Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles
Abstract
1. Introduction
2. Advantages and Limitations of Nano-Photosensitizers
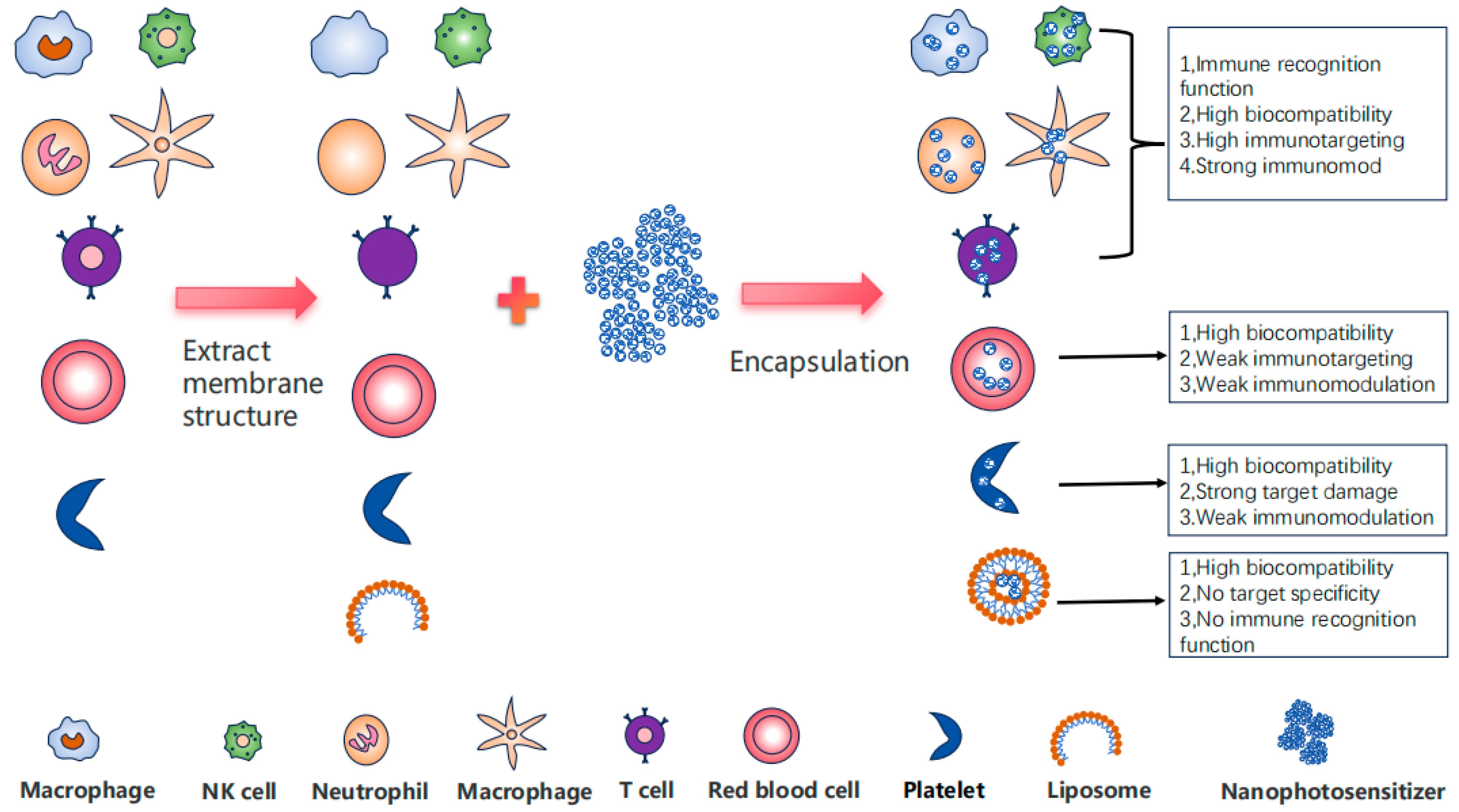
3. The Comprehensive Advantages of Immune Cell Membrane-Embedded Nanomaterials
4. Enhancement of the Stability and Targeting Efficacy of Nano-Photosensitizers via Immune Cell Membranes
4.1. Macrophage Membrane
4.2. Neutrophil Membrane
4.3. Other Immune Cell Membranes
5. The Immune Cell Membrane Endows the Nano-Photosensitizer with Additional Immune Properties
6. Nano-Photosensitizers Embedded in Immune Cell Membranes for Synergistic Therapy Against Tumors
7. Immunocyte Membrane Combined with Photodynamic Therapy for Precise Regulation of Chemotherapy
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. JNCI J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [PubMed]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Raj, J.G.J.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neuro-Oncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase to Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Fan, L.; Jiang, Z.; Xiong, Y.; Xu, Z.; Yang, X.; Gu, D.; Ainiwaer, M.; Li, L.; Liu, J.; Chen, F. Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 17404. [Google Scholar] [CrossRef]
- Modi, S.K.; Mohapatra, P.; Bhatt, P.; Singh, A.; Parmar, A.S.; Roy, A.; Joshi, V.; Singh, M.S. Targeting tumor microenvironment with photodynamic nanomedicine. Med. Res. Rev. 2025, 45, 66–96. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar]
- Gouirand, V.; Guillaumond, F.; Vasseur, S. Influence of the Tumor Microenvironment on Cancer Cells Metabolic Reprogramming. Front. Oncol. 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Feng, L.; Chao, Y.; Yi, X.; Dong, Z.; Yang, K.; Tan, W.; Liu, Z.; Chen, M. G-Quadruplex-Based Nanoscale Coordination Polymers to Modulate Tumor Hypoxia and Achieve Nuclear-Targeted Drug Delivery for Enhanced Photodynamic Therapy. Nano Lett. 2018, 18, 6867–6875. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Li, Z.; Lai, X.; Fu, S.; Ren, L.; Cai, H.; Zhang, H.; Gu, Z.; Ma, X.; Luo, K. Immunogenic Cell Death Activates the Tumor Immune Microenvironment to Boost the Immunotherapy Efficiency. Adv. Sci. 2022, 9, e2201734. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Li, X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori J. 2018, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Diederich, M. Stress-induced cellular responses in immunogenic cell death: Implications for cancer immunotherapy. Biochem. Pharmacol. 2018, 153, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Fucikova, J.; Becht, E.; Iribarren, K.; Goc, J.; Remark, R.; Damotte, D.; Alifano, M.; Devi, P.; Biton, J.; Germain, C.; et al. Calreticulin Expression in Human Non-Small Cell Lung Cancers Correlates with Increased Accumulation of Antitumor Immune Cells and Favorable Prognosis. Cancer Res. 2016, 76, 1746–1756. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Vandecasteele, K.; Bachert, C.; Krysko, O.; Krysko, D.V. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv. Exp. Med. Biol. 2016, 930, 133–149. [Google Scholar]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Chen, W.R.; Wang, X. DC vaccine generated by ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma. OncoImmunology 2016, 5, e1072674. [Google Scholar] [CrossRef]
- Rothe, F.; Patties, I.; Kortmann, R.-D.; Glasow, A. Immunomodulatory Effects by Photodynamic Treatment of Glioblastoma Cells In Vitro. Molecules 2022, 27, 3384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Li, F.; Sheng, J.; Xu, C.; Li, D.; Yu, H.; Liu, W. Combination of Chemotherapy and Photodynamic Therapy with Oxygen Self-Supply in the Form of Mutual Assistance for Cancer Therapy. Int. J. Nanomed. 2021, 16, 3679–3694. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Sun, J.; Gong, H.; Cao, Y.; Duan, L.; Yi, S.; Ying, B.; Xiao, B. Near-Infrared-Enpowered Nanomotor-Mediated Targeted Chemotherapy and Mitochondrial Phototherapy to Boost Systematic Antitumor Immunity. Adv. Health Mater. 2022, 11, e2200255. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.-M.; Li, Y.-H.; Cai, S.-J.; Yin, X.-B.; He, X.-W.; Zhang, Y.-K. Fluorescent Imaging-Guided Chemotherapy-and-Photodynamic Dual Therapy with Nanoscale Porphyrin Metal-Organic Framework. Small 2017, 13, 1603459. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, F.; Chen, B.; Tang, H.; Zeng, X.; Cai, D.; Zhu, M.; Long, R.; Yang, D.; Kankala, R.K.; et al. Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2020, 189, 110842. [Google Scholar] [CrossRef]
- Ye, S.; Wang, F.; Fan, Z.; Zhu, Q.; Tian, H.; Zhang, Y.; Jiang, B.; Hou, Z.; Li, Y.; Su, G. Light/pH-Triggered Biomimetic Red Blood Cell Membranes Camouflaged Small Molecular Drug Assemblies for Imaging-Guided Combinational Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 15262–15275. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, C.; Peng, Y.; Hu, Z.; Zheng, S.; Chen, W.; Wen, L. Biomimetic lipid nanoparticles for homologous-targeting and enhanced photodynamic therapy against glioma. Eur. J. Pharm. Sci. 2023, 190, 106574. [Google Scholar] [CrossRef]
- Dai, J.; Wu, M.; Wang, Q.; Ding, S.; Dong, X.; Xue, L.; Zhu, Q.; Zhou, J.; Xia, F.; Wang, S.; et al. Red blood cell membrane-camouflaged nanoparticles loaded with AIEgen and Poly(I:C) for enhanced tumoral photodynamic-immunotherapy. Natl. Sci. Rev. 2021, 8, nwab039. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Yang, Z.; Guan, L.; Wang, Z.; Liu, A.; Yang, B.; Tang, L.; Lin, Q. Cancer cell membrane biomimetic nanosystem for homologous targeted dual-mode imaging and combined therapy. J. Colloid Interface Sci. 2023, 652, 770–779. [Google Scholar] [CrossRef]
- Jin, F.; Qi, J.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Xu, X.; Ying, X.; Ji, J.; et al. Cancer-cell-biomimetic Upconversion nanoparticles combining chemo-photodynamic therapy and CD73 blockade for metastatic triple-negative breast cancer. J. Control. Release 2021, 337, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Xu, J. Recent Advances in Organic Molecular-to-Supramolecular Self-Assembled Room-Temperature Phosphorescent Materials for Biomedical Applications. ACS Appl. Bio Mater. 2023, 6, 4572–4585. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, H.; Jiang, P.; Zhang, K.Y.; Liu, S.; Yang, T.; Zhao, Q.; Yang, L.; Lv, W.; Yu, Q.; et al. Multifunctional Phosphorescent Conjugated Polymer Dots for Hypoxia Imaging and Photodynamic Therapy of Cancer Cells. Adv. Sci. 2016, 3, 201500155. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale metal–organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.; Yuan, S.; Zhou, H. Photochromic metal–organic frameworks: Reversible control of singlet oxygen generation. Angew. Chem. Int. Ed. 2015, 54, 430–435. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal–Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.-Y.; Li, L.; Li, Z.; Fu, S.; Xu, Y.; Zheng, B.-Y.; Ke, M.; Li, X.; Huang, J.-D. A Sulfur-Bridging Sulfonate-Modified Zinc(II) Phthalocyanine Nanoliposome Possessing Hybrid Type I and Type II Photoreactions with Efficient Photodynamic Anticancer Effects. Molecules 2023, 28, 2250. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [PubMed]
- Sun, L.; Zhao, Y.; Peng, H.; Zhou, J.; Zhang, Q.; Yan, J.; Liu, Y.; Guo, S.; Wu, X.; Li, B. Carbon dots as a novel photosensitizer for photodynamic therapy of cancer and bacterial infectious diseases: Recent advances. J. Nanobiotechnol. 2024, 22, 210. [Google Scholar] [CrossRef]
- Wen, Y.; Jia, Q.; Nan, F.; Zheng, X.; Liu, W.; Wu, J.; Ren, H.; Ge, J.; Wang, P. Pheophytin Derived Near-Infrared-Light Responsive Carbon Dot Assembly as a New Phototheranotic Agent for Bioimaging and Photodynamic Therapy. Chem. Asian J. 2019, 14, 2162–2168. [Google Scholar] [CrossRef]
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic therapy of melanoma skin cancer using carbon dot—Chlorin e6—Hyaluronate conjugate. Acta Biomater. 2015, 26, 295–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; Nan, F.; Wang, J.; Liang, K.; Li, J.; Xue, X.; Ren, H.; Liu, W.; Ge, J.; et al. Carbon dots nanophotosensitizers with tunable reactive oxygen species generation for mitochondrion-targeted type I/II photodynamic therapy. Biomaterials 2023, 293, 121953. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Idoudi, S.; Fernandes, Q.; Al-Zaidan, L.; Uddin, S.; Dermime, S.; Billa, N.; Merhi, M. Nano-vitamin C: A promising candidate for therapeutic applications. Biomed. Pharmacother. 2023, 158, 114093. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, S.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding The Benefits and Risks of Sustainable Nanomaterials in a Research Environment. Chimia 2024, 78, 397–402. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, 2006484. [Google Scholar] [CrossRef]
- Liang, S.; Wang, M.; Wang, J.; Chen, G. Red-Blood-Cell-Membrane-Coated Metal-Drug Nanoparticles for Enhanced Chemotherapy. ChemBioChem 2021, 22, 3184–3189. [Google Scholar]
- Meng, D.; Yang, S.; Yang, Y.; Zhang, L.; Cui, L. Synergistic chemotherapy and phototherapy based on red blood cell biomimetic nanomaterials. J. Control. Release 2022, 352, 146–162. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef]
- Fang, M.; Liu, R.; Fang, Y.; Zhang, D.; Kong, B. Emerging platelet-based drug delivery systems. Biomed. Pharmacother. 2024, 177, 117131. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, Q.; Jiang, C.; Gu, Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 2019, 58, 81–91. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Zhou, H.; Wang, W.; Zhang, C.; Zhang, L.; Qu, Y.; Li, W.; Liu, X.; Zhao, M.; et al. Decoy Nanozymes Enable Multitarget Blockade of Proinflammatory Cascades for the Treatment of Multi-Drug-Resistant Bacterial Sepsis. Research 2022, 2022, 9767643. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Q.; Shi, M.; Yin, C.; Zhao, Z.; Shen, K.; Qiu, Y.; Xiao, Y.; Zhao, Y.; Yang, X.; et al. Fe3O4@TiO2-Laden Neutrophils Activate Innate Immunity via Photosensitive Reactive Oxygen Species Release. Nano Lett. 2020, 20, 261–271. [Google Scholar]
- Park, K.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef]
- Svennerholm, K.; Park, K.-S.; Wikström, J.; Lässer, C.; Crescitelli, R.; Shelke, G.V.; Jang, S.C.; Suzuki, S.; Bandeira, E.; Olofsson, C.S.; et al. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci. Rep. 2017, 7, 17434. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, Y.; Zou, K.; Lan, Z.; Cheng, G.; Mai, Q.; Cui, H.; Meng, Q.; Chen, T.; Rao, L.; et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact. Mater. 2023, 19, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-D.; Xiao, M.-T. Red blood cell membrane nanoparticles for tumor phototherapy. Colloids Surf. B Biointerfaces 2022, 220, 112895. [Google Scholar] [CrossRef]
- Peng, T.; Yao, J. Development and application of bionic systems consisting of tumor-cell membranes. J. Zhejiang Univ. Sci. B 2022, 23, 770–777. [Google Scholar] [CrossRef]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Sun, M.; Wang, J.; Wang, C.; Sun, Y. Cell Membrane-Camouflaged Nanocarriers for Cancer Diagnostic and Therapeutic. Front. Pharmacol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Cao, Z.; Li, P.; Li, Y.; Zhang, M.; Hao, M.; Li, W.; Mao, X.; Mo, L.; Yang, C.; Ding, X.; et al. Encapsulation of Nano-Bortezomib in Apoptotic Stem Cell-Derived Vesicles for the Treatment of Multiple Myeloma. Small 2023, 19, e2301748. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Yoo, H.; Park, S.; Kim, J.; Yum, K.; Kim, K.; Kim, H. Overcoming anticancer resistance by photodynamic therapy-related efflux pump deactivation and ultrasound-mediated improved drug delivery efficiency. Nano Converg. 2020, 7, 30. [Google Scholar] [CrossRef]
- Pang, E.; Xing, X.; Zhao, S.; Tan, Q.; Pan, T.; Yu, T.; Gan, Y.; Wang, B.; Tan, S.; Zhang, Y.; et al. Lysosome- and plasma membrane-accumulative and tumor-targetable polythiophene nanoparticles for enhanced sonodynamic therapy. J. Mater. Chem. B 2023, 11, 6123–6130. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mousavi, S.M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Encapsulation of Berberis vulgaris Anthocyanins into Nanoliposome Composed of Rapeseed Lecithin: A Comprehensive Study on Physicochemical Characteristics and Biocompatibility. Foods 2021, 10, 492. [Google Scholar] [CrossRef]
- Agrawal, Y.; Patel, V. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Schnell, U.; Cirulli, V.; Giepmans, B.N. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Han, Y.; Pan, H.; Li, W.; Chen, Z.; Ma, A.; Yin, T.; Liang, R.; Chen, F.; Ma, Y.; Jin, Y.; et al. T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy. Adv. Sci. 2019, 6, 1900251. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, J.; Hu, X.; Koh, K.; Chen, H. Current methods and emerging approaches for detection of programmed death ligand 1. Biosens. Bioelectron. 2022, 208, 114179. [Google Scholar] [CrossRef]
- Neupane, K.R.; Ramon, G.S.; Harvey, B.; Chun, B.; Aryal, S.P.; Masud, A.A.; McCorkle, J.R.; Kolesar, J.M.; Kekenes-Huskey, P.M.; Richards, C.I. Programming Cell-Derived Vesicles with Enhanced Immunomodulatory Properties. Adv. Health Mater. 2023, 12, e2301163. [Google Scholar] [CrossRef]
- Tian, J.-W.; Zhang, H.-J.; Li, S.-Y.; Guo, Y.-L.; Chen, G.; Yu, Z.-L. Tumor Cell-derived Extracellular Vesicles in Modulating Phenotypes and Immune Functions of Macrophages: Mechanisms and Therapeutic Applications. J. Cancer 2023, 14, 1321–1334. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Dastgheyb, R.M.; Li, Z. Tumor-derived extracellular vesicles modulate innate immune responses to affect tumor progression. Front. Immunol. 2022, 13, 1045624. [Google Scholar] [CrossRef]
- Hu, M.; Deng, F.; Song, X.; Zhao, H.; Yan, F. The crosstalk between immune cells and tumor pyroptosis: Advancing cancer immunotherapy strategies. J. Exp. Clin. Cancer Res. 2024, 43, 190. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Wang, C.; Wu, S. Research update on cell membrane camouflaged nanoparticles for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 944518. [Google Scholar] [CrossRef]
- Madsen, S.J.; Christie, C.; Hong, S.J.; Trinidad, A.; Peng, Q.; Uzal, F.A.; Hirschberg, H. Nanoparticle-loaded macrophage-mediated photothermal therapy: Potential for glioma treatment. Lasers Med. Sci. 2015, 30, 1357–1365. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Gao, M.; Yang, T.; Qin, W.; Wang, Q.; Huang, M.; Peng, H.; Shao, M.; Yao, W.; Yi, X.; Sun, G.; et al. Cell Membrane-Anchoring Nano-Photosensitizer for Light-Controlled Calcium-Overload and Tumor-Specific Synergistic Therapy. Small 2022, 18, e2204689. [Google Scholar]
- Xie, F.; Liu, Z.; Wang, P.; Cai, M.; Li, Y.; Yan, J.; Lin, Q.; Luo, F. Self-Delivering Nanodrugs Developed via Small-Molecule-Directed Assembly and Macrophage Cloaking for Sonodynamic-Augmented Immunotherapy. Adv. Healthc. Mater. 2022, 11, e2102770. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Hu, W.; Wang, Y.; Chou, T.; Zhang, Q.; Zhang, B.; Yu, Z.; Yang, Y.; Ren, L.; et al. Camouflaged Gold Nanodendrites Enable Synergistic Photodynamic Therapy and NIR Biowindow II Photothermal Therapy and Multimodal Imaging. ACS Appl. Mater. Interfaces 2021, 13, 10778–10795. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lei, T.; Wang, Y.; Cao, J.; Yang, X.; Qin, L.; Liu, R.; Zhou, Y.; Tong, F.; Umeshappa, C.S.; et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 2020, 255, 120159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, W.; Qiao, L.; Gao, M.; Zhou, M.; Zhang, H.; Sun, Q.; Yao, W.; Yang, T.; Ren, X.; et al. Biomimetic Nanophotosensitizer Amplifies Immunogenic Pyroptosis and Triggers Synergistic Cancer Therapy. Adv. Healthc. Mater. 2023, 12, e2301641. [Google Scholar] [CrossRef]
- Chen, C.; Song, M.; Du, Y.; Yu, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, F. Tumor-Associated-Macrophage-Membrane-Coated Nanoparticles for Improved Photodynamic Immunotherapy. Nano Lett. 2021, 21, 5522–5531. [Google Scholar] [PubMed]
- Meng, Z.; Wang, T.; Hu, Y.; Ouyang, H.; Wang, Q.; Wu, M.; Zhou, J.; Lou, X.; Wang, S.; Dai, J.; et al. Macrophage Membrane-Camouflaged Aggregation-Induced Emission Nanoparticles Enhance Photodynamic-Immunotherapy to Delay Postoperative Tumor Recurrence. Adv. Health Mater. 2023, 13, e2302156. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Zhang, J.; Zheng, C.; Ding, K.; Xiao, H.; Wang, L.; Zhang, Z. C–C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles To Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Yang, S.; Lai, X.; Liu, H.; Gao, Y.; Feng, H.; Zhu, M.; Yuan, Y.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef]
- Yu, Y.; Song, M.; Chen, C.; Du, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, S. Bortezomib-Encapsulated CuS/Carbon Dot Nanocomposites for Enhanced Photothermal Therapy via Stabilization of Polyubiquitinated Substrates in the Proteasomal Degradation Pathway. ACS Nano 2020, 14, 10688–10703. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Xu, X.; Zhang, W.; He, Z. Enhanced breast cancer treatment using phototherapy and RNS therapy with macrophage membrane-coated liposomes. Colloids Surf. B Biointerfaces 2024, 239, 113961. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Z.; Mu, Q.; Yu, Z.; Jacobson, O.; Li, L.; Yang, W.; Huang, C.; Kang, F.; Fan, W.; et al. Targeting Neutrophils for Enhanced Cancer Theranostics. Adv. Mater. 2020, 32, e2002739. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018, 9, 4777. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef] [PubMed]
- Selezneva, A.; Gibb, A.J.; Willis, D. The contribution of ion channels to shaping macrophage behaviour. Front. Pharmacol. 2022, 13, 970234. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Behrens, L.M.; van Egmond, M.; Berg, T.K.v.D. Neutrophils as immune effector cells in antibody therapy in cancer. Immunol. Rev. 2022, 314, 280–301. [Google Scholar] [CrossRef]
- Pereira-Veiga, T.; Schneegans, S.; Pantel, K.; Wikman, H. Circulating tumor cell-blood cell crosstalk: Biology and clinical relevance. Cell Rep. 2022, 40, 111298. [Google Scholar] [CrossRef]
- Sionov, R.V.; Lamagna, C.; Granot, Z. Recognition of Tumor Nidogen-1 by Neutrophil C-Type Lectin Receptors. Biomedicines 2022, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Fainsod-Levi, T.; Zelter, T.; Polyansky, L.; Pham, C.T.; Granot, Z. Neutrophil Cathepsin G and Tumor Cell RAGE Facilitate Neutrophil Anti-Tumor Cytotoxicity. OncoImmunology 2019, 8, e1624129. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, Y.; Du, R.; Hu, D.; Xie, J.; Zhang, J.; Deng, G.; Liang, N.; Tian, T.; Käsmann, L.; et al. Radiotherapy programs neutrophils to an antitumor phenotype by inducing mesenchymal-epithelial transition. Transl. Lung Cancer Res. 2021, 10, 1424–1443. [Google Scholar] [CrossRef]
- Fan, D.; Wang, S.; Huang, R.; Liu, X.; He, H.; Zhang, G. Light-Assisted “Nano-Neutrophils” with High Drug Loading for Targeted Cancer Therapy. Int. J. Nanomed. 2023, 18, 6487–6502. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Q.; Li, C.; Zeng, X.; Zhang, X.-Z. Near infrared light-triggered metal ion and photodynamic therapy based on AgNPs/porphyrinic MOFs for tumors and pathogens elimination. Biomaterials 2020, 248, 120029. [Google Scholar] [CrossRef]
- Qin, W.; Qiao, L.; Wang, Q.; Gao, M.; Zhou, M.; Sun, Q.; Zhang, H.; Yang, T.; Shan, G.; Yao, W.; et al. Advancing Precision: A Controllable Self-Synergistic Nanoplatform Initiating Pyroptosis-Based Immunogenic Cell Death Cascade for Targeted Tumor Therapy. ACS Nano 2024, 18, 1582–1598. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Hu, G.; Wu, X.; Nie, Y.; Wu, H.; Kong, D.; Ning, X. Multistage targeted “Photoactive neutrophil” for enhancing synergistic photo-chemotherapy. Biomaterials 2021, 279, 121224. [Google Scholar] [CrossRef]
- Kyrysyuk, O.; Wucherpfennig, K.W. Designing Cancer Immunotherapies That Engage T Cells and NK Cells. Annu. Rev. Immunol. 2023, 41, 17–38. [Google Scholar] [CrossRef]
- Wang, W.; Wu, F.; Mohammadniaei, M.; Zhang, M.; Li, Y.; Sun, Y.; Tang, B.Z. Genetically edited T-cell membrane coated AIEgen nanoparticles effectively prevents glioblastoma recurrence. Biomaterials 2023, 293, 121981. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, D.; Li, J.; Chen, X.; Xie, W.; Jiang, X.; Wu, L.; Wang, G.; Xiao, Y.; Liu, Z.; et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 2020, 10, 1281–1295. [Google Scholar] [CrossRef]
- Shi, C.; Jian, C.; Wang, L.; Gao, C.; Yang, T.; Fu, Z.; Wu, T. Dendritic cell hybrid nanovaccine for mild heat inspired cancer immunotherapy. J. Nanobiotechnol. 2023, 21, 347. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar]
- Lan, Z.; Liu, W.-J.; Yin, W.-W.; Yang, S.-R.; Cui, H.; Zou, K.-L.; Cheng, G.-W.; Chen, H.; Han, Y.-H.; Rao, L.; et al. Biomimetic MDSCs membrane coated black phosphorus nanosheets system for photothermal therapy/photodynamic therapy synergized chemotherapy of cancer. J. Nanobiotechnol. 2024, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Mo, F.; Chen-Mayfield, T.-J.; Saini, A.; LaMere, A.M.; Hu, Q. Chemically engineering cells for precision medicine. Chem. Soc. Rev. 2023, 52, 1068–1102. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Li, Z.; Peng, Y.; Tan, H.; Wang, C.; Huang, G.; Li, W.; Ma, G.; Wei, W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct. Target. Ther. 2022, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Le, X.T.; Kim, J.; Lee, H.; Nguyen, N.T.; Lee, W.T.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Macrophage-reprogramming upconverting nanoparticles for enhanced TAM-mediated antitumor therapy of hypoxic breast cancer. J. Control. Release 2023, 360, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, J.; Li, Y.; Lin, X.; Chu, Y.; Wang, W.; Huang, S.; Li, W.; Peng, J.; Liu, C.; et al. Aggregation-Induced-Emission Photosensitizer-Loaded Nano-Superartificial Dendritic Cells with Directly Presenting Tumor Antigens and Reversed Immunosuppression for Photodynamically Boosted Immunotherapy. Adv. Mater. 2023, 35, e2208555. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zou, M.; Liu, T.; Zeng, J.; Li, X.; Yu, W.; Li, C.; Ye, J.; Song, W.; Feng, J.; et al. Expandable Immunotherapeutic Nanoplatforms Engineered from Cytomembranes of Hybrid Cells Derived from Cancer and Dendritic Cells. Adv. Mater. 2019, 31, e1900499. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, Y.; Tang, Q.; Lin, J.; Huang, P. Biomimetic Nanoemulsion for Synergistic Photodynamic-Immunotherapy Against Hypoxic Breast Tumor. Angew. Chem. Int. Ed. 2021, 60, 10647–10653. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Brandstadter, J.D.; Yang, Y. Natural killer cell responses to viral infection. J. Innate Immun. 2011, 3, 274–279. [Google Scholar] [CrossRef]
- Arnon, T.I.; Markel, G.; Mandelboim, O. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 2006, 16, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Wehner, R.; Dietze, K.; Bachmann, M.; Schmitz, M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J. Innate Immun. 2011, 3, 258–263. [Google Scholar] [CrossRef]
- Tarazona, R.; Lopez-Sejas, N.; Guerrero, B.; Hassouneh, F.; Valhondo, I.; Pera, A.; Sanchez-Correa, B.; Pastor, N.; Duran, E.; Alonso, C.; et al. Current progress in NK cell biology and NK cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2020, 69, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lu, G.; Nie, W.; Huang, L.; Zhang, Y.; Fan, W.; Wu, G.; Liu, H.; Xie, H. Self-Activatable Photo-Extracellular Vesicle for Synergistic Trimodal Anticancer Therapy. Adv. Mater. 2021, 33, e2005562. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, S.; Zhang, M.; Lin, X.; Jin, X.; Zhang, M.; Liu, Y.; Wang, Y.; Shi, K. A Trojan Horse Delivery Vehicle Carrying siRNA Nanotherapeutics with Multiple Tumor Microenvironment Responsiveness Elicits Robust Antitumor Immune Responses In Situ via a “Self-Synergistic” Approach. Adv. Healthc. Mater. 2023, 12, e2301401. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Deng, T.; Zhu, Q.; Wang, J.; Shi, W.; Liu, Q.; Yu, Q.; Deng, W.; Yu, J.; Wang, Q.; et al. Photothermal Therapy Mediated Hybrid Membrane Derived Nano-formulation for Enhanced Cancer Therapy. AAPS PharmSciTech 2023, 24, 146. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Cao, Y.; Wang, Y.; Wang, F.; Zhang, F.; Zheng, S. Biodegradable Hypocrellin B nanoparticles coated with neutrophil membranes for hepatocellular carcinoma photodynamics therapy effectively via JUNB/ROS signaling. Int. Immunopharmacol. 2021, 99, 107624. [Google Scholar] [CrossRef]
- Xu, X.; Deng, G.; Sun, Z.; Luo, Y.; Liu, J.; Yu, X.; Zhao, Y.; Gong, P.; Liu, G.; Zhang, P.; et al. A Biomimetic Aggregation-Induced Emission Photosensitizer with Antigen-Presenting and Hitchhiking Function for Lipid Droplet Targeted Photodynamic Immunotherapy. Adv. Mater. 2021, 33, 2102322. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, J. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 16114–16121. [Google Scholar]
- Gao, G.; Jiang, Y.W.; Sun, W.; Guo, Y.; Jia, H.R.; Yu, X.W.; Pan, G.Y.; Wu, F.G. Molecular Targeting-Mediated Mild-Temperature Photothermal Therapy with a Smart Albumin-Based Nanodrug. Small 2019, 15, e1900501. [Google Scholar]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 1703588. [Google Scholar] [CrossRef] [PubMed]




| Investigators | Nanoplatform | Average Particle Size (nm) (±S.D.) | Zeta Potential (mV) (±S.D.) | PDI | Photosensitizer | Membrane Structure | Other Drugs | Synergistic Treatment Modalities | Target Disease |
|---|---|---|---|---|---|---|---|---|---|
| Gao et al. [99] | CMA nPS | ~200 | ~15 | 0.262 | DBCO-TAPP | azide-modified macrophage cell membrane with a VSV-G-modified NIH3T3 cell membrane | -- | PDT + Ca2+ overload | Pulmonary carcinoma |
| Xie et al. [100] | HB-NLG8189@MPCM | 232.46 ± (6.52) | −26.96 ± (4.02) | -- | chlorine6-C15-ethyl ester (HB) | Macrophage membrane | indoleamine-(2,3)-dioxygenase (IDO) pathway inhibitor | SDT + immunotherapy | Triple-Negative Breast Cancer |
| Sun et al. [101] | AuND-TPP-ICG@MCM | 135.1 ± (3.0) | −3.3 ± (0.1) | -- | indocyanine green | macrophage cell membrane | multifunctional gold nanodendrite; triphenylphosphonium | PDT + PTT | Breast cancer |
| Hu et al. [102] | (C/I) BP@B-A (D) &M1m | 117.0 ± (7.5) | -- | -- | Chlorin e6 | M1 macrophage cell membranes | Doxorubicin; indoleamine 2,3-dioxygenase 1 inhibitor | PDT + Chemotherapy + ICD | Breast cancer; Cutaneous melanoma |
| Wang et al. [103] | M-Cu-T | 172 ± (1) | −11 ± (1) | -- | meso-tetra (4-aminophenyl) porphyrin | M1-Raw264.7 cell membranes | Cu2+ | PDT + immunotherapy | Colon cancer; Lung cancer |
| Chen et al. [104] | NPR@TAMM | 91 ± (4) | −20 | -- | NaYF4:Yb | tumor-associated macrophage membrane | Rose Bengal (NPR) | PDT + ICD | Breast cancer |
| Meng et al. [105] | M@PFC | 200 | ~−25 | -- | PF3-PPh3 | Macrophage membrane | immune adjuvant (CpG) | Immunotherapy + PDT | Breast cancer |
| Zhao et al. [106] | M@BS-QE NP | 155.3 | −19.1 | 0.312 | bismuth selenide nano-Particles | Macrophage membrane | quercetin | PTT + Chemotherapy | Breast cancer |
| Liu et al. [107] | nano-Pt/VP@MLipo | 140 | −16.7 | -- | verteporfin | RAW264.7 macrophage brane | Platinum Nanoparticles | Chemophototherapy | Breast cancer |
| Yu et al. [108] | CuSCDB @ MMT 7 | 222.5 ± 20.1 | −18± 1.8 | -- | CuSCDs | macrophage membrane hybridized with T7 peptide | Bortezomib | PTT + Chemotherapy | Breast cancer |
| Wang et al. [109] | IR 780-NO-PFH-Lip@M | -- | -- | -- | near-infrared fluorescent dye(IR780) | macrophage cell membranes | Diazeniumdiolate; perfluorocarbon | reactive nitrogen species therapy + PTT/PDT | Breast cancer |
| Fan et al. [121] | NMPC-NPs | 165 | −12.6 | -- | Chlorin e6 | neutrophil membrane | Paclitaxel (PTX) dimeric prodrug | PDT + Chemotherapy | Breast cancer |
| Zhang et al. [122] | PAM | 220 | −15 | -- | porphyrinic porous coordination network | neutrophil membrane | silver nanoparticles | PDT + metal ions therapy | Colon cancer |
| Qin et al. [123] | I-L@NM | 61 ± (6) | −10 | -- | indocyanine green | neutrophil membrane | β-Lapachone; | PTT + Chemotherapy | Colon cancer |
| Xu et al. [124] | PAN | 91.25 ± (0.34) | −40.21 ± (3.12) | -- | Chlorin e6 | neutrophil membrane | cationic RGD-apoptotic peptide conjugate | PDT + Chemotherapy | Squamous cell carcinoma of skin; Tongue squamous cell carcinoma |
| Wang et al. [126] | CM@AIE NPs | 107 | 7 | -- | AIE-gens | genetically engineered CAR T-cell membrane | -- | PTT | glioblastoma |
| Ma et al. [127] | CIM | 110 | −6.7 | 0.288 | near-infrared fluorescent dye (IR780) | GPC3 targeting CAR-T-cellmembranes | mesoporous silica nanoparticles | PTT | Hepatocellular carcinoma |
| Shi et al. [128] | LDC@ZnP NPs | 30 | −10 | -- | Melanin | dendritic cell membrane | Adpgk, zinc phosphate nanoparticles | PTT + immunotherapy | Colon cancer |
| Lan et al. [131] | BDM | 264 | −23.5 | -- | Black phosphorous | myeloid-derived suppressor cell membrane | Decitabine | PTT + PDT + Chemotherapy | Oral squamous cell carcinoma |
| Zhao et al. [73] | GNR@SiO2@MnO2@MDSCs (GSMM) | 129 | −35.43 | -- | Gold nanorod | myeloid-derived suppressor cells membrane | MnO2 | PTT+ CDT | Cutaneous melanoma; Breast cancer |
| Investigators | Nanoplatform | Average Particle Size (nm) (±S.D.) | Zeta Potential (mV) (±S.D.) | PDI | Photosensitizer | Membrane Structure | Other Drugs | Synergistic Treatment Modalities | Target Disease |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al. [134] | CCA-M1EVs | 100 | -- | -- | chlorin e6 | M1-like macrophage-derived extracellular vesicles | CPPO; banoxantrone | CDT + Chemotherapy | Glioblastoma multiforme |
| Yoon et al. [135] | UCNPs@mSiO2PFC/Ce6@RAW-Man/PTX | 61.3 ± 1.1 | −11.6 | -- | chlorin e6 | macrophage membranes | perfluorocarbon; paclitaxel | PDT + immunotherapy | Breast cancer |
| Du et al. [69] | MCeC@MΦ | 71.2 ± (1.9) | −40 | -- | chlorin e6 | macrophage membranes | cerium oxide nanocatalyst | PDT + immunotherapy | Multi-drug-resistant bacterial sepsis |
| Zhang et al. [70] | Neu-FTO | 300 | 15 | -- | TiO2 | neutrophil membrane | Fe3O4 | PDT + immunotherapy | Infection |
| Sun et al. [136] | saDC@ Fs-NP | 110 ± (2.5) | −9.35 ± (0.68) | -- | AIE photosensitizer (FS) | superartificial dendritic cells membranes | -- | PDT + immunotherapy | Breast cancer |
| Liu et al. [137] | PCN@ FM | ~160 | −32 | 0.11 | porphyrin-based Zr-MOF (PCN-224) | cytomembranes of hybrid cells acquired from the fusion of cancer and dendritic cells | -- | PDT + immunotherapy | Breast cancer |
| Zhang et al. [138] | PHD@PM | 150 | −14.5 | -- | sinoporphyrin sodium | PD-1-expressing HEK293T-cell membranes | human serum albumin-perfluoro-tributylamine nanoemulsion | PDT + immunotherapy | Breast cancer |
| Deng et al. [76] | NK-NP | 80 ± (1.5) | -- | 0.105 | TCPP | natural killer cell membrane. | -- | PDT + immunotherapy | Breast cancer |
| Investigators | Nanoplatform | Average Particle Size (nm) (±S.D.) | Zeta Potential (mV) (±S.D.) | PDI | Photosensitizer | Membrane Structure | Other Drugs | Synergistic Treatment Modalities | Target Disease |
|---|---|---|---|---|---|---|---|---|---|
| Fang et al. [145] | siRNA/ICG@DSeSPm | ~116 | −13 ± (2) | -- | indocyanine green | macrophage membrane | siPDL1 | PDT + immunotherapy | Breast cancer |
| Ding et al. [144] | M1 CCD | 165 ± (31) | -- | -- | chlorin e6 | M1 macrophage-derived extracellular vesicles | CPPO | PDT + Chemotherapy | Breast cancer |
| Cao et al. [146] | EG@EMHMNPs | 230 ± (50) | −38.26 ± (0.36) | -- | Emodin | EMHM | glycyrrhizin | PDT + Chemotherapy | melanoma |
| Steen J. Madsen et al. [97] | Ma-AuNS | -- | -- | -- | gold–silica nanoshells (AuNS) | Rat alveolar macrophages membrane (Ma) | -- | PTT | Glioma |
| Zhang et al. [147] | NM-HB NPs | 140 | −24.8 | -- | Hypocrellins (HB) | neutrophil membrane | -- | PDT | Hepatocellular carcinoma |
| Xu et al. [148] | DC@AIEdots | 113.2 | −12.8 | 0.121 | MeTIND-4 | dendritic cell membrane | -- | PDT + immunotherapy | Breast cancer |
| Types of Membrane Carriers | Core Functions and Mechanisms | Advantages | Adapt to Diseases | Compatibility | Evaluation |
|---|---|---|---|---|---|
| Macrophage Cell Membrane | Targeting chemokine receptors (such as CCR2/CXCR4) in the tumor microenvironment; inducing M2 to M1 macrophage polarization, enhancing ROS production and immune response. | Highly efficient tumor targeting; Synergistic PDT and immunotherapy; Improving the hypoxic microenvironment of tumors | Solid tumors (such as glioma, breast cancer);Combined PDT/chemotherapy/immunotherapy | ★★★★★ | Macrophage membranes perform best in enhancing the efficacy of photodynamic therapy (PDT) and reshaping the immune microenvironment, especially for ROS-dependent photosensitizers. |
| Neutrophil Cell Membrane | Inflammation targeting (CXCR1/CXCR2 receptors); Evading immune clearance, prolonging circulation time; Inducing M1 macrophage polarization | High biocompatibility; Penetrate the inflammatory barrier; Support multiple laser treatments | Infection-related tumors or metastases; PDT combined with antibacterial/anti-inflammatory therapy | ★★★★☆ | It is suitable for scenarios requiring long cycles and combined multi-mode treatments, but the ability to generate ROS depends on the design of the photosensitizer. |
| T Cell Membrane | PD-1/PD-L1 blockade reverses T-cell exhaustion; penetrates the blood–brain barrier (BBB); activates the DC-T cell axis | Brain tumor-specific delivery; Immune checkpoint blockade enhancement; Activation of systemic anti-tumor immunity | Glioma, metastatic brain tumor; PDT combined with immune checkpoint inhibitors | ★★★★☆ | It has unique advantages for brain tumors, but the stability of membrane proteins needs to be optimized to maintain the function of PD-1. |
| Dendritic Cell Membrane | MHC-I/II and co-stimulatory molecules (CD80/CD86) activate T cells; induce tumor antigen-specific immune responses. | Efficient T-cell activation; natural antigen-presenting function; support the transformation of “cold tumors” to “hot tumors” | Low immunogenic tumors (such as pancreatic cancer); PDT combined with personalized vaccine therapy | ★★★☆☆ | It needs to be combined with tumor antigen loading technology, is suitable for customized photoimmunotherapy, but has a relatively high preparation complexity. |
| NK Cell Membrane | NKG2D/DNAM-1 mediates tumor recognition; induces M1 macrophage polarization; and synergizes with PDT to enhance the distant effect. | Innate immune activation; inhibition of tumor metastasis; long-lasting immune memory | Highly metastatic tumors (such as melanoma); PDT combined with adoptive cell therapy | ★★★★☆ | It has performed outstandingly in the control of metastatic foci, but the issue of large-scale preparation of NK membrane proteins needs to be addressed. |
| MDSCs Cell Membrane | Tumor chemokine receptor targeting; Evading immune surveillance | High tumor accumulation efficiency; low immunogenicity | Immunosuppressive tumor microenvironment; PDT combined with immunosuppression reversal therapy | ★★☆☆☆ | The potential remains to be verified. It is applicable to highly immune-escape tumors, but it may aggravate immune suppression and thus requires careful design. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Huang, Y.; Wen, Y.; Zou, Y.; Nie, K.; Liu, Z.; Li, X.; Zou, H.; Wang, Y. Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles. Pharmaceutics 2025, 17, 481. https://doi.org/10.3390/pharmaceutics17040481
He Z, Huang Y, Wen Y, Zou Y, Nie K, Liu Z, Li X, Zou H, Wang Y. Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles. Pharmaceutics. 2025; 17(4):481. https://doi.org/10.3390/pharmaceutics17040481
Chicago/Turabian StyleHe, Zhaoyang, Yunpeng Huang, Yu Wen, Yufeng Zou, Kai Nie, Zhongtao Liu, Xiong Li, Heng Zou, and Yongxiang Wang. 2025. "Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles" Pharmaceutics 17, no. 4: 481. https://doi.org/10.3390/pharmaceutics17040481
APA StyleHe, Z., Huang, Y., Wen, Y., Zou, Y., Nie, K., Liu, Z., Li, X., Zou, H., & Wang, Y. (2025). Tumor Treatment by Nano-Photodynamic Agents Embedded in Immune Cell Membrane-Derived Vesicles. Pharmaceutics, 17(4), 481. https://doi.org/10.3390/pharmaceutics17040481





