Investigation of Nano Spray-Dried, Hyaluronic Acid-Modified Polymeric Micelles for Nasal Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification of Vinpocetine via High-Performance Liquid Chromatography
2.3. Formulation of Polymeric Micelles via Nano Spray-Drying
2.4. Characterization of the Nano Spray-Dried Powders
2.4.1. Characterization of Morphology via Scanning Electron Microscopy
2.4.2. Determination of Particle Size via Laser Diffraction
2.4.3. Characterization of Crystallinity via X-Ray Powder Diffraction
2.5. Characterization of the Polymeric Micelles in Liquid State
2.5.1. Determination of Micelle Size, Size Distribution, and Zeta Potential
2.5.2. Determination of Encapsulation Efficiency
2.5.3. Determination of Thermodynamic Solubility
2.5.4. Determination of Viscosity
2.6. Nasal Applicability Studies
2.6.1. In Vitro Mucoadhesion Study
2.6.2. In Vitro Drug Release Study
2.6.3. In Vitro Drug Permeation Study
2.7. Stability Studies
3. Results
3.1. Characterization of the Nano Spray-Dried Powders
3.1.1. Determination of Particle Size and Size Distribution
3.1.2. Characterization of Morphology
3.1.3. Characterization of Crystallinity via X-Ray Powder Diffraction
3.2. Characterization of the Polymeric Micelles in a Liquid State
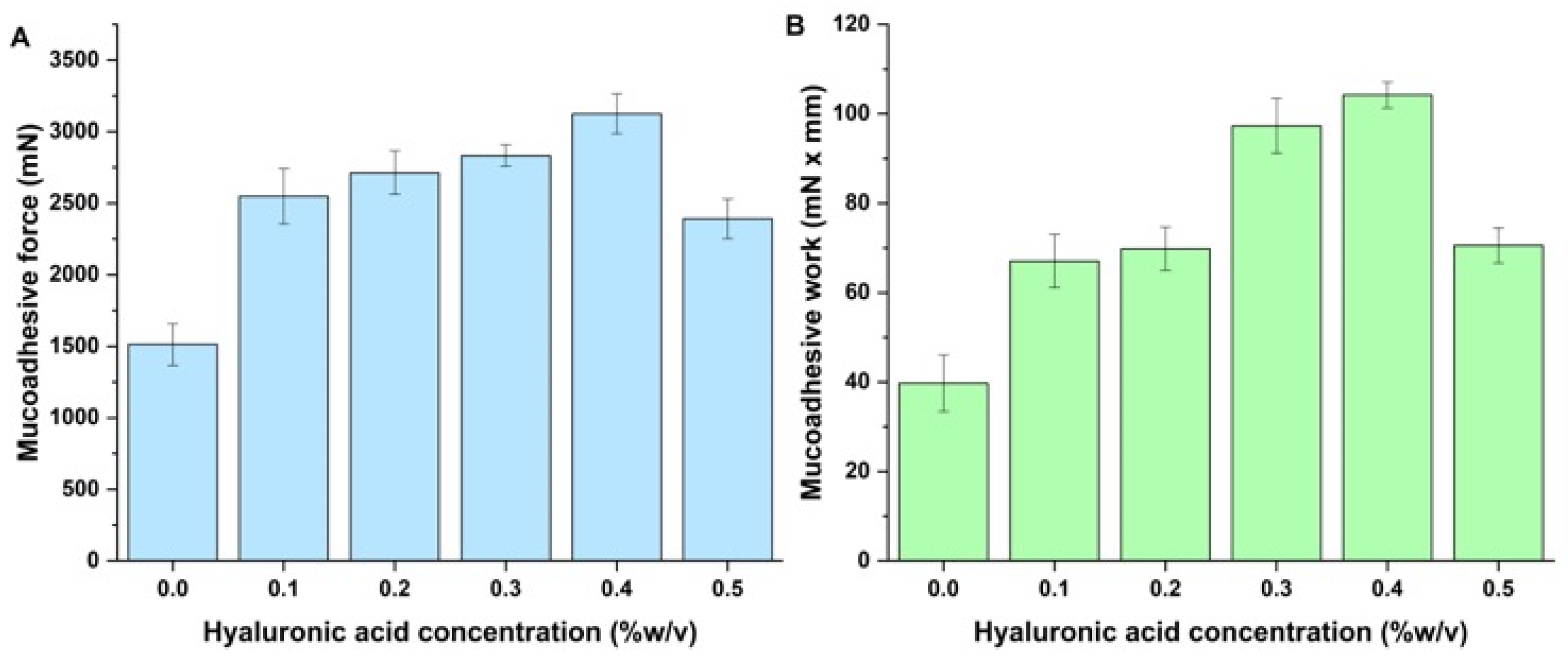
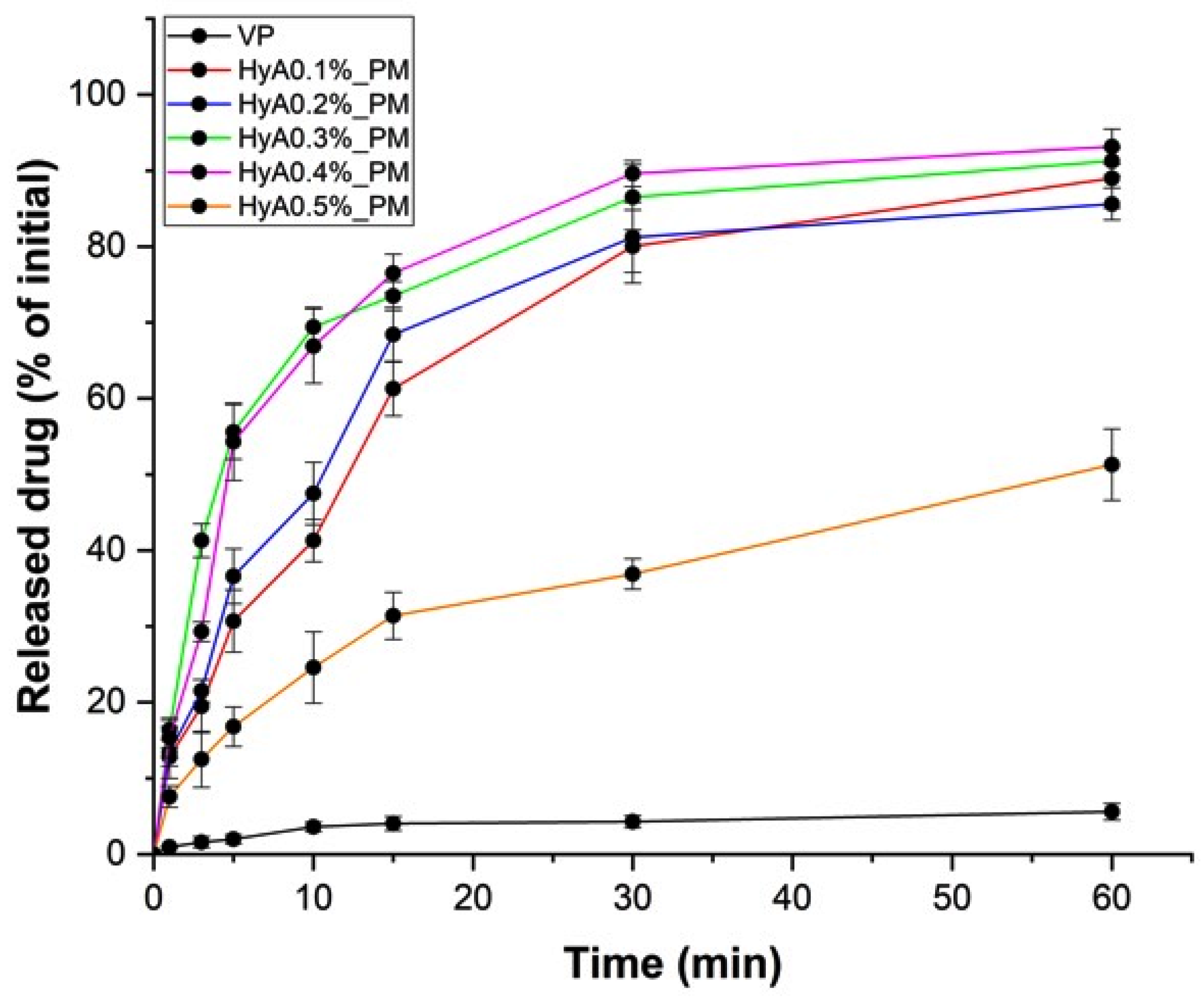
3.3. In Vitro Nasal Applicability Studies
3.4. Stability Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| D[0.5] | Average particle size |
| DH | Average hydrodynamic diameter, micelle size |
| DLS | Dynamic light scattering |
| D-TRE | D-trehalose dihydrate |
| EE | Encapsulation efficiency |
| HPLC | High-performance liquid chromatography |
| HyA | Hyaluronic acid |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| P 188 | Poloxamer 188 |
| PBS | Phosphate buffered saline |
| PCL-PVAc-PEG | Poly(vinyl caprolactam)-poly(vinyl acetate)-poly(ethylene glycol) |
| PdI | Polydispersity index |
| PEG-PPG-PEG | Poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) |
| RH | Relative humidity |
| SEM | Scanning electron microscopy |
| SNES | Simulated nasal electrolyte solution |
| SP | Soluplus |
| VP | Vinpocetine |
| XRPD | X-ray powder diffraction |
| ζ | Zeta potential |
References
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Jogi, G.; Shah, N.; Athalye, M.N.; Bamaniya, N.; Vora, L.K.; Cláudia Paiva-Santos, A. Advanced Particulate Carrier-Mediated Technologies for Nasal Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 74, 103569. [Google Scholar] [CrossRef]
- Williams, G.; Suman, J.D. In Vitro Anatomical Models for Nasal Drug Delivery. Pharmaceutics 2022, 14, 1353. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Afshari, R.; Khalaji, S.; Rabiee, M.; Bagherzadeh, M.; Fatahi, Y.; Dinarvand, R.; Tahriri, M.; Tayebi, L.; et al. Polymeric Nanoparticles for Nasal Drug Delivery to the Brain: Relevance to Alzheimer’s Disease. Adv. Ther. 2021, 4, 2000076. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in Nasal Drug Delivery Systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-Time Quantitative Monitoring of in Vitro Nasal Drug Delivery by a Nasal Epithelial Mucosa-on-a-Chip Model. Expert Opin. Drug Deliv. 2021, 18, 803–818. [Google Scholar] [CrossRef]
- Rai, G.; Gauba, P.; Dang, S. Recent Advances in Nanotechnology for Intra-Nasal Drug Delivery and Clinical Applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104726. [Google Scholar] [CrossRef]
- Huang, C.-W.; Chuang, C.-P.; Chen, Y.-J.; Wang, H.-Y.; Lin, J.-J.; Huang, C.-Y.; Wei, K.-C.; Huang, F.-T. Integrin A2β1-Targeting Ferritin Nanocarrier Traverses the Blood–Brain Barrier for Effective Glioma Chemotherapy. J. Nanobiotechnol. 2021, 19, 180. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Kapoor, B.; Jha, N.K.; Gupta, P.K.; Gupta, G.; Chellappan, D.K.; Devkota, H.P.; Prasher, P.; Ansari, M.S.; et al. Advances in Designing of Polymeric Micelles for Biomedical Application in Brain Related Diseases. Chem.-Biol. Interact. 2022, 361, 109960. [Google Scholar] [CrossRef]
- Rajput, A.; Pingale, P.; Dhapte-Pawar, V. Nasal Delivery of Neurotherapeutics via Nanocarriers: Facets, Aspects, and Prospects. Front. Pharmacol. 2022, 13, 979682. [Google Scholar] [CrossRef]
- Sastri, K.T.; Gupta, N.V.; M, S.; Chakraborty, S.; Kumar, H.; Chand, P.; Balamuralidhara, V.; Gowda, D.V. Nanocarrier Facilitated Drug Delivery to the Brain through Intranasal Route: A Promising Approach to Transcend Bio-Obstacles and Alleviate Neurodegenerative Conditions. J. Drug Deliv. Sci. Technol. 2022, 75, 103656. [Google Scholar] [CrossRef]
- Abo El-Enin, H.A.; Ahmed, M.F.; Naguib, I.A.; El-Far, S.W.; Ghoneim, M.M.; Alsalahat, I.; Abdel-Bar, H.M. Utilization of Polymeric Micelles as a Lucrative Platform for Efficient Brain Deposition of Olanzapine as an Antischizophrenic Drug via Intranasal Delivery. Pharmaceuticals 2022, 15, 249. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Y.; Zhang, J.; Zhan, X.; Zhu, S.; Yang, H.; Wang, G. Multiple Stimuli-Responsive Polymeric Micelles for Controlled Release. Soft Matter 2013, 9, 370–373. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Xuan, Y.; Zhi, D.; Wang, W.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. pH-Sensitive Hyaluronic Acid-Targeted Prodrug Micelles Constructed via a One-Step Reaction for Enhanced Chemotherapy. Int. J. Biol. Macromol. 2022, 206, 489–500. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Zhang, Z.; Wang, L.; Fan, Y.; Liu, X.; Liu, X.; Ya, H.; Zhang, Y.; Xu, Y. Hyaluronic Acid Micelles for Promoting the Skin Permeation and Deposition of Curcumin. Int. J. Nanomed. 2022, 17, 4009–4022. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Suzuki, K.; Yoshizaki, Y.; Horii, K.; Murase, N.; Kuzuya, A.; Ohya, Y. Preparation of Hyaluronic Acid-Coated Polymeric Micelles for Nasal Vaccine Delivery. Biomater. Sci. 2022, 10, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Chopde, S.; Datir, R.; Deshmukh, G.; Dhotre, A.; Patil, M. Nanoparticle Formation by Nanospray Drying & Its Application in Nanoencapsulation of Food Bioactive Ingredients. J. Agric. Food Res. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA Nanoparticles Prepared by Nano Spray Drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Panda, P.K.; Ramachandran, A.; Panda, P.; Sharawat, I.K. Safety and Efficacy of Vinpocetine as a Neuroprotective Agent in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Neurocrit. Care 2022, 37, 314–325. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.; Al-Gareeb, A.; Naji, M.; Al-Mamorry, F. Role of Vinpocetine in Ischemic Stroke and Poststroke Outcomes: A Critical Review. Brain Circ. 2020, 6, 1. [Google Scholar] [CrossRef]
- Sipos, B.; Csóka, I.; Budai-Szűcs, M.; Kozma, G.; Berkesi, D.; Kónya, Z.; Balogh, G.T.; Katona, G. Development of Dexamethasone-Loaded Mixed Polymeric Micelles for Nasal Delivery. Eur. J. Pharm. Sci. 2021, 166, 105960. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Harmonised Guideline: Q2(R2)—Validation of Analytical Procedures. 2022. Available online: https://www.Ich.Org/Page/Quality-Guidelines (accessed on 13 April 2025).
- Katona, G.; Sipos, B.; Ambrus, R.; Csóka, I.; Szabó-Révész, P. Characterizing the Drug-Release Enhancement Effect of Surfactants on Megestrol-Acetate-Loaded Granules. Pharmaceuticals 2022, 15, 113. [Google Scholar] [CrossRef]
- Sipos, B.; Katona, G.; Szarvas, F.M.; Budai-Szűcs, M.; Ambrus, R.; Csóka, I. Development of Vinpocetine-Loaded Nasal Polymeric Micelles via Nano-Spray-Drying. Pharmaceuticals 2023, 16, 1447. [Google Scholar] [CrossRef]
- Keck, T.; Leiacker, R.; Riechelmann, H.; Rettinger, G. Temperature Profile in the Nasal Cavity. Laryngoscope 2000, 110, 651–654. [Google Scholar] [CrossRef]
- ICH. Stability Testing of New Drug Substances and Drug Products Q1A(R2); ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Aguilera-Garrido, A.; Molina-Bolívar, J.A.; Gálvez-Ruiz, M.J.; Galisteo-González, F. Mucoadhesive Properties of Liquid Lipid Nanocapsules Enhanced by Hyaluronic Acid. J. Mol. Liq. 2019, 296, 111965. [Google Scholar] [CrossRef]
- Guarise, C.; Acquasaliente, L.; Pasut, G.; Pavan, M.; Soato, M.; Garofolin, G.; Beninatto, R.; Giacomel, E.; Sartori, E.; Galesso, D. The Role of High Molecular Weight Hyaluronic Acid in Mucoadhesion on an Ocular Surface Model. J. Mech. Behav. Biomed. Mater. 2023, 143, 105908. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Erman, L.; Partenhauser, A. Evaluation of Modified Hyaluronic Acid in Terms of Rheology, Enzymatic Degradation and Mucoadhesion. Int. J. Biol. Macromol. 2019, 123, 1204–1210. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic Acid Targeting of CD44 for Cancer Therapy: From Receptor Biology to Nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Peach, R.; Hollenbaugh, D.; Stamenkovic, I.; Aruffo, A. Identification of Hyaluronic Acid Binding Sites in the Extracellular Domain of CD44. J. Cell Biol. 1993, 122, 257–264. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Chen, C.-T.; Yang, J.-C.; Tsai, T. Spray-Dried Microparticles Containing Polymeric Micelles Encapsulating Hematoporphyrin. AAPS J. 2010, 12, 138–146. [Google Scholar] [CrossRef]
- Dattani, S.; Li, X.; Lampa, C.; Barriscale, A.; Damadzadeh, B.; Lechuga-Ballesteros, D.; Jasti, B.R. Development of Spray-Dried Micelles, Liposomes, and Solid Lipid Nanoparticles for Enhanced Stability. Pharmaceutics 2025, 17, 122. [Google Scholar] [CrossRef]
- Fatnassi, M.; Jacquart, S.; Brouillet, F.; Rey, C.; Combes, C.; Girod Fullana, S. Optimization of Spray-Dried Hyaluronic Acid Microspheres to Formulate Drug-Loaded Bone Substitute Materials. Powder Technol. 2014, 255, 44–51. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Agnello, S.; Fiorica, C.; Pitarresi, G.; Puleio, R.; Loria, G.R.; Giammona, G. Spray Dried Hyaluronic Acid Microparticles for Adhesion Controlled Aggregation and Potential Stimulation of Stem Cells. Int. J. Pharm. 2017, 519, 332–342. [Google Scholar] [CrossRef]
- Horvát, S.; Fehér, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Tóth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kürti, L.; et al. Sodium Hyaluronate as a Mucoadhesive Component in Nasal Formulation Enhances Delivery of Molecules to Brain Tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef]
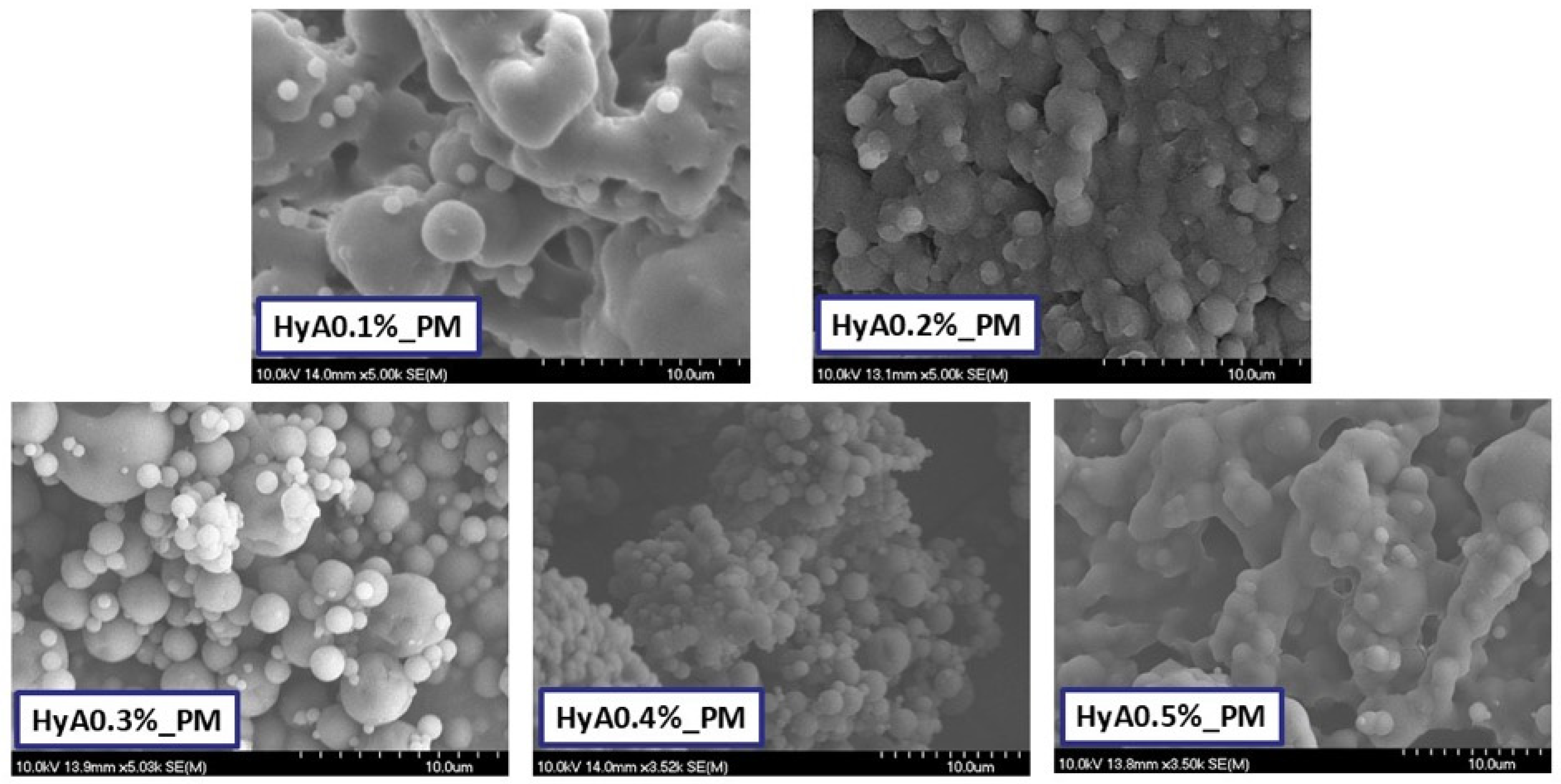




| HyA Concentration (% w/v) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|
| D[0.5] (µm) | 11.41 ± 2.40 | 7.34 ± 0.91 | 5.62 ± 1.64 | 4.15 ± 0.41 | 28.51 ± 6.41 |
| Span | 2.55 ± 0.54 | 2.26 ± 0.17 | 1.54 ± 0.23 | 1.06 ± 0.05 | 3.17 ± 0.76 |
| Yield (%) | 75.3 ± 2.4 | 71.2 ± 4.1 | 80.4 ± 3.9 | 84.5 ± 2.2 | 53.2 ± 2.7 |
| HyA Concentration (% w/v) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|
| D[0.5] (µm) | 14.27 ± 3.12 | 6.98 ± 1.24 | 6.11 ± 2.49 | 4.87 ± 0.89 | 36.75 ± 7.74 |
| HyA Concentration (% w/v) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|
| DH (nm) | 75.4 ± 7.4 | 102.3 ± 5.4 | 114.5 ± 3.7 | 107.3 ± 2.1 | 213.8 ± 14.5 |
| PdI | 0.415 ± 0.028 | 0.325 ± 0.017 | 0.365 ± 0.045 | 0.245 ± 0.007 | 0.564 ± 0.045 |
| ζ (mV) | −15.3 ± 2.4 | −23.1 ± 3.7 | −27.5 ± 1.5 | −34.5 ± 2.5 | −35.8 ± 6.5 |
| HyA Concentration (% w/v) | VP (0) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|---|
| EE% | - | 76.5 ± 4.5 | 81.2 ± 3.1 | 84.5 ± 2.6 | 89.5 ± 3.7 | 75.4 ± 6.7 |
| S25°C (µg/mL) | 2.39 ± 0.37 | 564.4 ± 31.2 | 607.7 ± 24.6 | 687 ± 19.6 | 769.4 ± 24.2 | 649.1 ± 38.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, B.; Mayer, L.; Budai-Szűcs, M.; Katona, G.; Ambrus, R.; Csóka, I. Investigation of Nano Spray-Dried, Hyaluronic Acid-Modified Polymeric Micelles for Nasal Administration. Pharmaceutics 2025, 17, 533. https://doi.org/10.3390/pharmaceutics17040533
Sipos B, Mayer L, Budai-Szűcs M, Katona G, Ambrus R, Csóka I. Investigation of Nano Spray-Dried, Hyaluronic Acid-Modified Polymeric Micelles for Nasal Administration. Pharmaceutics. 2025; 17(4):533. https://doi.org/10.3390/pharmaceutics17040533
Chicago/Turabian StyleSipos, Bence, Levente Mayer, Mária Budai-Szűcs, Gábor Katona, Rita Ambrus, and Ildikó Csóka. 2025. "Investigation of Nano Spray-Dried, Hyaluronic Acid-Modified Polymeric Micelles for Nasal Administration" Pharmaceutics 17, no. 4: 533. https://doi.org/10.3390/pharmaceutics17040533
APA StyleSipos, B., Mayer, L., Budai-Szűcs, M., Katona, G., Ambrus, R., & Csóka, I. (2025). Investigation of Nano Spray-Dried, Hyaluronic Acid-Modified Polymeric Micelles for Nasal Administration. Pharmaceutics, 17(4), 533. https://doi.org/10.3390/pharmaceutics17040533









