, L.; Ma, J. A PD-L1 Antibody-Conjugated PAMAM Dendrimer Nanosystem for Simultaneously Inhibiting Glycolysis and Promoting Immune Response in Fighting Breast Cancer. Adv. Mater. 2023, 35, e2305215. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lyu, Z.; Perles-Barbacaru, T.-A.; Huang, A.Y.-T.; Lian, B.; Jiang, Y.; Roussel, T.; Galanakou, C.; Giorgio, S.; Kao, C.-L.; et al. Modular Self-Assembling Dendrimer Nanosystems for Magnetic Resonance and Multimodality Imaging of Tumors. Adv. Mater. 2024, 36, e2308262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, X.; Mao, K.; Chen, H.; Cong, X.; Liu, F.; Wang, J.; Liu, S.; Xin, Y.; Zhu, G.; et al. Maleimide as the PEG End-Group Promotes Macrophage-Targeted Drug Delivery of PEGylated Nanoparticles in Vivo by Enhancing Interaction with Circulating Erythrocytes. Biomaterials 2023, 300, 122187. [Google Scholar] [CrossRef] [PubMed]
- Schnorenberg, M.R.; Hawley, K.M.; Thomas-Toth, A.T.; Watkins, E.A.; Tian, Y.; Ting, J.M.; Leak, L.B.; Kucera, I.M.; Raczy, M.M.; Kung, A.L.; et al. Targeted Polymersome Delivery of a Stapled Peptide for Drugging the Tumor Protein P53:BCL-2-Family Axis in Diffuse Large B-Cell Lymphoma. ACS Nano 2023, 17, 23374–23390. [Google Scholar] [PubMed]
- Valsalakumari, R.; Pandya, A.D.; Prasmickaite, L.; Kvalvaag, A.; Myrann, A.G.; Åslund, A.K.O.; Kjos, M.S.; Fontecha-Cuenca, C.; Haroon, H.B.; Ribeiro, A.R.S.; et al. Preclinical Efficacy of Cabazitaxel Loaded Poly(2-Alkyl Cyanoacrylate) Nanoparticle Variants. Int. J. Nanomed. 2024, 19, 3009–3029. [Google Scholar]
- Park, J.S.; Seo, J.H.; Jeong, M.Y.; Yang, I.G.; Kim, J.S.; Kim, J.H.; Ho, M.J.; Jin, S.G.; Choi, M.K.; Choi, Y.S.; et al. Carboxymethyl Cellulose-Based Rotigotine Nanocrystals-Loaded Hydrogel for Increased Transdermal Delivery with Alleviated Skin Irritation. Carbohydr. Polym. 2024, 338, 122197. [Google Scholar]
- Rong, M.; Liu, D.; Xu, X.; Li, A.; Bai, Y.; Yang, G.; Liu, K.; Zhang, Z.; Wang, L.; Wang, K.; et al. A Superparamagnetic Composite Hydrogel Scaffold as In Vivo Dynamic Monitorable Theranostic Platform for Osteoarthritis Regeneration. Adv. Mater. 2024, 36, e2405641. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Lei, F.; Gao, K.; Li, J.; Liu, R.; Karpf, A.R.; Bronich, T.K. Ligand-Installed Polymeric Nanocarriers for Combination Chemotherapy of EGFR-Positive Ovarian Cancer. J. Control Release 2023, 360, 872–887. [Google Scholar] [PubMed]
- Gao, Z.; He, T.; Zhang, P.; Li, X.; Zhang, Y.; Lin, J.; Hao, J.; Huang, P.; Cui, J. Polypeptide-Based Theranostics with Tumor-Microenvironment-Activatable Cascade Reaction for Chemo-Ferroptosis Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20271–20280. [Google Scholar]
- Han, J.; Choi, S.; Hong, J.; Gang, D.; Lee, S.; Shin, K.; Ko, J.; Kim, J.-U.; Hwang, N.S.; An, Y.-H.; et al. Superoxide Dismutase-Mimetic Polyphenol-Based Carbon Dots for Multimodal Bioimaging and Treatment of Atopic Dermatitis. ACS Appl. Mater. Interfaces 2024, 16, 24308–24320. [Google Scholar]
- Baek, G.W.; Kim, Y.J.; Kim, J.; Chang, J.H.; Kim, U.; An, S.; Park, J.; Yu, S.; Bae, W.K.; Lim, J.; et al. Memristive Switching Mechanism in Colloidal InP/ZnSe/ZnS Quantum Dot-Based Synaptic Devices for Neuromorphic Computing. Nano Lett. 2024, 24, 5855–5861. [Google Scholar]
- Balamur, R.; Eren, G.O.; Kaleli, H.N.; Karatum, O.; Kaya, L.; Hasanreisoglu, M.; Nizamoglu, S. A Retina-Inspired Optoelectronic Synapse Using Quantum Dots for Neuromorphic Photostimulation of Neurons. Adv. Sci. 2024, 11, e2401753. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Son, S.E.; Seong, G.H. Functionalized Ultra-Fine Bimetallic PtRu Alloy Nanoparticle with High Peroxidase-Mimicking Activity for Rapid and Sensitive Colorimetric Quantification of C-Reactive Protein. Mikrochim. Acta 2021, 188, 119. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Lin, G.; Stephen, Z.R.; Chung, S.; Wang, H.; Patton, V.K.; Gebhart, R.N.; Zhang, M. In Vivo Serum Enabled Production of Ultrafine Nanotherapeutics for Cancer Treatment. Mater Today 2020, 38, 10–23. [Google Scholar] [CrossRef]
- Thangudu, S.; Yu, C.-C.; Lee, C.-L.; Liao, M.-C.; Su, C.-H. Magnetic, Biocompatible FeCO3 Nanoparticles for T2-Weighted Magnetic Resonance Imaging of In Vivo Lung Tumors. J. Nanobiotechnol. 2022, 20, 157. [Google Scholar] [CrossRef]
- Nafee, N.; Gaber, D.M.; Abouelfetouh, A.; Alseqely, M.; Empting, M.; Schneider, M. Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road? Int. J. Nanomed. 2024, 19, 3861–3890. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial-Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-Associated microRNAs and Their Roles in Cancer Stemness and Drug Resistance. Cancer Commun 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor Microenvironment and Epithelial Mesenchymal Transition as Targets to Overcome Tumor Multidrug Resistance. Drug Resist. Updat. 2020, 53, 100715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, Z.-Y.; Li, S.; Liu, S.; Xiao, C.; Li, Z.; Zhang, B.-X.; Chen, X.-P.; Yang, X. Targeting Transforming Growth Factor-β Signaling for Enhanced Cancer Chemotherapy. Theranostics 2021, 11, 1345–1363. [Google Scholar] [CrossRef]
- Cai, H.-P.; Wang, J.; Xi, S.-Y.; Ni, X.-R.; Chen, Y.-S.; Yu, Y.-J.; Cen, Z.-W.; Yu, Z.-H.; Chen, F.-R.; Guo, C.-C.; et al. Tenascin-Cmediated Vasculogenic Mimicry Formation via Regulation of MMP2/MMP9 in Glioma. Cell Death Dis. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lu, K.V.; Petritsch, C.; Liu, P.; Ganss, R.; Passegué, E.; Song, H.; Vandenberg, S.; Johnson, R.S.; Werb, Z.; et al. HIF1alpha Induces the Recruitment of Bone Marrow-Derived Vascular Modulatory Cells to Regulate Tumor Angiogenesis and Invasion. Cancer Cell 2008, 13, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Lu, Z.; Xu, S.; Li, M.; Wang, X.; Zhang, Z.; He, Q. Self-Delivery Micellar Nanoparticles Prevent Premetastatic Niche Formation by Interfering with the Early Recruitment and Vascular Destruction of Granulocytic Myeloid-Derived Suppressor Cells. Nano Lett. 2020, 20, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive Angiogenesis in Human Metastatic Malignant Melanoma. Am. J. Pathol. 2021, 191, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Bassani, B.; D’Urso, D.G.; Pitaku, I.; Cassinotti, E.; Pelosi, G.; Boni, L.; Dominioni, L.; Noonan, D.M.; Mortara, L.; et al. Angiogenin and the MMP9-TIMP2 Axis Are Up-Regulated in Proangiogenic, Decidual NK-like Cells from Patients with Colorectal Cancer. FASEB J. 2018, 32, 5365–5377. [Google Scholar] [CrossRef]
- Ross, R. Cell Biology of Atherosclerosis. Annu. Rev. Physiol. 1995, 57, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tian, H.; Jin, F.; Zhang, X.; Su, S.; Liu, Y.; Wen, Z.; He, X.; Li, X.; Duan, C. CypD Induced ROS Output Promotes Intracranial Aneurysm Formation and Rupture by 8-OHdG/NLRP3/MMP9 Pathway. Redox Biol. 2023, 67, 102887. [Google Scholar] [CrossRef] [PubMed]
- Meteva, D.; Vinci, R.; Seppelt, C.; Abdelwahed, Y.S.; Pedicino, D.; Nelles, G.; Skurk, C.; Haghikia, A.; Rauch-Kröhnert, U.; Gerhardt, T.; et al. Toll-like Receptor 2, Hyaluronan, and Neutrophils Play a Key Role in Plaque Erosion: The OPTICO-ACS Study. Eur. Heart J. 2023, 44, 3892–3907. [Google Scholar] [CrossRef]
- Tajali, R.; Eidi, A.; Ahmadi Tafti, H.; Pazouki, A.; Sharifi, A.M. Restoring the Angiogenic Capacity of the Human Diabetic Adipose-Derived Mesenchymal Stem Cells Primed with Deferoxamine as a Hypoxia Mimetic Agent: Role of HIF-1α. Adv. Pharm. Bull. 2023, 13, 350–360. [Google Scholar] [CrossRef]
- Venkataraman, L.; Sivaraman, B.; Vaidya, P.; Ramamurthi, A. Nanoparticulate Delivery of Agents for Induced Elastogenesis in Three-Dimensional Collagenous Matrices. J. Tissue Eng. Regen. Med. 2016, 10, 1041–1056. [Google Scholar] [CrossRef]
- Arnold, F.; Muzzio, N.; Patnaik, S.S.; Finol, E.A.; Romero, G. Pentagalloyl Glucose-Laden Poly(Lactide-Co-Glycolide) Nanoparticles for the Biomechanical Extracellular Matrix Stabilization of an In Vitro Abdominal Aortic Aneurysm Model. ACS Appl. Mater. Interfaces 2021, 13, 25771–25782. [Google Scholar] [CrossRef]
- Wang, H.; Keiser, J.A. Vascular Endothelial Growth Factor Upregulates the Expression of Matrix Metalloproteinases in Vascular Smooth Muscle Cells: Role of Flt-1. Circ. Res. 1998, 83, 832–840. [Google Scholar] [CrossRef]
- Gong, T.; Hong, Z.-Y.; Chen, C.-H.; Tsai, C.-Y.; Liao, L.-D.; Kong, K.V. Optical Interference-Free Surface-Enhanced Raman Scattering CO-Nanotags for Logical Multiplex Detection of Vascular Disease-Related Biomarkers. ACS Nano 2017, 11, 3365–3375. [Google Scholar] [CrossRef] [PubMed]
- Islam, Y.; Khalid, A.; Pluchino, S.; Sivakumaran, M.; Teixidò, M.; Leach, A.; Fatokun, A.A.; Downing, J.; Coxon, C.; Ehtezazi, T. Development of Brain Targeting Peptide Based MMP-9 Inhibiting Nanoparticles for the Treatment of Brain Diseases with Elevated MMP-9 Activity. J. Pharm. Sci. 2020, 109, 3134–3144. [Google Scholar] [CrossRef]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Takahashi, H.; Machida, T.; Wakigawa, T.; Harada, E.; Miyaji, H.; Koga, M.; Nishioku, T.; et al. Brain Pericytes among Cells Constituting the Blood-Brain Barrier Are Highly Sensitive to Tumor Necrosis Factor-α, Releasing Matrix Metalloproteinase-9 and Migrating in Vitro. J. Neuroinflamm. 2011, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, R.; Gongora, M.C.; Winkles, J.A.; Yepes, M. Tumor Necrosis Factor-like Weak Inducer of Apoptosis Increases the Permeability of the Neurovascular Unit through Nuclear Factor-Kappa B Pathway Activation. J. Neurosci. 2005, 25, 10094–10100. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin Protects Blood-Brain Barrier Integrity and Permeability by Inhibiting Matrix Metalloproteinase-9 via the NOTCH3/NF-κB Pathway. Aging 2019, 11, 11391–11415. [Google Scholar] [CrossRef]
- Machida, T.; Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Koga, M.; Nakamoto, K.; Yamauchi, A.; Kataoka, Y. Role of Thrombin-PAR1-PKCθ/δ Axis in Brain Pericytes in Thrombin-Induced MMP-9 Production and Blood-Brain Barrier Dysfunction in Vitro. Neuroscience 2017, 350, 146–157. [Google Scholar] [CrossRef]
- Chen, J.-T.; Chen, T.-G.; Chang, Y.-C.; Chen, C.-Y.; Chen, R.-M. Roles of NMDARs in Maintenance of the Mouse Cerebrovascular Endothelial Cell-Constructed Tight Junction Barrier. Toxicology 2016, 339, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-S.; Chen, X.; Li, W.-T.; Shen, J.-G. Targeting RNS/Caveolin-1/MMP Signaling Cascades to Protect against Cerebral Ischemia-Reperfusion Injuries: Potential Application for Drug Discovery. Acta Pharmacol. Sin. 2018, 39, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, D.; Lu, Z.; Man, J.; Zhang, Z.; Fu, X.; Cui, K.; Wang, J. Metformin Protects against Pericyte Apoptosis and Promotes Neurogenesis through Suppressing JNK P38 MAPK Signalling Activation in Ischemia/Reperfusion Injury. Neurosci. Lett. 2022, 783, 136708. [Google Scholar] [CrossRef]
- Pan, P.; Zhao, H.; Zhang, X.; Li, Q.; Qu, J.; Zuo, S.; Yang, F.; Liang, G.; Zhang, J.H.; Liu, X.; et al. Cyclophilin a Signaling Induces Pericyte-Associated Blood-Brain Barrier Disruption after Subarachnoid Hemorrhage. J. Neuroinflamm. 2020, 17, 16. [Google Scholar] [CrossRef]
- Yang, D.; Baumann, J.M.; Sun, Y.-Y.; Tang, M.; Dunn, R.S.; Akeson, A.L.; Kernie, S.G.; Kallapur, S.; Lindquist, D.M.; Huang, E.J.; et al. Overexpression of Vascular Endothelial Growth Factor in the Germinal Matrix Induces Neurovascular Proteases and Intraventricular Hemorrhage. Sci. Transl. Med. 2013, 5, 193ra90. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Munaut, C.; Jost, M.; Noël, A.; Werb, Z.; Foidart, J.-M.; Rakic, J.-M. Matrix Metalloproteinase-9 Contributes to Choroidal Neovascularization. Am. J. Pathol. 2002, 161, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic Degradation of VE-Cadherin Alters the Blood-Retinal Barrier in Diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Santos, J.M.; Zhong, Q. Sirt1, a Negative Regulator of Matrix Metalloproteinase-9 in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5653–5660. [Google Scholar] [CrossRef]
- Mishra, M.; Kowluru, R.A. Role of PARP-1 as a Novel Transcriptional Regulator of MMP-9 in Diabetic Retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1761–1769. [Google Scholar] [CrossRef]
- Kowluru, R.A. Role of Matrix Metalloproteinase-9 in the Development of Diabetic Retinopathy and Its Regulation by H-Ras. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4320–4326. [Google Scholar] [CrossRef]
- Lambert, V.; Wielockx, B.; Munaut, C.; Galopin, C.; Jost, M.; Itoh, T.; Werb, Z.; Baker, A.; Libert, C.; Krell, H.-W.; et al. MMP-2 and MMP-9 Synergize in Promoting Choroidal Neovascularization. FASEB J. 2003, 17, 2290–2292. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Noda, K.; Tagawa, Y.; Inafuku, S.; Dong, Y.; Fukuhara, J.; Dong, Z.; Ando, R.; Kanda, A.; Ishida, S. Genistein Attenuates Choroidal Neovascularization. J. Nutr. Biochem. 2014, 25, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-M.; Kang, J.-H.; Choi, J.-H.; Park, S.J.; Bai, S.; Im, S.-Y. Platelet-Activating Factor Induces Matrix Metalloproteinase-9 Expression through Ca(2+)- or PI3K-Dependent Signaling Pathway in a Human Vascular Endothelial Cell Line. FEBS Lett. 2005, 579, 6451–6458. [Google Scholar]
- Mishra, M.; Flaga, J.; Kowluru, R.A. Molecular Mechanism of Transcriptional Regulation of Matrix Metalloproteinase-9 in Diabetic Retinopathy. J. Cell Physiol. 2016, 231, 1709–1718. [Google Scholar] [CrossRef]
- Hsieh, M.-Y.; Chen, W.-Y.; Jiang, M.-J.; Cheng, B.-C.; Huang, T.-Y.; Chang, M.-S. Interleukin-20 Promotes Angiogenesis in a Direct and Indirect Manner. Genes. Immun. 2006, 7, 234–242. [Google Scholar] [CrossRef] [PubMed]
- De Lucca Camargo, L.; Babelova, A.; Mieth, A.; Weigert, A.; Mooz, J.; Rajalingam, K.; Heide, H.; Wittig, I.; Lopes, L.R.; Brandes, R.P. Endo-PDI Is Required for TNFα-Induced Angiogenesis. Free Radic. Biol. Med. 2013, 65, 1398–1407. [Google Scholar] [CrossRef]
- Miyoshi, A.; Koyama, S.; Sasagawa-Monden, M.; Kadoya, M.; Konishi, K.; Shoji, T.; Inaba, M.; Yamamoto, Y.; Koyama, H. JNK and ATF4 as Two Important Platforms for Tumor Necrosis Factor-α-Stimulated Shedding of Receptor for Advanced Glycation End Products. FASEB J. 2019, 33, 3575–3589. [Google Scholar]
- Yang, R.; Zhang, Y.; Huang, D.; Luo, X.; Zhang, L.; Zhu, X.; Zhang, X.; Liu, Z.; Han, J.-Y.; Xiong, J.-W. Miconazole Protects Blood Vessels from MMP9-Dependent Rupture and Hemorrhage. Dis. Model. Mech. 2017, 10, 337–348. [Google Scholar] [PubMed]
- Freundt, G.V.; von Samson-Himmelstjerna, F.A.; Nitz, J.-T.; Luedde, M.; Waltenberger, J.; Wieland, T.; Frey, N.; Preusch, M.; Hippe, H.-J. The Orphan Receptor GPRC5B Activates Pro-Inflammatory Signaling in the Vascular Wall via Fyn and NFκB. Biochem. Biophys. Res. Commun. 2022, 592, 60–66. [Google Scholar]
- Bou Khzam, L.; Boulahya, R.; Abou-Saleh, H.; Hachem, A.; Zaid, Y.; Merhi, Y. Soluble CD40 Ligand Stimulates the Pro-Angiogenic Function of Peripheral Blood Angiogenic Outgrowth Cells via Increased Release of Matrix Metalloproteinase-9. PLoS ONE 2013, 8, e84289. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Park, I.; Hong, I.-K.; Byun, H.-J.; Choi, J.; Kim, Y.-M.; Lee, H. Fibronectin and Vitronectin Induce AP-1-Mediated Matrix Metalloproteinase-9 Expression through Integrin α(5)β(1)/α(v)β(3)-Dependent Akt, ERK and JNK Signaling Pathways in Human Umbilical Vein Endothelial Cells. Cell Signal 2011, 23, 125–134. [Google Scholar] [CrossRef]
- Bhowmik, T.; Gomes, A. Down-Regulation of Cyclin-Dependent Kinase-4 and MAPK through Estrogen Receptor Mediated Cell Cycle Arrest in Human Breast Cancer Induced by Gold Nanoparticle Tagged Toxin Protein NKCT1. Chem. Biol. Interact. 2017, 268, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Park, I.-C.; Lee, H.-C.; Woo, S.-H.; Lee, J.-Y.; Hong, Y.-J.; Rhee, C.-H.; Lee, Y.-S.; Lee, S.-H.; Shim, B.-S.; et al. Protein Kinase C-Alpha Activation by Phorbol Ester Induces Secretion of Gelatinase B/MMP-9 through ERK 1/2 Pathway in Capillary Endothelial Cells. Int. J. Oncol. 2003, 22, 137–143. [Google Scholar]
- Renault, M.-A.; Roncalli, J.; Tongers, J.; Thorne, T.; Klyachko, E.; Misener, S.; Volpert, O.V.; Mehta, S.; Burg, A.; Luedemann, C.; et al. Sonic Hedgehog Induces Angiogenesis via Rho Kinase-Dependent Signaling in Endothelial Cells. J. Mol. Cell Cardiol. 2010, 49, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Patra, D.; Ghosh, P.; Banerjee, S.; Chowdhury, K.D.; Chakraborty, P.; Basu, A.; Sadhukhan, G.C. Activity of ROCKII Not ROCKI Promotes Pulmonary Metastasis of Melanoma Cells via Modulating Smad2/3-MMP9 and FAK-Src-VEGF Signalling. Cell Signal 2022, 97, 110389. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, A.J.; Mishra, M.; Kowluru, R.A. Crosstalk Between Histone and DNA Methylation in Regulation of Retinal Matrix Metalloproteinase-9 in Diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Olguín, P.; Dang, L.T.; He, D.; Thomas, S.; Chi, L.; Sukonnik, T.; Khyzha, N.; Dobenecker, M.-W.; Fish, J.E.; Bruneau, B.G. Ezh2-Mediated Repression of a Transcriptional Pathway Upstream of Mmp9 Maintains Integrity of the Developing Vasculature. Development 2014, 141, 4610–4617. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, T.; Huang, C.; Xu, Z.; Wang, L.; Jiang, E.; Wang, H.; Chen, Y.; Liu, K.; Shao, Z.; et al. Melanoma Cell-Secreted Exosomal miR-155-5p Induce Proangiogenic Switch of Cancer-Associated Fibroblasts via SOCS1/JAK2/STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2018, 37, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, S.-R.; Arai, K.; Lee, S.-R.; Tsuji, K.; Rebeck, G.W.; Lo, E.H. Lipoprotein Receptor-Mediated Induction of Matrix Metalloproteinase by Tissue Plasminogen Activator. Nat. Med. 2003, 9, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP2A-BP Encoded by LINC00665 Inhibits Triple-Negative Breast Cancer Progression. EMBO J. 2020, 39, e102190. [Google Scholar] [CrossRef]
- Mandel, A.; Larsson, P.; Sarwar, M.; Semenas, J.; Syed Khaja, A.S.; Persson, J.L. The Interplay between AR, EGF Receptor and MMP-9 Signaling Pathways in Invasive Prostate Cancer. Mol. Med. 2018, 24, 34. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Li, P.; Ding, M.; You, S.; Xu, Z.; Ye, J.; Chen, X.; Tan, M.; Wang, J.; et al. ROC1 Promotes the Malignant Progression of Bladder Cancer by Regulating P-IκBα/NF-κB Signaling. J. Exp. Clin. Cancer Res. 2021, 40, 158. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Wang, X.; Zheng, Y.; Jia, H.; Li, H.; Wang, J.; Wang, N.; Xiang, F.; Li, Y. Silencing of KIF3B Suppresses Breast Cancer Progression by Regulating EMT and Wnt/β-Catenin Signaling. Front. Oncol. 2020, 10, 597464. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guan, Z.; Ying, W.-Z.; Xing, D.; Ying, K.E.; Sanders, P.W. Matrix Metalloproteinase-9 Regulates Afferent Arteriolar Remodeling and Function in Hypertension-Induced Kidney Disease. Kidney Int. 2023, 104, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Wågsäter, D.; Zhu, C.; Björck, H.M.; Eriksson, P. Effects of PDGF-C and PDGF-D on Monocyte Migration and MMP-2 and MMP-9 Expression. Atherosclerosis 2009, 202, 415–423. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Miyamoto, T.; Shikata, E.; Yamaguchi, I.; Shimada, K.; Yagi, K.; Tada, Y.; Korai, M.; Kitazato, K.T.; Kanematsu, Y.; et al. Activation of the NLRP3/IL-1β/MMP-9 Pathway and Intracranial Aneurysm Rupture Associated with the Depletion of ERα and Sirt1 in Oophorectomized Rats. J. Neurosurg. 2023, 138, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.; Rakhshan, K.; Nikbakht, F.; Maleki-Ravasan, N.; Tahghighi, A.; Azizi, Y. Cardioprotective Effects of the Aqueous Extract of Echinops Cephalotes on Myocardial Ischemia-Reperfusion in Rats by Modulation of MMP-2, MMP-9, TIMP, and Oxidative Stress. Biomed. Pharmacother. 2024, 176, 116927. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Nayeem, N.; He, Y.; Morales, J.; Graham, D.; Klajn, R.; Contel, M.; O’Brien, S.; Ulijn, R.V. Self-Complementary Zwitterionic Peptides Direct Nanoparticle Assembly and Enable Enzymatic Selection of Endocytic Pathways. Adv. Mater. 2022, 34, e2104962. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, Y.; Yu, Y.; Hu, M.; Yang, C.; Zhang, Z. A Sequentially Responsive and Structure-Transformable Nanoparticle with a Comprehensively Improved “CAPIR Cascade” for Enhanced Antitumor Effect. Nanoscale 2019, 11, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.; Vandooren, J.; Locatelli, E.; Fiten, P.; Opdenakker, G.; Proost, P.; Krüger, A.; Lellouche, J.P.; Israel, L.L.; Shenkman, L.; et al. Matrix Metalloproteinase-9 (MMP-9) as an Activator of Nanosystems for Targeted Drug Delivery in Pancreatic Cancer. J. Control Release 2016, 239, 39–48. [Google Scholar] [CrossRef]
- Han, Q.-J.; Lan, X.-T.; Wen, Y.; Zhang, C.-Z.; Cleary, M.; Sayyed, Y.; Huang, G.; Tuo, X.; Yi, L.; Xi, Z.; et al. Matrix Metalloproteinase-9-Responsive Surface Charge-Reversible Nanocarrier to Enhance Endocytosis as Efficient Targeted Delivery System for Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2021, 10, e2002143. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Haldar, M.K.; Nahire, R.R.; Katti, P.; Ambre, A.H.; Muhonen, W.W.; Shabb, J.B.; Padi, S.K.R.; Singh, R.K.; Borowicz, P.P.; et al. Mmp-9 Responsive PEG Cleavable Nanovesicles for Efficient Delivery of Chemotherapeutics to Pancreatic Cancer. Mol. Pharm. 2014, 11, 2390–2399. [Google Scholar] [CrossRef]
- Hu, A.; Pu, Y.; Xu, N.; Yang, H.; Hu, X.; Sun, R.; Jin, R.; Nie, Y. Hierarchically Decorated Magnetic Nanoparticles Amplify the Oxidative Stress and Promote the Chemodynamic/Magnetic Hyperthermia/Immune Therapy. Acta Biomater. 2024, 173, 457–469. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Huang, Z.; Zuo, T.; Lu, Q.; Wu, G.; Shen, Q. Reducing Interstitial Fluid Pressure and Inhibiting Pulmonary Metastasis of Breast Cancer by Gelatin Modified Cationic Lipid Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 29457–29468. [Google Scholar] [CrossRef] [PubMed]
- Ehrsam, D.; Sieber, S.; Oufir, M.; Porta, F.; Hamburger, M.; Huwyler, J.; Meyer Zu Schwabedissen, H.E. Design, Synthesis, and Characterization of a Paclitaxel Formulation Activated by Extracellular MMP9. Bioconjug Chem. 2020, 31, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shen, N.; Ci, T.; Tang, Z.; Gu, Z.; Li, G.; Chen, X. Combretastatin A4 Nanodrug-Induced MMP9 Amplification Boosts Tumor-Selective Release of Doxorubicin Prodrug. Adv. Mater. 2019, 31, e1904278. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Liu, S.; Lu, Q.; He, J.; Zhou, Z.; Hu, Y. Enzyme Sensitive, Surface Engineered Nanoparticles for Enhanced Delivery of Camptothecin. J. Control Release 2015, 216, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Ehrsam, D.; Lengerke, C.; Meyer Zu Schwabedissen, H.E. Synthesis and Characterization of PDMS-PMOXA-Based Polymersomes Sensitive to MMP-9 for Application in Breast Cancer. Mol. Pharm. 2018, 15, 4884–4897. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-Mediated Release of Chemotherapeutics from Mesoporous Silica Nanoparticles to Ex Vivo Human and Mouse Lung Tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Nossier, A.I.; Mohammed, O.S.; Fakhr El-Deen, R.R.; Zaghloul, A.S.; Eissa, S. Gelatin-Modified Gold Nanoparticles for Direct Detection of Urinary Total Gelatinase Activity: Diagnostic Value in Bladder Cancer. Talanta 2016, 161, 511–519. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor Microenvironment-Activated Cancer Cell Membrane-Liposome Hybrid Nanoparticle-Mediated Synergistic Metabolic Therapy and Chemotherapy for Non-Small Cell Lung Cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Ma, B.; Ma, Y.; Cao, P.; Leng, X.; Huang, P.; Zhao, Y.; Ji, T.; Lu, X.; Liu, L. Doxorubicin and CpG Loaded Liposomal Spherical Nucleic Acid for Enhanced Cancer Treatment. J. Nanobiotechnol. 2022, 20, 140. [Google Scholar] [CrossRef]
- Battistella, C.; Callmann, C.E.; Thompson, M.P.; Yao, S.; Yeldandi, A.V.; Hayashi, T.; Carson, D.A.; Gianneschi, N.C. Delivery of Immunotherapeutic Nanoparticles to Tumors via Enzyme-Directed Assembly. Adv. Healthc. Mater. 2019, 8, e1901105. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, Y.; Liang, X.; Wusiman, Z.; Yin, Y.; Shen, Q. Synergistic Inhibition of Migration and Invasion of Breast Cancer Cells by Dual Docetaxel/Quercetin-Loaded Nanoparticles via Akt/MMP-9 Pathway. Int. J. Pharm. 2017, 523, 300–309. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Z.; Zhang, H.; Kong, H.; Li, X.; Wang, H.; Xie, W. An Efficient Trojan Delivery of Tetrandrine by Poly(N-Vinylpyrrolidone)-Block-Poly(ε-Caprolactone) (PVP-b-PCL) Nanoparticles Shows Enhanced Apoptotic Induction of Lung Cancer Cells and Inhibition of Its Migration and Invasion. Int. J. Nanomed. 2014, 9, 231–242. [Google Scholar]
- Sánchez-Rodríguez, C.; Palao-Suay, R.; Rodrigáñez, L.; Aguilar, M.R.; Martín-Saldaña, S.; San Román, J.; Sanz-Fernández, R. α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma. Biomolecules 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.H.; Osman, H.A.; Teleb, M.; Darwish, A.I.; Abu-Serie, M.M.; Khattab, S.N.; Haiba, N.S. Engineered S-Triazine-Based Dendrimer-Honokiol Conjugates as Targeted MMP-2/9 Inhibitors for Halting Hepatocellular Carcinoma. ChemMedChem 2021, 16, 3701–3719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhong, W.; Ren, X.; Sha, X.; Fang, X. Matrix Metalloproteinases-2/9-Sensitive Peptide-Conjugated Polymer Micelles for Site-Specific Release of Drugs and Enhancing Tumor Accumulation: Preparation and in Vitro and in Vivo Evaluation. Int. J. Nanomed. 2016, 11, 1643–1661. [Google Scholar]
- Stapf, M.; Teichgräber, U.; Hilger, I. Methotrexate-Coupled Nanoparticles and Magnetic Nanochemothermia for the Relapse-Free Treatment of T24 Bladder Tumors. Int. J. Nanomed. 2017, 12, 2793–2811. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, F.; Wu, Z.; Zhang, F.; Niu, K.; Kang, Y.; Liu, X.; Wang, Q.; Wang, Y.; Wang, Q. Inhibition of Cancer Cell Migration with CuS@ mSiO2-PEG Nanoparticles by Repressing MMP-2/MMP-9 Expression. Int. J. Nanomed. 2018, 13, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Lee, Y.-C.; Huang, C.-H.; Chang, L.-S. Gallic Acid-Capped Gold Nanoparticles Inhibit EGF-Induced MMP-9 Expression through Suppression of P300 Stabilization and NFκB/c-Jun Activation in Breast Cancer MDA-MB-231 Cells. Toxicol. Appl. Pharmacol. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Zhang, J.; Zheng, C.; Ding, K.; Xiao, H.; Wang, L.; Zhang, Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles to Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hu, J.; Wang, H.; Wang, C. Gold Nanoparticles Regulate the Antitumor Secretome and Have Potent Cytotoxic Effects against Prostate Cancer Cells. J. Appl. Toxicol. 2021, 41, 1286–1303. [Google Scholar] [CrossRef]
- Kang, S.; Zhou, G.; Yang, P.; Liu, Y.; Sun, B.; Huynh, T.; Meng, H.; Zhao, L.; Xing, G.; Chen, C.; et al. Molecular Mechanism of Pancreatic Tumor Metastasis Inhibition by Gd@C82(OH)22 and Its Implication for de Novo Design of Nanomedicine. Proc. Natl. Acad. Sci. USA 2012, 109, 15431–15436. [Google Scholar] [CrossRef]
- Chen, S.H.; Kang, S.-G.; Luo, J.; Zhou, R. Charging Nanoparticles: Increased Binding of Gd@C82(OH)22 Derivatives to Human MMP-9. Nanoscale 2018, 10, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Valdepérez, D.; Jin, Q.; Yang, B.; Li, Z.; Wu, Y.; Pelaz, B.; Parak, W.J.; Ji, J. Dual Enzymatic Reaction-Assisted Gemcitabine Delivery Systems for Programmed Pancreatic Cancer Therapy. ACS Nano 2017, 11, 1281–1291. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Lei, T.; Na, D.; Nie, M.; Yang, Y.; Xie, C.; He, Z.; Wang, J. Selective Mediation of Ovarian Cancer SKOV3 Cells Death by Pristine Carbon Quantum Dots/Cu2O Composite through Targeting Matrix Metalloproteinases, Angiogenic Cytokines and Cytoskeleton. J. Nanobiotechnol. 2021, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Akers, W.J.; Xu, B.; Lee, H.; Sudlow, G.P.; Fields, G.B.; Achilefu, S.; Edwards, W.B. Detection of MMP-2 and MMP-9 Activity in Vivo with a Triple-Helical Peptide Optical Probe. Bioconjugate Chem. 2012, 23, 656–663. [Google Scholar] [CrossRef]
- Black, K.C.L.; Akers, W.J.; Sudlow, G.; Xu, B.; Laforest, R.; Achilefu, S. Dual-Radiolabeled Nanoparticle SPECT Probes for Bioimaging. Nanoscale 2015, 7, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, A.M.; Kubelick, K.P.; Emelianov, S.Y. Gold Nanoparticles Conjugated with DNA Aptamer for Photoacoustic Detection of Human Matrix Metalloproteinase-9. Photoacoustics 2022, 25, 100307. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and Phenotypic Heterogeneity in Prostate Cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, S.; Xie, J.; Ding, B.; Wang, M.; Zhang, P.; Mi, P.; Wang, C.; Liu, R.; Zhang, T.; et al. Spatial Transcriptomics Reveals Heterogeneity of Macrophages in the Tumor Microenvironment of Granulomatous Slack Skin. J. Pathol. 2023, 261, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Rinaldi, B.; Berrino, L.; Rossi, F.; Galderisi, U.; Cipollaro, M. Novel Potential Targets for Prevention of Arterial Restenosis: Insights from the Pre-Clinical Research. Clin. Sci. 2014, 127, 615–634. [Google Scholar] [CrossRef]
- Francis, D.J.; Parish, C.R.; McGarry, M.; Santiago, F.S.; Lowe, H.C.; Brown, K.J.; Bingley, J.A.; Hayward, I.P.; Cowden, W.B.; Campbell, J.H.; et al. Blockade of Vascular Smooth Muscle Cell Proliferation and Intimal Thickening after Balloon Injury by the Sulfated Oligosaccharide PI-88: Phosphomannopentaose Sulfate Directly Binds FGF-2, Blocks Cellular Signaling, and Inhibits Proliferation. Circ. Res. 2003, 92, e70–e77. [Google Scholar] [CrossRef]
- Meneghini, B.C.; Tavares, E.R.; Guido, M.C.; Tavoni, T.M.; Stefani, H.A.; Kalil-Filho, R.; Maranhão, R.C. Lipid Core Nanoparticles as Vehicle for Docetaxel Reduces Atherosclerotic Lesion, Inflammation, Cell Death and Proliferation in an Atherosclerosis Rabbit Model. Vascul Pharmacol. 2019, 115, 46–54. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Wei, X.; Ma, L.; Sheng, J.; Lu, P. PEGylated Polyethylenimine Derivative-Mediated Local Delivery of the shSmad3 Inhibits Intimal Thickening after Vascular Injury. BioMed Res. Int. 2019, 2019, 8483765. [Google Scholar] [CrossRef]
- Correction to: Endothelial Foxp1 Regulates Neointimal Hyperplasia Via Matrix Metalloproteinase-9/Cyclin Dependent Kinase Inhibitor 1B Signal Pathway. J. Am. Heart Assoc. 2023, 12, e020899. [CrossRef]
- Ramirez-Carracedo, R.; Tesoro, L.; Hernandez, I.; Diez-Mata, J.; Filice, M.; Toro, R.; Rodriguez-Piñero, M.; Zamorano, J.L.; Saura, M.; Zaragoza, C. Non-Invasive Detection of Extracellular Matrix Metalloproteinase Inducer EMMPRIN, a New Therapeutic Target against Atherosclerosis, Inhibited by Endothelial Nitric Oxide. Int. J. Mol. Sci. 2018, 19, 3248. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.-P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef]
- Sarami Foroshani, M.; Sobhani, Z.S.; Mohammadi, M.T.; Aryafar, M. Fullerenol Nanoparticles Decrease Blood-Brain Barrier Interruption and Brain Edema during Cerebral Ischemia-Reperfusion Injury Probably by Reduction of Interleukin-6 and Matrix Metalloproteinase-9 Transcription. J. Stroke Cerebrovasc. Dis. 2018, 27, 3053–3065. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, L.-Y.; Hong, R.; Song, J.-X.; Guo, X.-J.; Zhou, W.; Hu, Z.-L.; Wang, W.; Wang, Y.-L.; Shen, J.-G.; et al. Momordica Charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharmacol. 2022, 13, 908830. [Google Scholar] [CrossRef]
- Miyagawa, T.; Chen, Z.-Y.; Chang, C.-Y.; Chen, K.-H.; Wang, Y.-K.; Liu, G.-S.; Tseng, C.-L. Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization Via Inhibition of VEGF Production. Pharmaceutics 2020, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ma, W.; Shi, L.; Chen, X.; Wu, R.; Zhang, Y.; Chen, H.; Chen, H. Poly(Lactic-Co-Glycolic Acid) Nanoparticle-Mediated Interleukin-12 Delivery for the Treatment of Diabetic Retinopathy. Int. J. Nanomed. 2019, 14, 6357–6369. [Google Scholar]
- Huang, K.; Liu, X.; Lv, Z.; Zhang, D.; Zhou, Y.; Lin, Z.; Guo, J. MMP9-Responsive Graphene Oxide Quantum Dot-Based Nano-in-Micro Drug Delivery System for Combinatorial Therapy of Choroidal Neovascularization. Small 2023, 19, e2207335. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.-G. Adenosine Receptor Signaling: A Key to Opening the Blood-Brain Door. Fluids Barriers CNS 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H. Receptor-Mediated Transcytosis of Macromolecules across the Blood-Brain Barrier. Expert. Opin. Drug Deliv. 2023, 20, 1699–1711. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pan, M.; Fiaz, M.; Hao, Y.; Yan, Y.; Sun, L.; Yan, F. Ultrasound-Mediated Blood-Brain Barrier Opening: An Effective Drug Delivery System for Theranostics of Brain Diseases. Adv. Drug Deliv. Rev. 2022, 190, 114539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Zhao, F.; Jiang, Z.; Cui, Y.; Ou, M.; Mei, L.; Wang, Q. Mitochondrial-Targeted and ROS-Responsive Nanocarrier via Nose-to-Brain Pathway for Ischemic Stroke Treatment. Acta Pharm. Sin. B 2023, 13, 5107–5120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S.; et al. Specific Anti-Glioma Targeted-Delivery Strategy of Engineered Small Extracellular Vesicles Dual-Functionalised by Angiopep-2 and TAT Peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar]
- Moghimi, S.M.; Haroon, H.B.; Yaghmur, A.; Hunter, A.C.; Papini, E.; Farhangrazi, Z.S.; Simberg, D.; Trohopoulos, P.N. Perspectives on Complement and Phagocytic Cell Responses to Nanoparticles: From Fundamentals to Adverse Reactions. J. Control Release 2023, 356, 115–129. [Google Scholar]
- Guo, C.; Yuan, H.; Wang, Y.; Feng, Y.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. The Interplay between PEGylated Nanoparticles and Blood Immune System. Adv. Drug Deliv. Rev. 2023, 200, 115044. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and Immunological Toxic Effects of Nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar]
- Lang, T.; Liu, Y.; Zheng, Z.; Ran, W.; Zhai, Y.; Yin, Q.; Zhang, P.; Li, Y. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31, e1806202. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An Updated Overview on Metal Nanoparticles Toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [PubMed]
- Ren, Q.; Ma, J.; Li, X.; Meng, Q.; Wu, S.; Xie, Y.; Qi, Y.; Liu, S.; Chen, R. Intestinal Toxicity of Metal Nanoparticles: Silver Nanoparticles Disorder the Intestinal Immune Microenvironment. ACS Appl. Mater. Interfaces 2023, 15, 27774–27788. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control Release 2022, 351, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Kim, Y.-H.; Chou, M.; Hwang, J.; Cheon, E.-J.; Lee, H.-J.; Chung, S.-H. Evaluation of the Efficacy and Safety of A Novel 0.05% Cyclosporin A Topical Nanoemulsion in Primary Sjögren’s Syndrome Dry Eye. Ocul. Immunol. Inflamm. 2020, 28, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sanapalli, B.K.R.; Yele, V.; Jupudi, S.; Karri, V.V.S.R. Ligand-Based Pharmacophore Modeling and Molecular Dynamic Simulation Approaches to Identify Putative MMP-9 Inhibitors. RSC Adv. 2021, 11, 26820–26831. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Li, M.; Chen, Y.; Song, S.; Yu, H.; Zhang, P.; Xiao, C.; Lv, G.; Chen, X. Immunosuppressive Enzyme-Responsive Nanoparticles for Enhanced Accumulation in Liver Allograft to Overcome Acute Rejection. Biomaterials 2024, 306, 122476. [Google Scholar] [CrossRef]
- Patrick, P.S.; Stuckey, D.J.; Zhu, H.; Kalber, T.L.; Iftikhar, H.; Southern, P.; Bear, J.C.; Lythgoe, M.F.; Hattersley, S.R.; Pankhurst, Q.A. Improved Tumour Delivery of Iron Oxide Nanoparticles for Magnetic Hyperthermia Therapy of Melanoma via Ultrasound Guidance and 111In SPECT Quantification. Nanoscale 2024, 16, 19715–19729. [Google Scholar] [CrossRef]
- Liu, P.; Hu, Q. Engineering Cells for Cancer Therapy. Acc. Chem. Res. 2024, 57, 2358–2371. [Google Scholar] [CrossRef]

| Classification By Function | Types of Human MMPs | Main Substrate | Main Cell Source |
|---|---|---|---|
| Collagenases | 1 | Collagen, Gelatin | Platelets, Macrophages, Endothelium, Smooth Muscle Cell (SMCs), Fibroblasts |
| 8 | Collagen, Gelatin, Aggrecan | Macrophages, Neutrophils | |
| 13 | Collagen, Gelatin, Fibronectin | SMCs, Macrophages, Varicose Veins, Breast Cancer | |
| Gelatinases | 2 | Platelets, Leukocytes, Endothelium, Vascular Smooth Muscle (VSM), Collagen, Gelatin | Adventitia, Aortic Aneurysm, Varicose Veins |
| 9 | Collagen, Gelatin, Elastin | Microvessels, Macrophages, Neutrophils, Endothelium, VSM, Adventitia, Aortic Aneurysm | |
| Stromelysins | 3 | Extracellular Matrix (ECM), pro-MMP | Endothelium, Intima, VSM, Platelets, Coronary Artery Disease, Synovial Fibroblasts |
| 10 | ECM, pro-MMP | Atherosclerosis, Uterus, Arthritis, Carcinoma Cells | |
| 11 | Insulin-Like Growth Factor Binding Protein, etc. | Brain, Uterus, Angiogenesis | |
| Matrilysins | 26 | ECM, pro-MMP | Endothelium, Intima, VSM, Uterus |
| Membrane-Anchored | 14 | Fibronectin, Laminin, Gelatin | Breast Cancer, Endometrial Tumors |
| 15 | Collagen, pro-MMP-2, pro-MMP-13, Fibronectin | VSM, Fibroblasts, Platelets, Brain, Uterus, Angiogenesis | |
| 16 | pro-MMP-2, Fibronectin | Fibroblasts, Leukocytes | |
| 17 | pro-MMP-2, Fibronectin | Leukocytes, Angiogenesis | |
| 24 | pro-MMP-2 | Brain, Breast Cancer | |
| 25 | pro-MMP-2, Fibronectin | Leukocytes, Lung, Pancreas, Kidney, Brain, Astrocytoma, Glioblastoma | |
| Metalloelastase | 12 | Fibronectin, Tenascin-C | Leukocytes, Anaplastic Astrocytomas, Glioblastomas |
| Enamelysin | 20 | Elastin, Fibronectin, Laminin | SMCs, Fibroblasts, Macrophages |
| Epilysin | 28 | Amelogenin, Dentin Sialophosphoprotein | Tooth Enamel |
| Classification by Material | Applications in Medicine | References |
|---|---|---|
| Metal Nanoparticles | ||
| Gold Nanoparticles | Drug delivery | [24] |
| Cancer treatment | [25] | |
| Diagnostics | [26] | |
| Silver Nanoparticles | Antimicrobial coatings | [27] |
| Wound dressings | [28] | |
| Platinum Nanoparticles | Cancer therapy | [29] |
| Metal Oxide Nanoparticles | ||
| Titanium Dioxide | Photocatalysis | [30] |
| Zinc Oxide | Antibacterial agents | [31] |
| Iron Oxide | Magnetic resonance imaging contrast agents | [32] |
| Drug delivery | [33] | |
| Ceramic Nanoparticles | ||
| Silica Nanoparticles | Drug delivery | [34] |
| Catalysis | [35] | |
| Biosensors | [36] | |
| Alumina Nanoparticles | Coating | [37] |
| Carbon-Based Nanoparticles | ||
| Carbon Nanotubes | Electronics | [38] |
| Conductive materials | [39] | |
| Drug delivery | [40] | |
| Fullerenes | Antioxidants | [41] |
| Drug delivery | [42] | |
| Graphene | Flexible electronics | [43] |
| Sensors | [44] | |
| Polymeric Nanoparticles | ||
| Poly(Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles | Drug delivery | [45] |
| Vaccine delivery | [46] | |
| Cancer therapy | [47] | |
| Polycaprolactone (PCL) Nanoparticles | Drug delivery | [48] |
| Tissue engineering | [49] | |
| Gene delivery | [50] | |
| Polystyrene (PS) Nanoparticles | Diagnostics | [51] |
| Research tools | [52] | |
| Chitosan Nanoparticles | Drug delivery | [53] |
| Gene delivery | [54] | |
| Antimicrobial agents | [55] | |
| Poly(N-Isopropylacrylamide) (PNIPAM) Nanoparticles | Drug delivery | [56] |
| Smart materials | [57] | |
| Poly(Methyl Methacrylate) (PMMA) Nanoparticles | Drug delivery | [58] |
| Bone cement and other base material | [59] | |
| Dendrimers | Drug delivery | [60] |
| Gene delivery | [61] | |
| Imaging | [62] | |
| Polyethylene Glycol (PEG) Nanoparticles | Drug delivery | [63] |
| Protein delivery | [64] | |
| Poly(Alkyl Cyanoacrylate) (PACA) Nanoparticles | Drug delivery | [65] |
| Hydrogel Nanoparticles | Drug delivery | [66] |
| Tissue engineering | [67] | |
| Polypeptide-Based Nanoparticles | Drug delivery | [68] |
| Theranostics | [69] | |
| Classification by Size | Applications in Medicine | References |
|---|---|---|
| Quantum Dots (2–10 nm) | Bioimaging | [70] |
| Quantum computing | [71] | |
| Photovoltaics | [72] | |
| Ultra-Fine Particles (1–100 nm) | Catalysis | [73] |
| Drug delivery | [74] | |
| Imaging | [75] | |
| Fine Particles (100–1000 nm) | Coatings | [76] |
| Molecules | Signaling Pathways and Mechanisms | Regulatory Effects on MMP-9 Expression | References |
|---|---|---|---|
| NF-κB | Binding directly to promoter | Upregulation | |
| Platelet-Activating Factor (PAF) | Ca2+/PI3K, ERK pathways | Upregulation | [112] |
| AP-1 | Binding directly to promoter | Upregulation | |
| Poly ADP Ribosyltransferase-1 (PARP-1) | Formation of transcription complex in promoter | Upregulation | [108] |
| Sirt-1 | Reducing the binding of AP-1 and PARP-1 to promoter | Downregulation | [108,113] |
| IL-20 | JNK, ERK1/2, P38 MAPK pathways | Upregulation | [114] |
| TNF-α | JNK, ERK1/2, AP-1 pathways | Upregulation | [115,116] |
| Miconazole | ERK pathway | Downregulation | [117] |
| Transient Receptor Potential Vanilloid 4 (TRPV-4) | ERK pathway | Downregulation | [10] |
| G-Protein Coupled Receptor 5B (GPRC5B) | ERK1/2, NF-κB pathways | Upregulation | [118] |
| Soluble CD40 Ligands | P38 MAPK pathway | Upregulation | [119] |
| Tenascin-C (TNC) | Akt | Upregulation | [82] |
| Fibronectin | JNK, ERK, PI3K/Akt pathways, AP-1 | Upregulation | [120] |
| Estrogen Receptor | ERK, P38 MAPK, PI3K/Akt pathways, NF-κB | Downregulation | [121] |
| Thrombin Receptor (PAR-1) | PKCθ/Akt, PKCδ/ERK pathways | Upregulation | [99] |
| PKC-A | ERK1/2 pathways | Upregulation | [122] |
| Sonic Hedgehog (Shh) | Rho, ROCK | Upregulation | [123] |
| Smads | ROCKII | Upregulation | [124] |
| Ezh2 | Promoting promoter methylation in retinal endothelial cells | Upregulation | [125] |
| Inhibiting activation in mouse embryos | Downregulation | [126] | |
| miR-155 | SOCS1/JAK2/STAT3 pathway | Upregulation | [127] |
| Tissue Plasminogen Activator (tPA) | Low-density lipoprotein receptor-associated protein (LRP) | Upregulation | [128] |
| MMP-9-Related Signaling Pathways | Corresponding Biological Effects | References |
|---|---|---|
| Tumor progression | ||
| VEGF/VEGFR | Stimulation of angiogenesis and tumor growth | [83] |
| TGF-β | Initially functions as the tumor suppressor, but later promotes metastasis | [129] |
| EGFR | Enhances tumor cell proliferation and survival | [130] |
| MAPK | Essential for cell proliferation, differentiation, and survival | [121] |
| NF-κB | Promotes tumor progression, angiogenesis, and metastasis | [131] |
| PI3K/Akt/mTOR | Critical for cell growth, survival, and metabolism; frequently dysregulated in cancer | [82] |
| Wnt/β-catenin | Influences cell proliferation, differentiation, and stem cell renewal; dysregulation can drive tumor development | [132] |
| Vascular diseases | ||
| TGF-β | Stimulates ECM production, normally restraining vascular smooth muscle cell proliferation; MMP-9 disrupts balance, leading to vascular remodeling and lesion formation | [133] |
| PDGF | Promotes VSMC migration and proliferation, resulting in neointima formation | [134] |
| TNF-α | Induces inflammation and ECM restructuring within the vessel wall | [118] |
| Interleukin | Contributes to the inflammatory response within vascular lesions | [135] |
| Oxidative stress | Triggers ECM degradation, contributing to vascular damage | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, Z. Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases. Pharmaceutics 2025, 17, 479. https://doi.org/10.3390/pharmaceutics17040479
Li X, Xu Z. Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases. Pharmaceutics. 2025; 17(4):479. https://doi.org/10.3390/pharmaceutics17040479
Chicago/Turabian StyleLi, Xuying, and Zhuping Xu. 2025. "Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases" Pharmaceutics 17, no. 4: 479. https://doi.org/10.3390/pharmaceutics17040479
APA StyleLi, X., & Xu, Z. (2025). Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases. Pharmaceutics, 17(4), 479. https://doi.org/10.3390/pharmaceutics17040479








