A Bionic “Trojan Horse”-like Nanovesicle Delivery System Hybridized with BCG Cytoplasmic Membrane and Melanoma Cell Membrane for Cancer Immunotherapy
Abstract
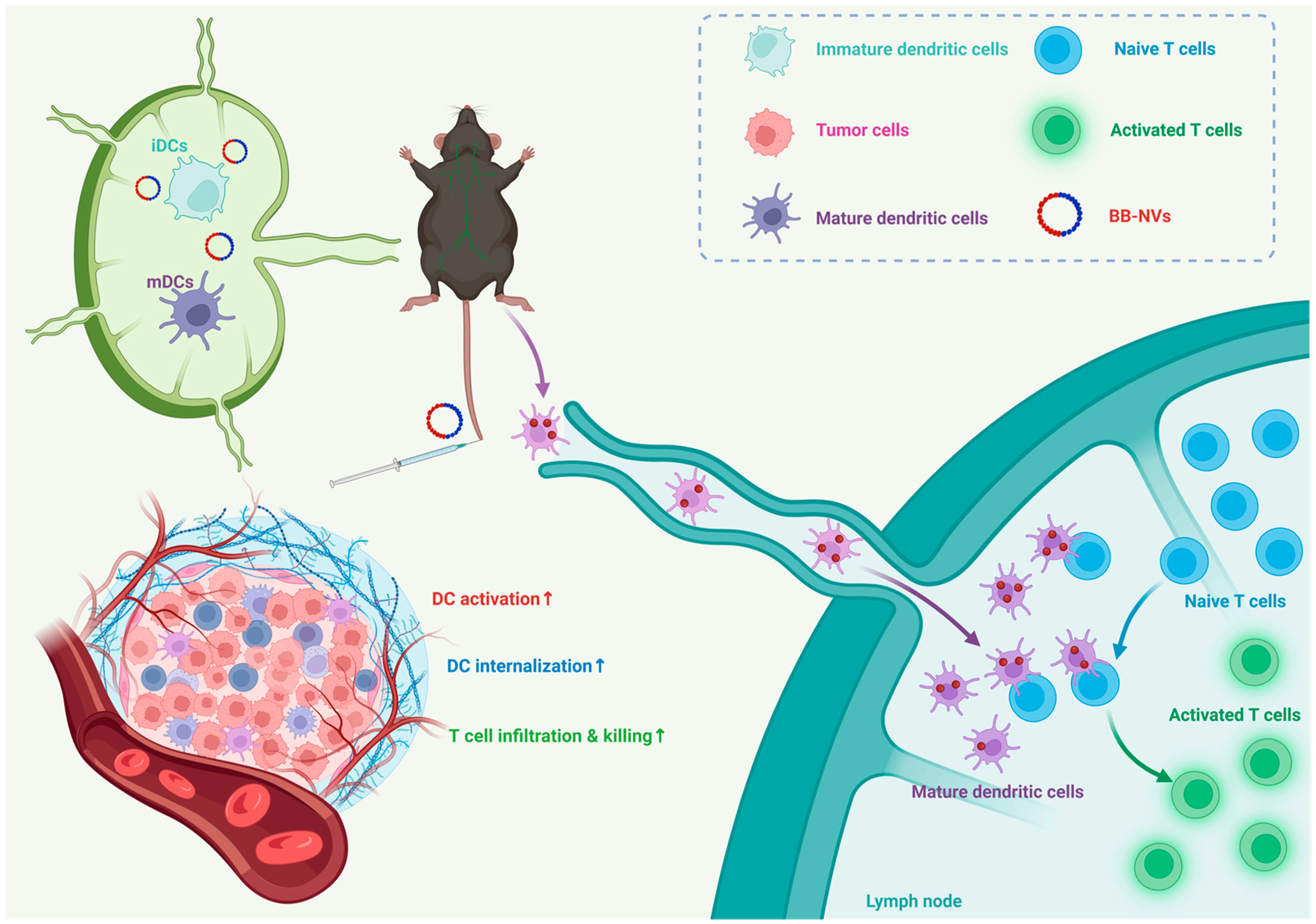
1. Introduction
2. Materials and Methods
3. Results
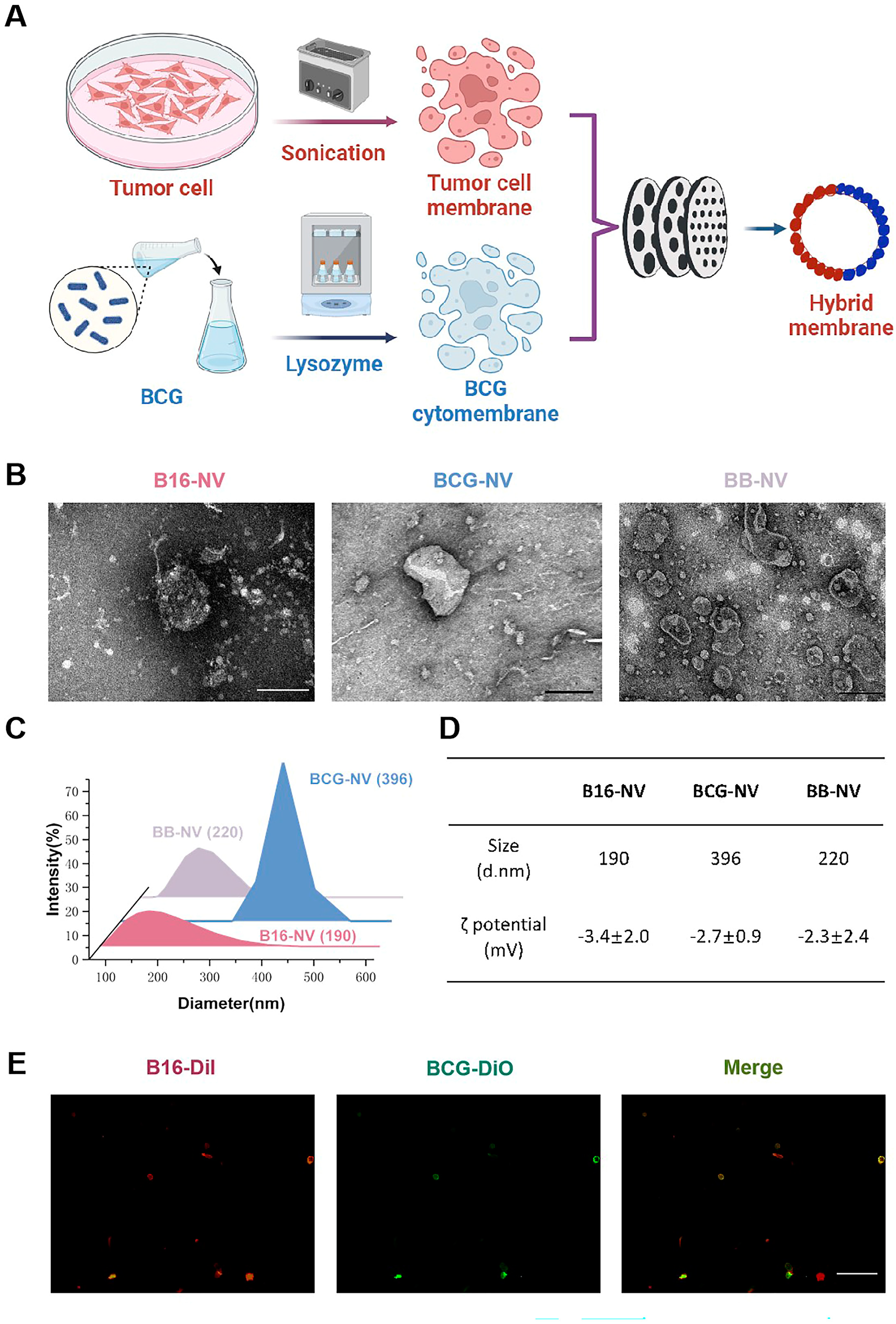
3.1. Preparation and Characterization of B16-NVs, BCG-NVs, and BB-NVs
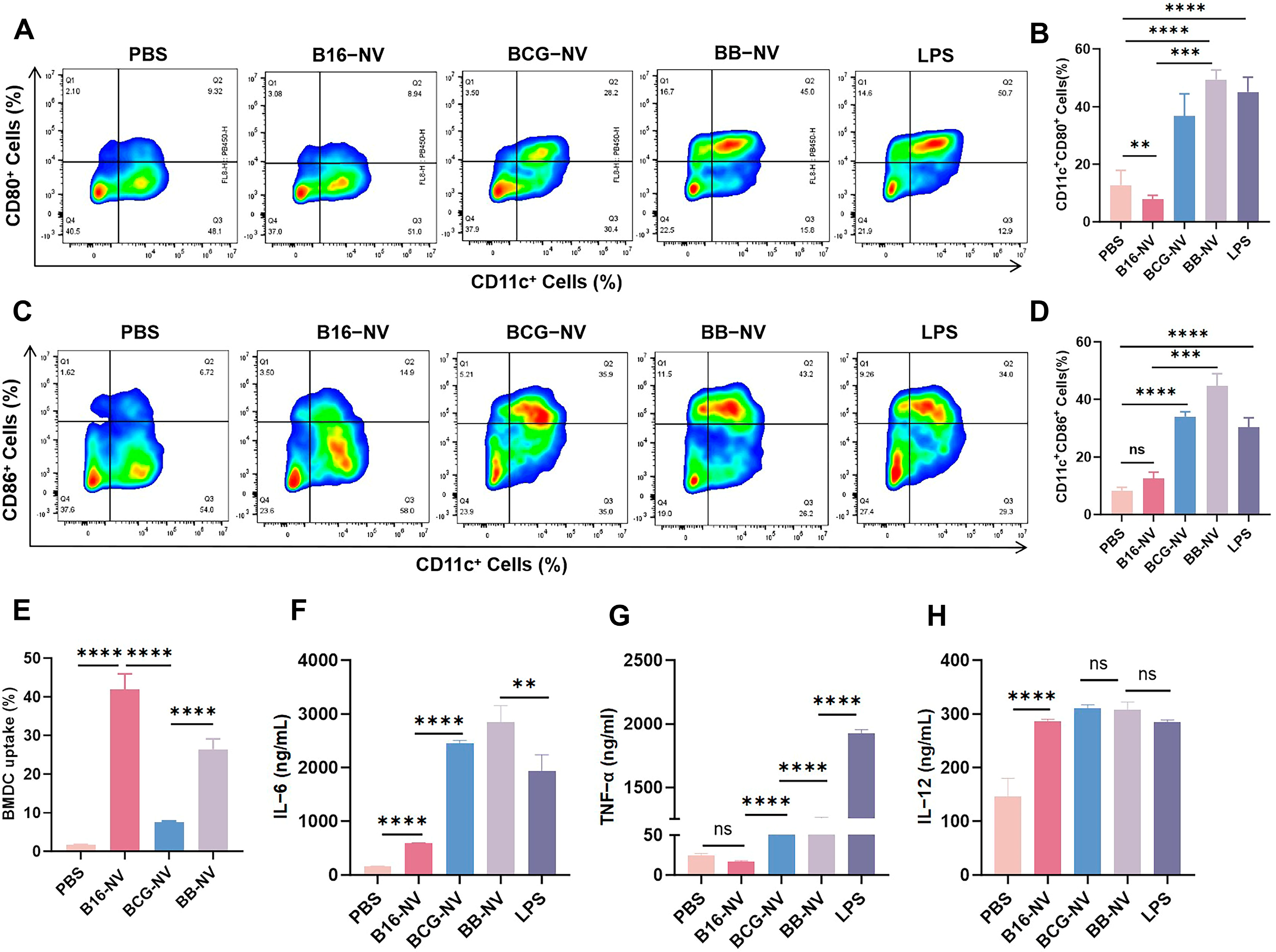
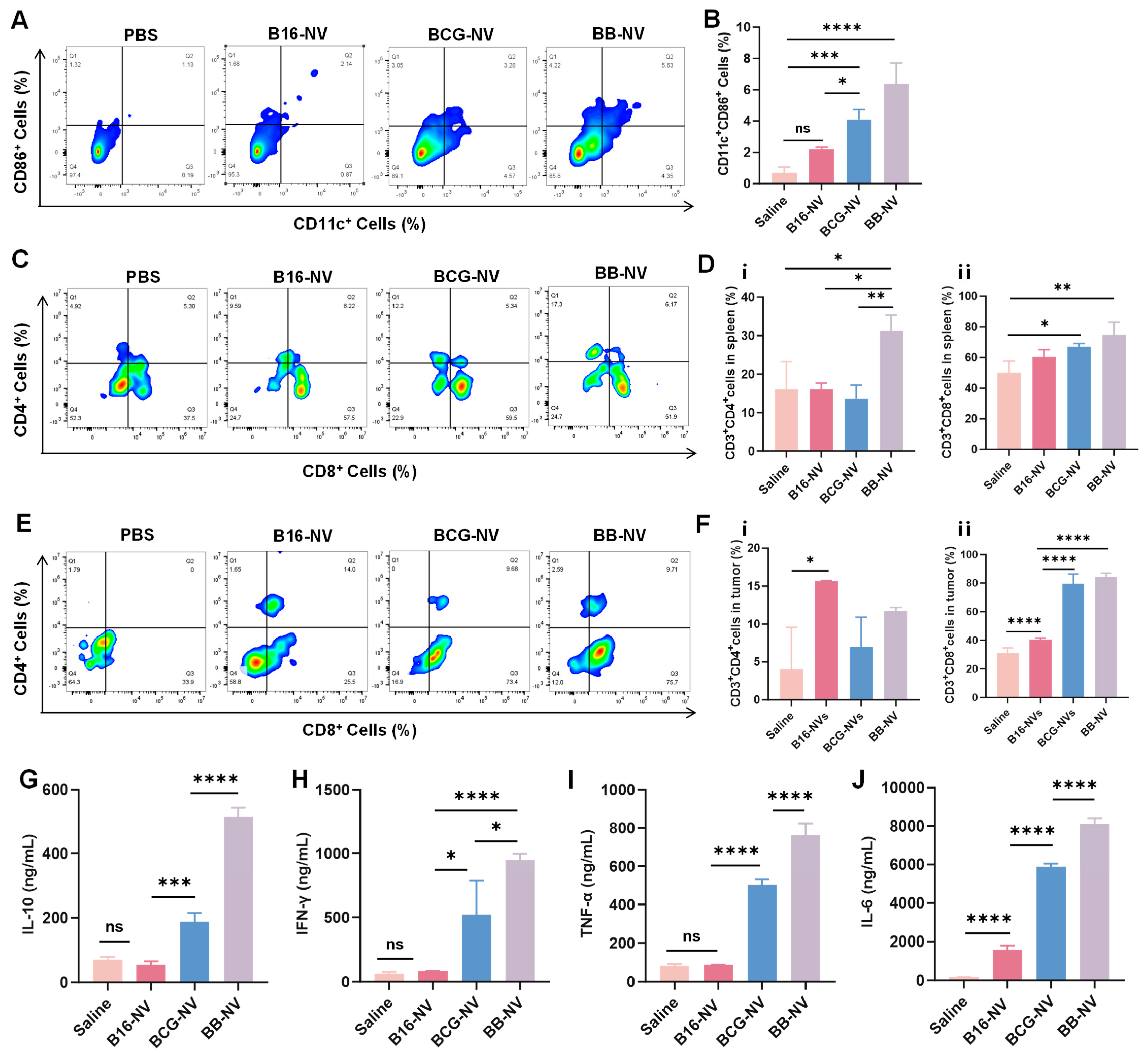
3.2. BB-NVs Increase Tumor Antigen Uptake and Activate Bone Marrow-Derived Dendritic Cells (BMDCs) by Co-Delivery of Antigen and Adjuvant
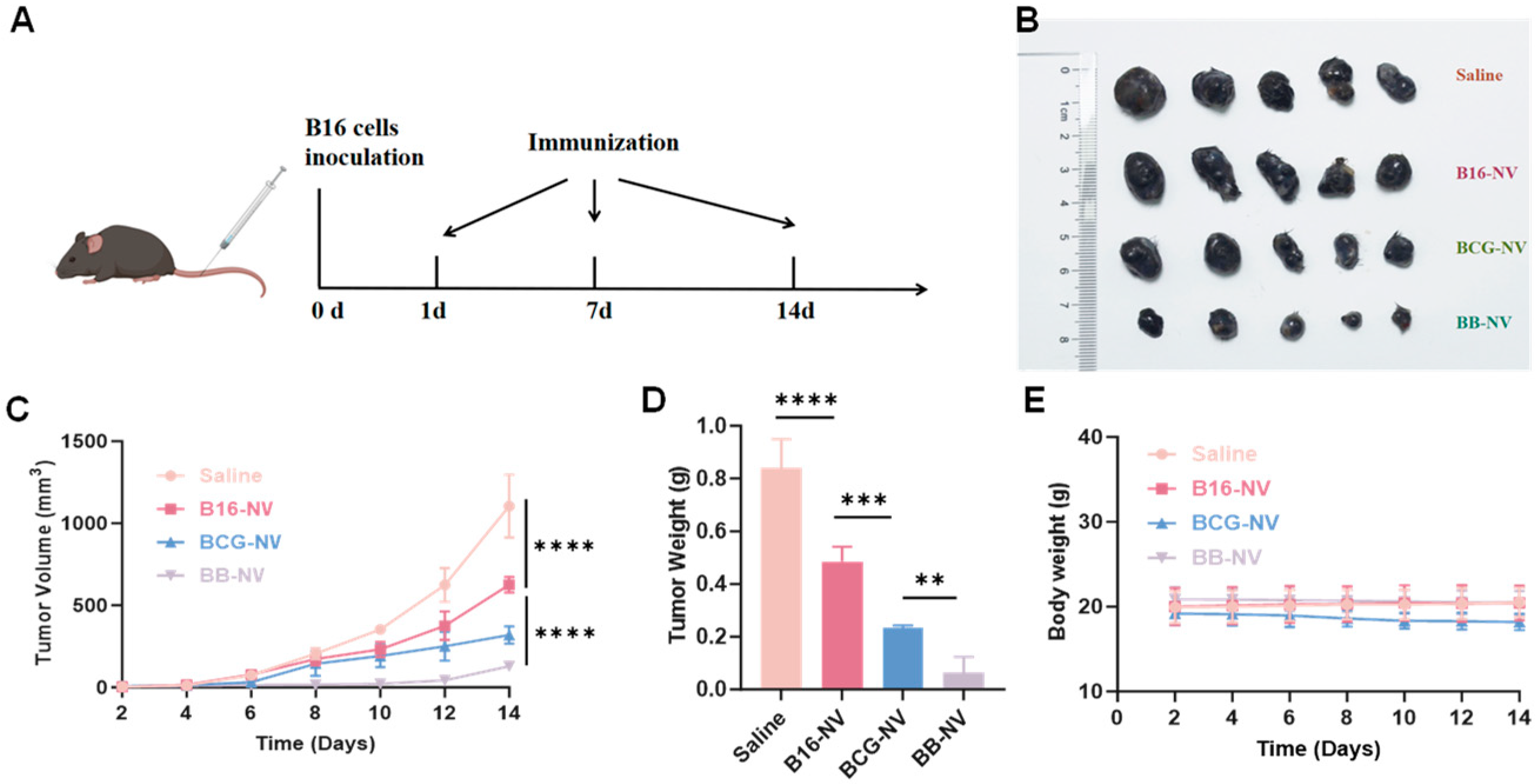
3.3. BB-NVs Vaccination Inhibits Tumor Progression in Murine B16 Tumor Model
3.4. BB-NVs Activate Immune Cells and Promote Anti-Tumor Cytokine Secretion in Mice
3.5. Biosafety Assessment of B16-NVs, BCG-NVs, and BB-NVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-Presenting Cells |
| BCG | Bacillus Calmette–Guérin |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DCs | Dendritic Cells |
| FBS | Fetal Bovine Serum |
| PBS | Phosphate-Buffered Saline |
| TEM | Transmission Electron Microscopy |
| DLS | Dynamic Light Scattering |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FITC | Fluoresce-Activated Cell Sorting |
| H&E | Hematoxylin and Eosin Staining |
| SEM | Scanning Electron Microscopy |
| °C | Celsius |
References
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.K.; Schwab, R.D.; Brookens, S.K.; Posey, A.D. Advances in cancer immunotherapies. Cell 2023, 186, 1814–1814.e1. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, H.; Ma, L.; Shi, Y.; Ji, M.; Sun, X.; Ma, D.; Zhou, W.; Huang, T.; Zhang, D. The quest for nanoparticle-powered vaccines in cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Ramirez-Valdez, R.A.; Khalilnezhad, A.; Khalilnezhad, S.; Dillon, M.; Hermans, D.; Fussell, S.; Tobin, K.K.; Dutertre, C.-A.; Lynn, G.M.; et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell 2022, 185, 4317–4332.e15. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, X.; Liu, L.; Zhao, C.; Zhang, J.; Yang, S.; Pan, P.; Huang, Q.; Zhao, X.; Tian, R.; et al. Genetically Engineered Cytomembrane Nanovaccines for Cancer Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2400068. [Google Scholar] [CrossRef]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, M.; Tijani, A.O.; Puri, A.; Chablani, L. Adjuvants in cutaneous vaccination: A comprehensive analysis. J. Control. Release 2024, 369, 475–492. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Le, Q.-V.; Lee, J.; Lee, H.; Shim, G.; Oh, Y.-K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B 2021, 11, 2096–2113. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, M.; Xue, C.; Wang, Y. Recent Advances in the Development of Membrane-derived Vesicles for Cancer Immunotherapy. Curr. Drug Deliv. 2024, 21, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ju, D.; Zhang, X. Cell Membrane-Derived Vesicle: A Novel Vehicle for Cancer Immunotherapy. Front. Immunol. 2022, 13, 923598. [Google Scholar] [CrossRef]
- Marianecci, C.; Carafa, M. Smart Nanovesicles for Drug Targeting and Delivery. Pharmaceutics 2019, 11, 147. [Google Scholar] [CrossRef]
- Cheng, Q.; Kang, Y.; Yao, B.; Dong, J.; Zhu, Y.; He, Y.; Ji, X. Genetically Engineered-Cell-Membrane Nanovesicles for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2302131. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Y.; Wu, S.; Yan, M.; Cao, Y.; Mou, N.; Wang, G.; Sun, D.; Wu, W. Cell membrane camouflaged biomimetic nanoparticles: Focusing on tumor theranostics. Mater. Today Bio 2022, 14, 100228. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, J. Application of Biomimetic Nanoparticles based on the Cell Membrane in Tumor Therapy. Curr. Top. Med. Chem. 2023, 23, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Jakubechova, J.; Altanerova, U.; Altaner, C. Tumor-targeted suicide gene-directed enzyme prodrug therapy mediated by extracellular vesicles. Neoplasma 2023, 70, 333–339. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.-C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Benitez, M.L.R.; Bender, C.B.; Oliveira, T.L.; Schachtschneider, K.M.; Collares, T.; Seixas, F.K. Mycobacterium bovis BCG in metastatic melanoma therapy. Appl. Microbiol. Biotechnol. 2019, 103, 7903–7916. [Google Scholar] [CrossRef]
- Ramalingam, S.; Gunasekaran, K.; Arora, H.; Muruganandam, M.; Nagaraju, S.; Padmanabhan, P. Disseminated BCG Infection after intravesical BCG Immunotherapy of Bladder Cancer. QJM Int. J. Med. 2021, 114, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, Y.-L.; Men, Y.; Jiao, Y.-Z.; Chen, S. Kinetic cation effect in alkaline hydrogen electrocatalysis and double layer proton transfer. Nat. Commun. 2025, 16, 1844. [Google Scholar] [CrossRef]
- Storgard, R.; Markova, A. Cutaneous hypersensitivity reactions to immune checkpoint inhibitors. J. Allergy Clin. Immunol. Pract. 2024, 12, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, J.; Chen, Y.; Liu, Y.; Li, X.; Li, H.; Cao, P. Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis. Adv. Sci. 2024, 11, e2308235. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Zhang, Z.; Dai, Y.; Chen, X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 2019, 48, 3771–3810. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Ma, S.; Wang, Y.; Huang, Z.; Qu, H.; Yao, H.; Zhang, Y.; Wu, G.; Huang, L.; et al. A Minimalist Binary Vaccine Carrier for Personalized Postoperative Cancer Vaccine Therapy. Adv. Mater. 2022, 34, 2109254. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular vesicles, genetic programmers. Nat. Cell Biol. 2024, 26, 22–23. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, R.; Nie, G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat. Protoc. 2022, 17, 2240–2274. [Google Scholar] [CrossRef]
- Kremenovic, M.; Chan, A.A.; Feng, B.; Bäriswyl, L.; Robatel, S.; Gruber, T.; Tang, L.; Lee, D.J.; Schenk, M. BCG hydrogel promotes CTSS-mediated antigen processing and presentation, thereby suppressing metastasis and prolonging survival in melanoma. J. Immunother. Cancer 2022, 10, e004133. [Google Scholar] [CrossRef]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Means, T.K.; Heldwein, K.A.; Keen, M.A.; Hill, P.J.; Belisle, J.T.; Fenton, M.J. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 2001, 69, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Jung, J.; Lee, Y.; Kim, T.-Y.; Choi, S.-Y.; Park, J.; Kim, D.-S.; Kwon, H.-J. Immunostimulatory oligodeoxynucleotide isolated from genome wide screening of Mycobacterium bovis chromosomal DNA. Mol. Immunol. 2006, 43, 2107–2118. [Google Scholar] [CrossRef]
- Tsuji, S.; Matsumoto, M.; Takeuchi, O.; Akira, S.; Azuma, I.; Hayashi, A.; Toyoshima, K.; Seya, T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: Involvement of toll-like receptors. Infect. Immun. 2000, 68, 6883–6890. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Salome, B.; Daza, J.; Farkas, A.; Bhardwaj, N.; Horowitz, A. Bacillus Calmette-Guerin (BCG): Its fight against pathogens and cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 39, 121–129. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Chen, K.; Hu, T.; Wang, Y.; Wang, J.; Lv, C.; Xu, J.; Zhang, X.; Li, A.; Chen, B.; et al. A Bionic “Trojan Horse”-like Nanovesicle Delivery System Hybridized with BCG Cytoplasmic Membrane and Melanoma Cell Membrane for Cancer Immunotherapy. Pharmaceutics 2025, 17, 507. https://doi.org/10.3390/pharmaceutics17040507
Xiao Y, Chen K, Hu T, Wang Y, Wang J, Lv C, Xu J, Zhang X, Li A, Chen B, et al. A Bionic “Trojan Horse”-like Nanovesicle Delivery System Hybridized with BCG Cytoplasmic Membrane and Melanoma Cell Membrane for Cancer Immunotherapy. Pharmaceutics. 2025; 17(4):507. https://doi.org/10.3390/pharmaceutics17040507
Chicago/Turabian StyleXiao, Yuai, Kexin Chen, Tianchi Hu, Yuchong Wang, Jing Wang, Chuan Lv, Jianguo Xu, Xinyi Zhang, Ang Li, Bingdi Chen, and et al. 2025. "A Bionic “Trojan Horse”-like Nanovesicle Delivery System Hybridized with BCG Cytoplasmic Membrane and Melanoma Cell Membrane for Cancer Immunotherapy" Pharmaceutics 17, no. 4: 507. https://doi.org/10.3390/pharmaceutics17040507
APA StyleXiao, Y., Chen, K., Hu, T., Wang, Y., Wang, J., Lv, C., Xu, J., Zhang, X., Li, A., Chen, B., Zhu, J., Wu, M., & Xue, C. (2025). A Bionic “Trojan Horse”-like Nanovesicle Delivery System Hybridized with BCG Cytoplasmic Membrane and Melanoma Cell Membrane for Cancer Immunotherapy. Pharmaceutics, 17(4), 507. https://doi.org/10.3390/pharmaceutics17040507





