Development of GSH-Stimuli-Responsive Micelles Using a Targeted Paclitaxel Prodrug for Enhanced Anticancer Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
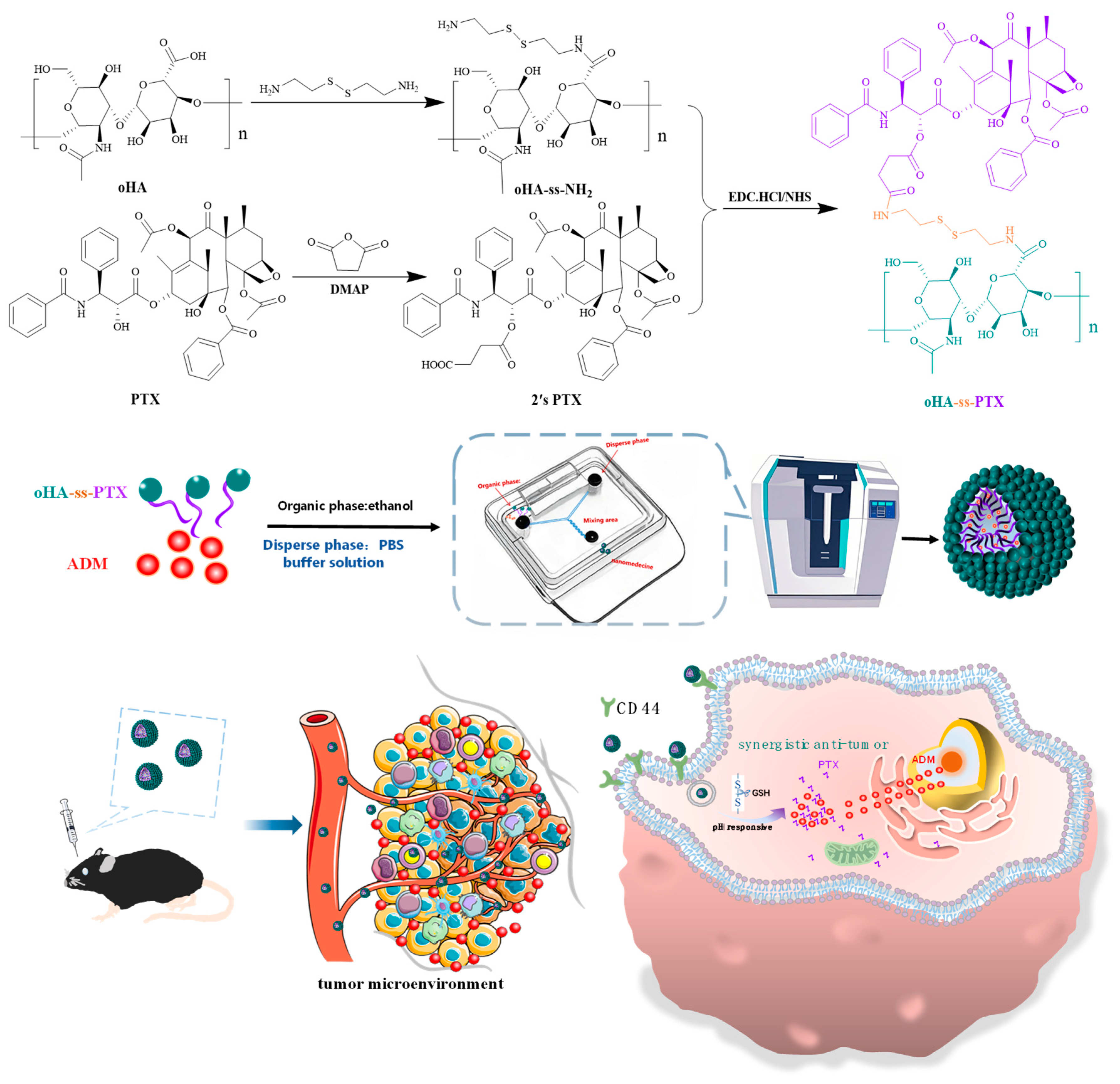
2.2. Synthesis of Amphiphilic Prodrug oHA-ss-PTX
2.2.1. Synthesis of Redox-Sensitive oHA-ss-NH2
2.2.2. Carboxylation of Paclitaxel (2′sPTX)
2.2.3. Synthesis of Amphiphilic oHA-ss-PTX
2.3. Preparation of oHA-ss-PTX Micelles and ADM/oHA-ss-PTX Micelles
2.4. Characterization of oHA-ss-PTX Micelles and ADM/oHA-ss-PTX Micelles
2.5. Loading Content and Encapsulation Efficiency of Micelles
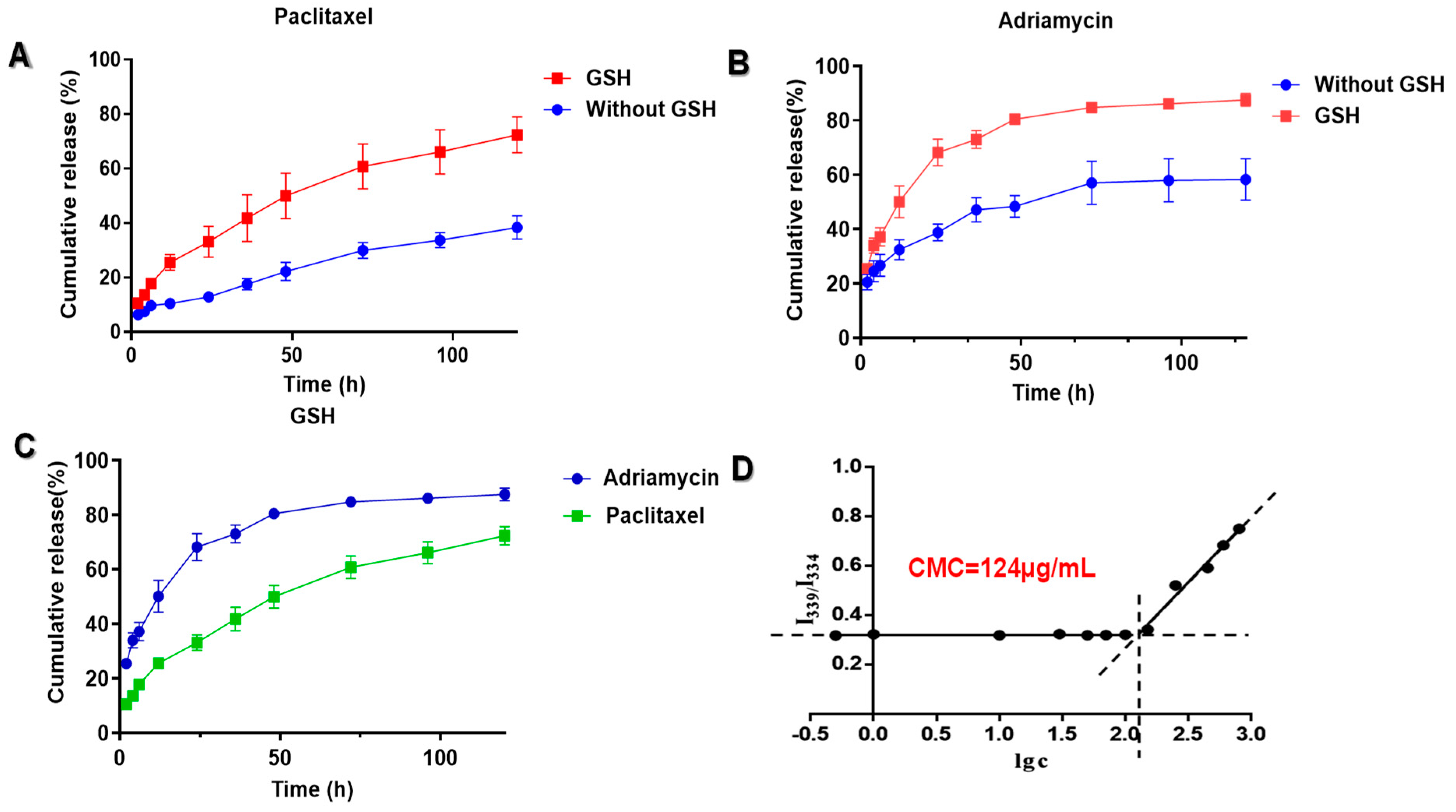
2.6. Drugs Release of Micelles by Glutathione In Vitro
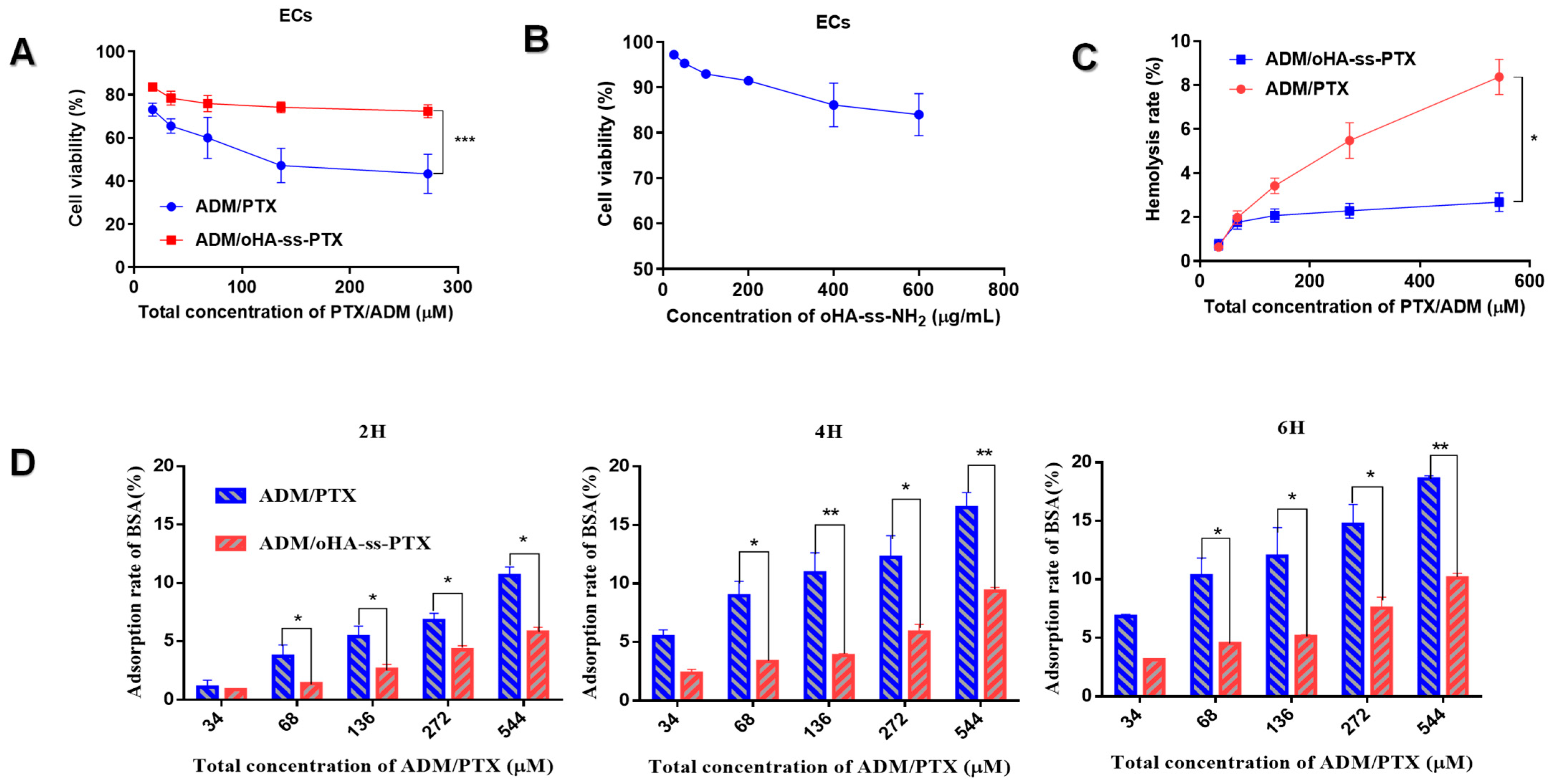
2.7. Toxicity of oHA-ss-NH2 and ADM/oHA-ss-PTX to ECs
2.8. Hemolysis Testing
2.9. Bovine Serum Albumin (BSA) Adsorption
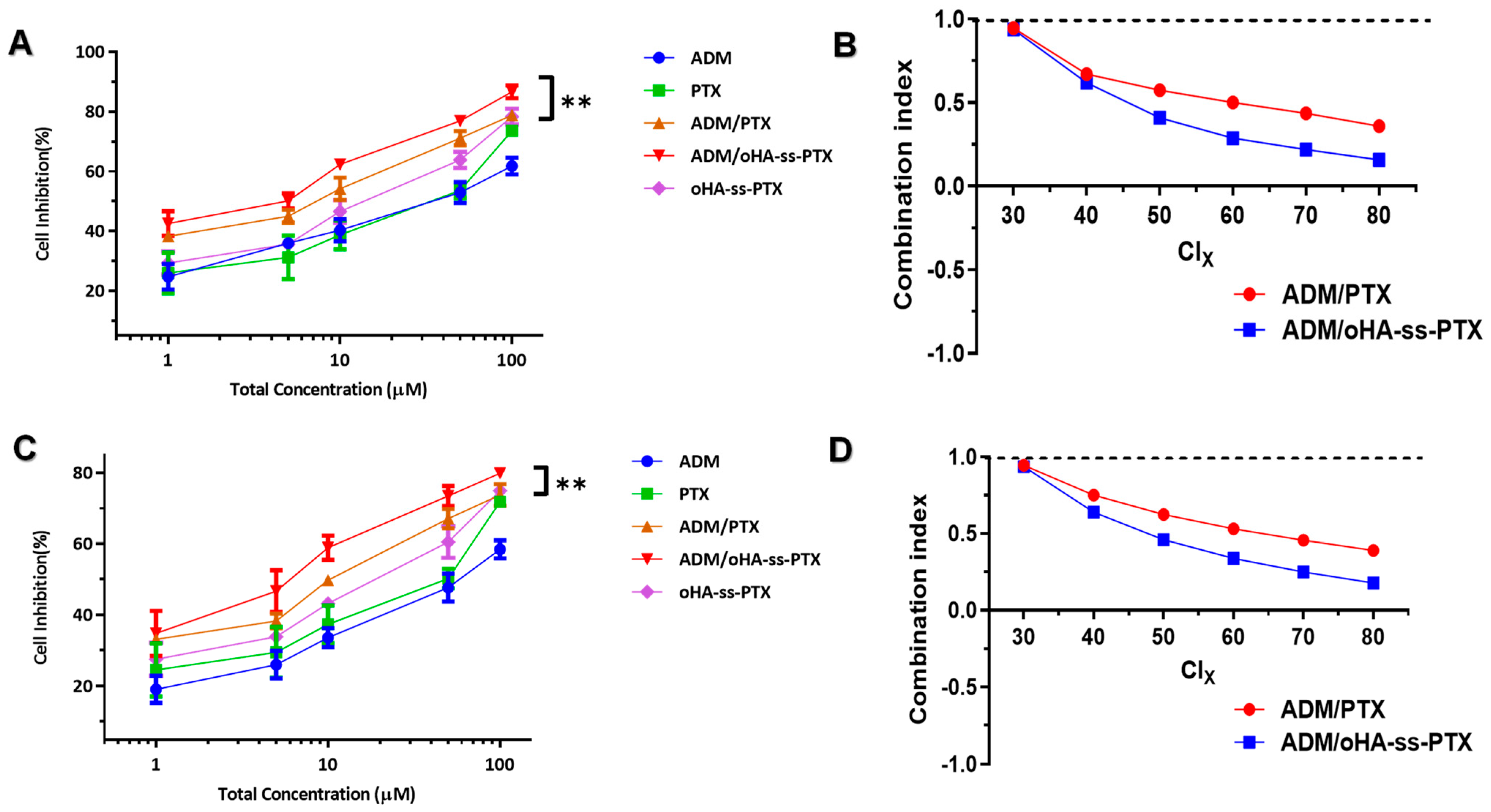
2.10. Evaluation of Anticancer Effect In Vitro
2.11. Evaluation of Synergistic Antitumor Effect In Vitro
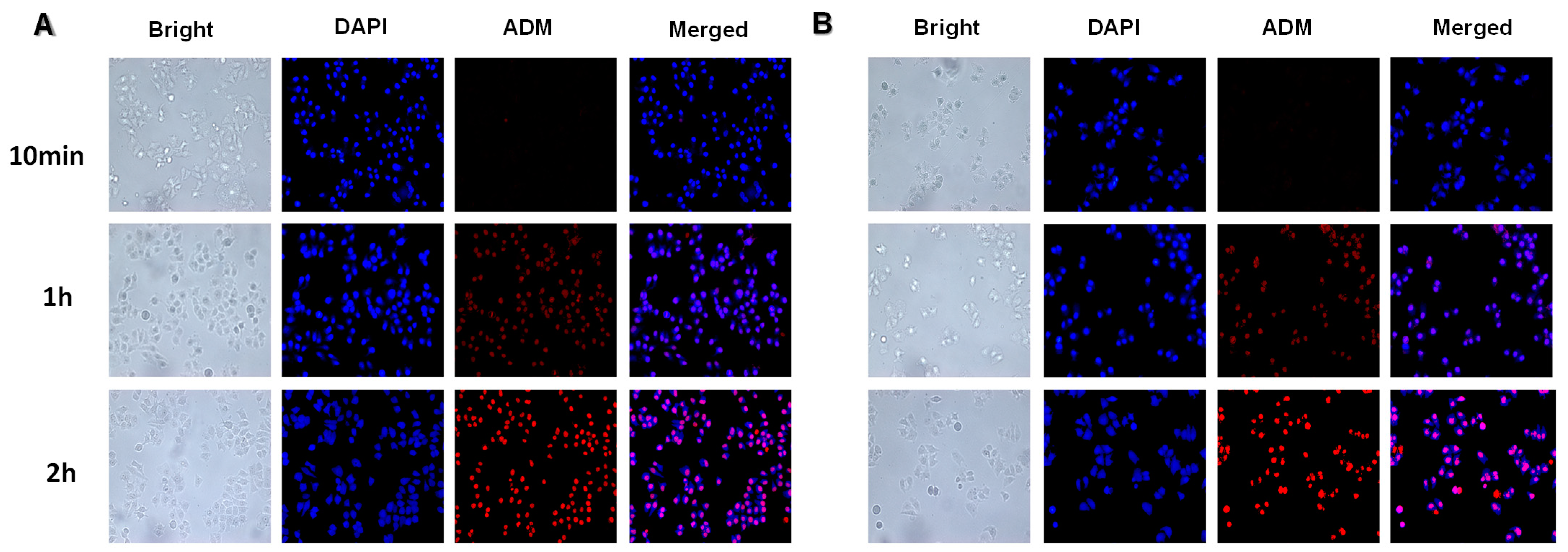
2.12. Cellular Internalization on Targeting of ADM/oHA-ss-PTX
2.13. In Vitro Migration and Invasion
2.14. In Vivo Biodistribution of Micelles
2.15. In Vivo Antitumor Efficacy
2.16. Organ Damage Assays
2.17. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of oHA-ss-PTX
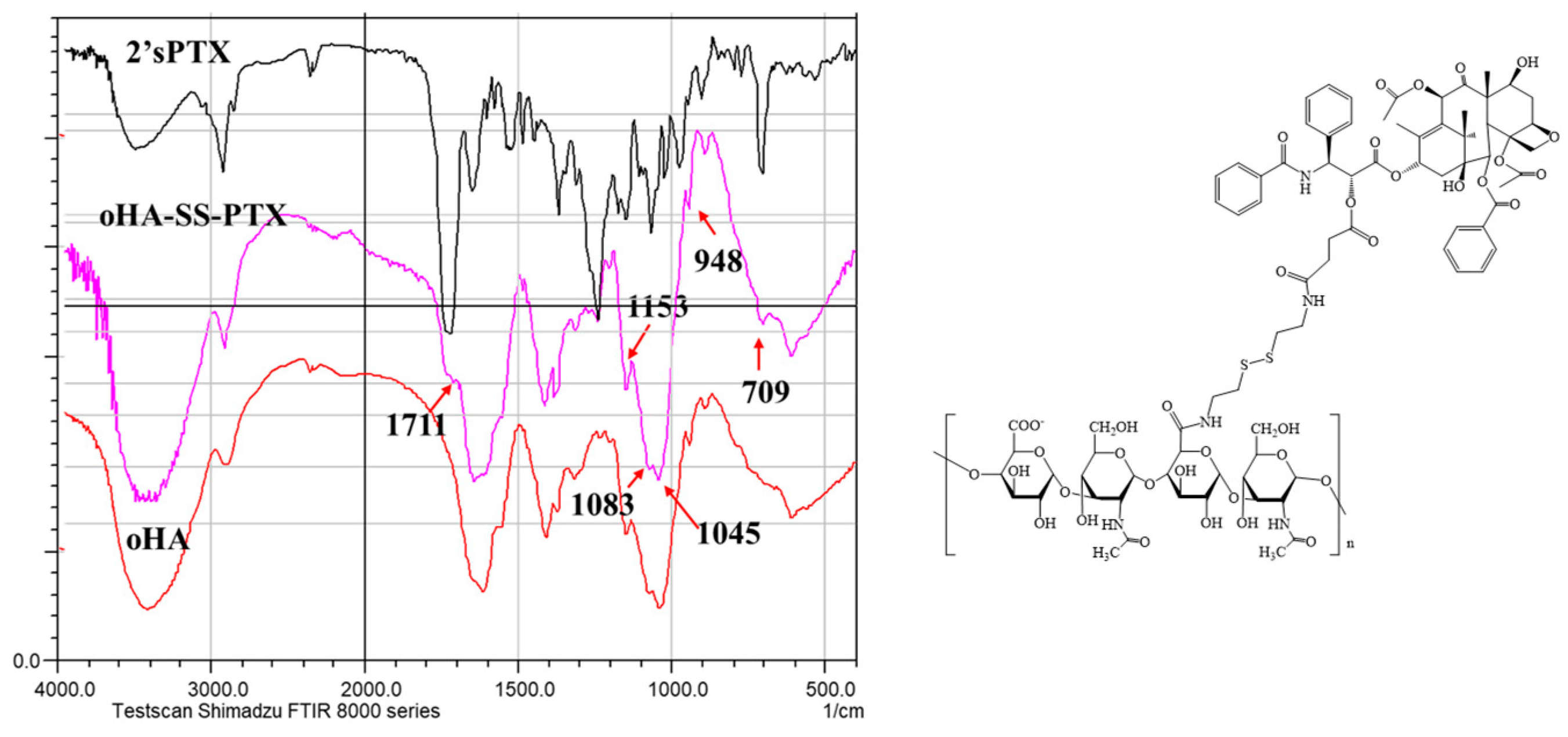
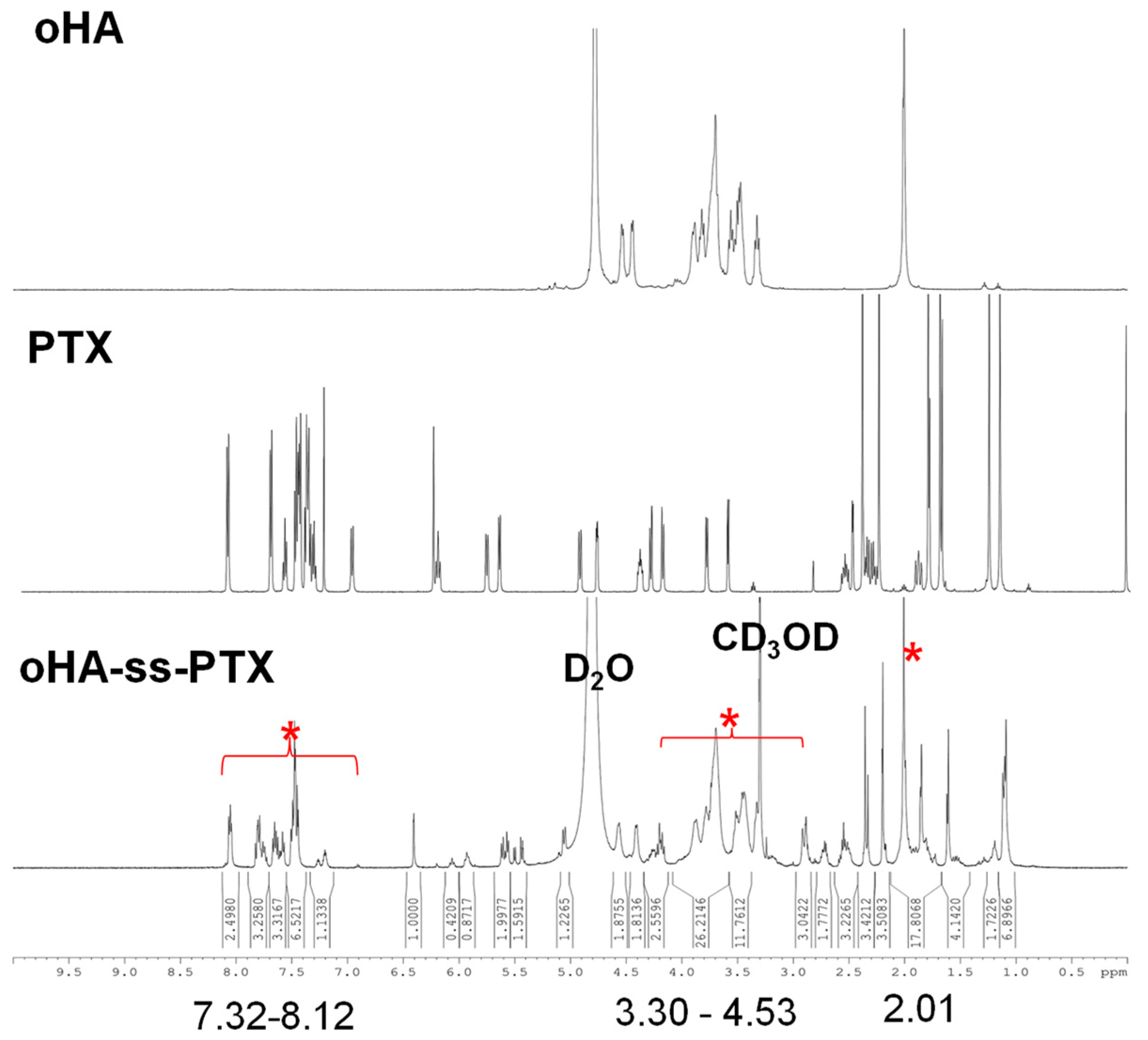
3.1.1. Analysis of FTIR and 1HNMR of oHA-ss-NH2
3.1.2. Analysis of FTIR and 1HNMR of 2′sPTX
3.1.3. Analysis of FTIR and 1HNMR of oHA-ss-PTX
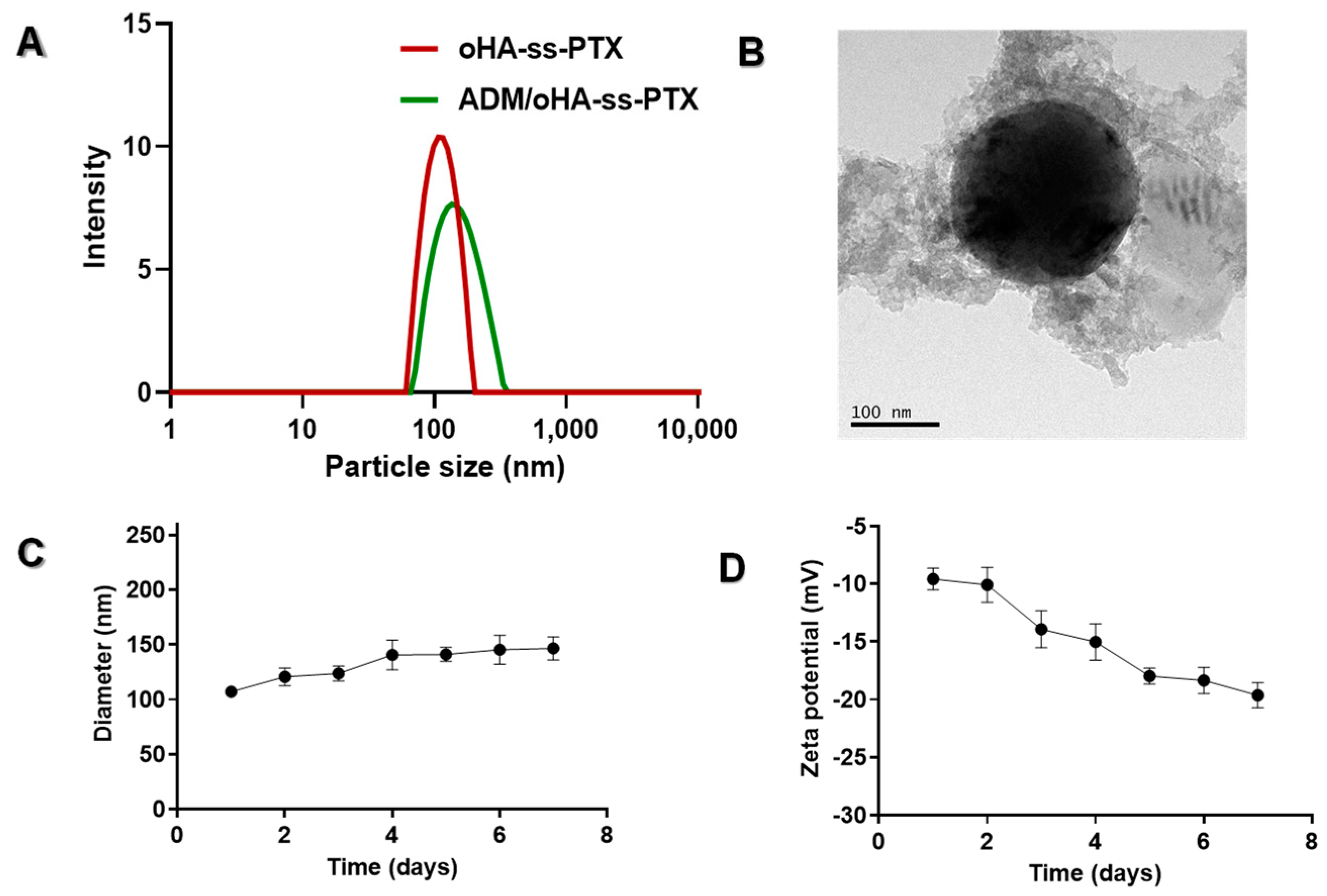
3.2. Characterization of Micelles
3.2.1. Preparation Process Screening of Prodrug oHA-ss-PTX Micelles
3.2.2. Physicochemical Characteristics of the ADM/oHA-ss-PTX Micelles
3.2.3. Stability Evaluation of ADM/oHA-ss-PTX Micelles
3.3. In Vitro Drug Release of ADM/oHA-ss-PTX Micelles
3.4. Biocompatibility of ADM/oHA-ss-PTX Micelles
3.4.1. Cytotoxicity of oHA-ss-NH2 and ADM/oHA-ss-PTX
3.4.2. Hemolysis Rate of ADM/oHA-ss-PTX Micelles
3.4.3. BSA Adsorption of ADM/oHA-ss-PTX Micelles
3.5. Cellular Proliferation Inhibitions and Cell Apoptosis
3.6. Cellular Internalization
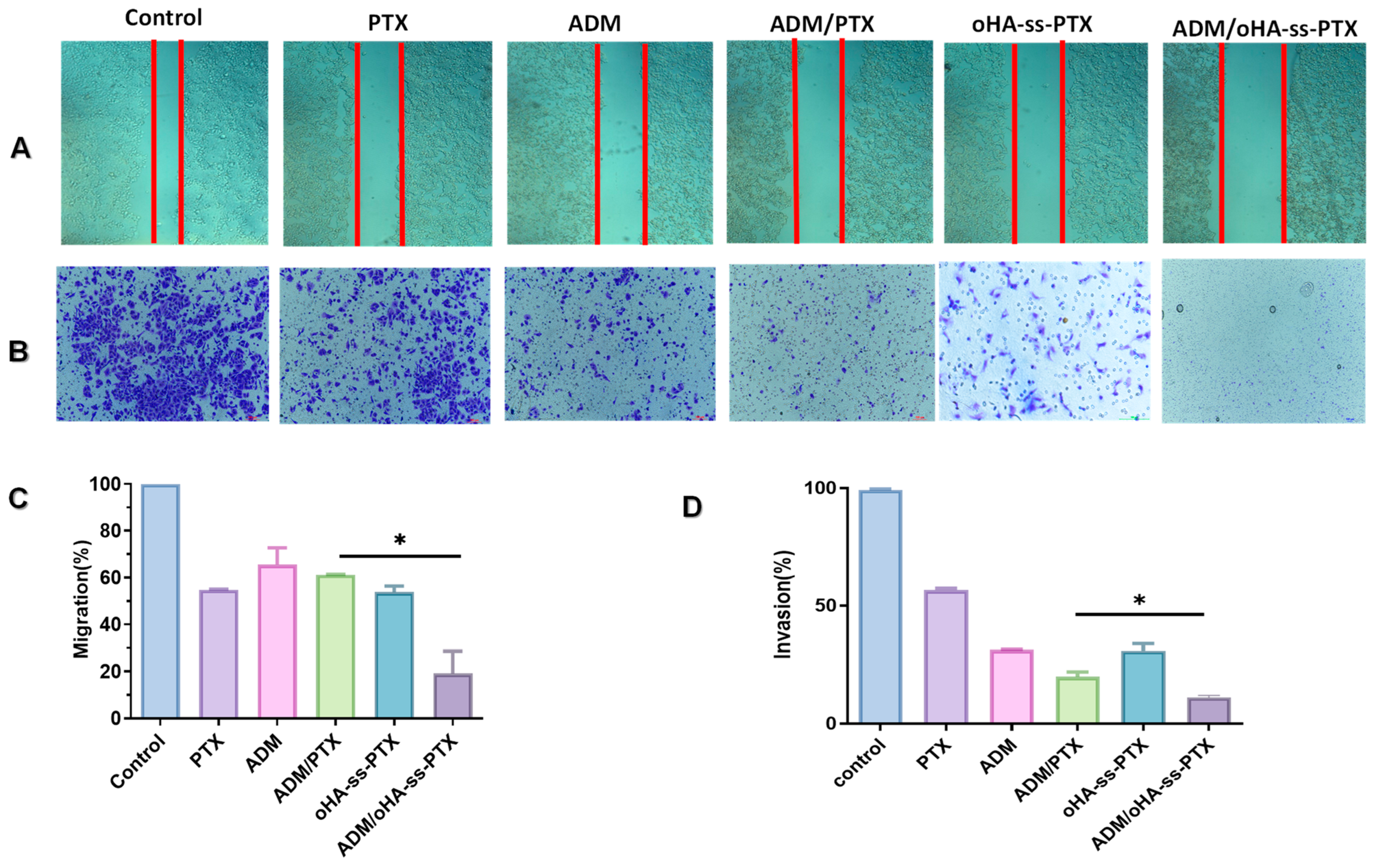
3.7. In Vitro Antimetastatic Effects
3.8. In Vivo Fluorescence Imaging
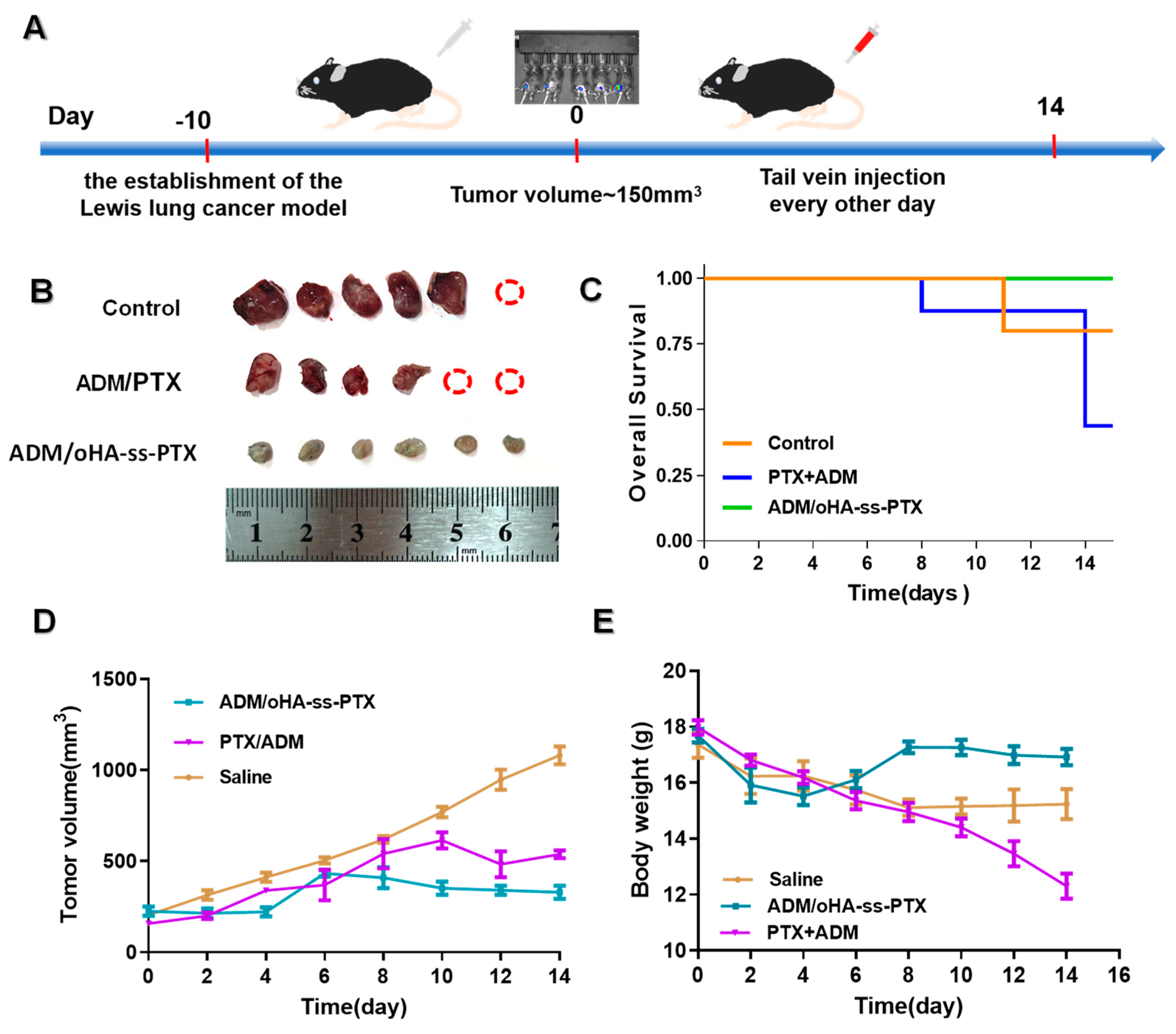
3.9. In Vivo Antitumor Efficacies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- McGinnis, C.S.; Miao, Z.; Superville, D.; Yao, W.; Goga, A.; Reticker-Flynn, N.E.; Winkler, J.; Satpathy, A.T. The temporal progression of lung immune remodeling during breast cancer metastasis. Cancer Cell 2024, 42, 1018–1031.e6. [Google Scholar] [CrossRef]
- Ma, W.; Kang, G.Y.; Sun, L.; Meng, C.; Liu, Y.; Zheng, Z.; Jiang, M.C.; Wang, D.; Pun, S.H.; Yu, C.Y.; et al. Multicyclic topology-enhanced anticancer drug delivery. J. Control. Release 2022, 345, 278–291. [Google Scholar] [CrossRef]
- Qin, Y.; Ling, X.; Li, Y.; Wang, J.; Wang, J.; Rong, Z.; Cheng, Y.; Tao, Z.; Zhang, H.; Wei, H.; et al. Histidine phosphatase-ferroptosis crosstalk modulation for efficient hepatocellular carcinoma treatment. J. Nanobiotechnol. 2024, 22, 622. [Google Scholar] [CrossRef]
- Monteran, L.; Ershaid, N.; Doron, H.; Zait, Y.; Scharff, Y.; Ben-Yosef, S.; Avivi, C.; Barshack, I.; Sonnenblick, A.; Erez, N. Chemotherapy-induced complement signaling modulates immunosuppression and metastatic relapse in breast cancer. Nat. Commun. 2022, 13, 5797. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Y.; Wang, Z.; Wang, H.; Zhang, D.; Cai, J.; Zhang, B.; Zhang, J.; Huang, M.; Pircher, A.; et al. Tumor immune microenvironment analysis of non-small cell lung cancer development through multiplex immunofluorescence. Transl. Lung Cancer Res. 2024, 13, 2395–2410. [Google Scholar] [CrossRef]
- Wu, T.Y.; Cao, W.J.; Li, Z.L.; Gong, Y.C.; Xiong, X.Y. Co-Delivery of paclitaxel and doxorubicin in folate-Targeted pluronic/ploy (D,L-lactide-b-glycolide) polymersomes. J. Biomater. Appl. 2023, 37, 1555–1567. [Google Scholar] [CrossRef]
- Rezaei, N.; Arki, M.K.; Miri-Lavasani, Z.; Solhi, R.; Khoramipour, M.; Rashedi, H.; Aghdaei, H.A.; Hossein-Khannazer, N.; Mostafavi, E.; Vosough, M. Co-delivery of doxorubicin and paclitaxel via noisome nanocarriers attenuates cancerous phenotypes in gastric cancer cells. Eur. J. Pharm. Biopharm. 2023, 188, 33–47. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-Delivery of Paclitaxel and Doxorubicin by pH-Responsive Prodrug Micelles for Cancer Therapy. Int. J. Nanomed. 2020, 15, 3319–3331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, H.; Guo, X.; Hou, M.; Zhang, X.; Li, S.; Wang, C.; Zhao, G.; Li, W.; Zhang, X.; et al. Calcium Orthophosphate in Liposomes for Co-Delivery of Doxorubicin Hydrochloride/Paclitaxel in Breast Cancer. Mol. Pharm. 2023, 20, 3914–3924. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Hu, C.; Wang, J.; Gao, X.; Xia, J.; Li, W.; Song, P.; Zhang, W.; Ge, F.; Zhu, L. Pluronic-Based Nanoparticles for Delivery of Doxorubicin to the Tumor Microenvironment by Binding to Macrophages. ACS Nano 2024, 18, 14441–14456. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, X.; Kang, L.; Feng, S.; Li, Y.; Zhao, G. Peptide-modified nanoparticles for doxorubicin delivery: Strategies to overcome chemoresistance and perspectives on carbohydrate polymers. Int. J. Biol. Macromol. 2025, 299, 140143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, S.; Liu, Y.; Sun, W.; Zhou, T.; Zhao, L.; Li, Z.; Duan, Y. Ultrasound targeted microbubble destruction assisted exosomal delivery of siHmox1 effectively inhibits doxorubicin-induced cardiomyocyte ferroptosis. J. Nanobiotechnol. 2024, 22, 531. [Google Scholar] [CrossRef]
- Guo, C.; Yin, J.; Chen, D. Co-encapsulation of curcumin and resveratrol into novel nutraceutical hyalurosomes nano-food delivery system based on oligo-hyaluronic acid-curcumin polymer. Carbohydr. Polym. 2018, 181, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, B.; Feng, Y.; Zhao, L.; Wang, Q.; He, H.; Yin, T.; Zhang, Y.; Yang, L.; Gou, J.; et al. Dual-Targeted Self-Adjuvant Heterocyclic Lipidoid@Polyester Hybrid Nanovaccines for Boosting Cancer Immunotherapy. ACS Nano 2024, 18, 15557–15575. [Google Scholar] [CrossRef]
- Shao, W.; Yang, Y.; Shen, W.; Ren, L.; Wang, W.W.; Zhu, P. Hyaluronic acid-conjugated methotrexate and 5-fluorouracil for targeted drug delivery. Int. J. Biol. Macromol. 2024, 273, 132671. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Qi, Z.; Meng, X.; Hu, M.; Liu, X.; Song, Y.; Deng, Y. Deuterated colchicine liposomes based on oligomeric hyaluronic acid modification enhance anti-tumor effect and reduce systemic toxicity. Int. J. Pharm. 2023, 632, 122578. [Google Scholar] [CrossRef]
- Chi, R.; Pan, L.; Yang, Z.; Yang, X.; Xia, H.; Lin, D.; Hao, J.; Si, X.; Yan, D.; Li, H.; et al. Oxaliplatin-loaded amphiphilic hyaluronic acid nanohydrogel formed via interfacial reactions enhances the therapeutic effect of targeted tumor. Int. J. Biol. Macromol. 2025, 284, 138118. [Google Scholar] [CrossRef]
- Ke, C.; Wang, D.; Sun, Y.; Qiao, D.; Ye, H.; Zeng, X. Immunostimulatory and antiangiogenic activities of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2013, 58, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant activity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef]
- Kang, R.; Song, M.; Fang, Z.; Liu, K. Nano-composite hydrogels of Cu-Apa micelles for anti-vasculogenic mimicry. J. Drug Target. 2023, 31, 166–178. [Google Scholar] [CrossRef]
- Guo, C.; Su, Y.; Wang, B.; Liu, K. Novel polysaccharide building hybrid nanoparticles: Remodelling TAMs to target ERα-positive breast cancer. J. Drug Target. 2022, 30, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fu, C.; Liu, H.; Yin, Y.; Cao, Y.; Feng, J.; Zhang, A.; Wang, W. Chemoimmunotherapeutic Nanogel for Pre- and Postsurgical Treatment of Malignant Melanoma by Reprogramming Tumor-Associated Macrophages. Nano Lett. 2024, 24, 1717–1728. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Lai, W.; Gao, Y.; Liao, S.; Ning, Q.; Tang, S. Tumor Microenvironment Responsive RNA Drug Delivery Systems: Intelligent Platforms for Sophisticated Release. Mol. Pharm. 2024, 21, 4217–4237. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, e1803549. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Wang, C.; Galarraga, J.H.; Puré, E.; Han, L.; Burdick, J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 2019, 222, 119451. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Sandra, F.C.; Park, D.Y.; Lee, J.Y.; Oh, K.S.; Kim, D.; Byun, Y.; Kim, I.S.; Kwon, I.C.; Kim, S.Y.; et al. Doxorubicin/Heparin Composite Nanoparticles for Caspase-Activated Prodrug Chemotherapy. Biomaterials 2016, 101, 131–142. [Google Scholar] [CrossRef]
- Ding, J.; Xiao, C.; Li, Y.; Cheng, Y.; Wang, N.; He, C.; Zhuang, X.; Zhu, X.; Chen, X. Efficacious hepatoma-targeted nanomedicine self-assembled from galactopeptide and doxorubicin driven by two-stage physical interactions. J. Control. Release 2013, 169, 193–203. [Google Scholar] [CrossRef]
- Biancacci, I.; Sun, Q.; Möckel, D.; Gremse, F.; Rosenhain, S.; Kiessling, F.; Bartneck, M.; Hu, Q.; Thewissen, M.; Storm, G.; et al. Optical imaging of the whole-body to cellular biodistribution of clinical-stage PEG-b-pHPMA-based core-crosslinked polymeric micelles. J. Control. Release 2020, 328, 805–816. [Google Scholar] [CrossRef]
- Attia, A.B.; Yang, C.; Tan, J.P.; Gao, S.; Williams, D.F.; Hedrick, J.L.; Yang, Y.Y. The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials 2013, 34, 3132–3140. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Luo, Z.; Wang, S.; Lin, J.; Xie, Z.; Li, M.; Li, C.; Cao, H.; Huang, Q.; et al. Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J. Cell Physiol. 2019, 234, 6611–6623. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Hwang, I.; Zhang, J.; Chen, Z.; Han, J.; Jeon, J.; Koo, B.K.; Kim, S.; Lee, J.E.; Kim, Y.; et al. Exploration of drug resistance mechanisms in triple negative breast cancer cells using a microfluidic device and patient tissues. Elife 2024, 12, RP88830. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cheng, H.; Teng, M.; Zhang, H.; Chen, H.; Qu, S.; Liu, G. Optimizing interventional therapy: A homogeneous lipiodol formulation of Tirapazamine and Sorafenib responsive to post-embolization microenvironment. J. Control. Release 2025, 379, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, H.; Li, Q.; Cai, J.; Miao, Z.; Xie, P.; Tang, S.; He, D. Carrier-free nanoparticles based on self-assembly of 5-FU and copper-genistein complexes for the combined treatment of hepatocellular carcinoma. Drug Deliv. Transl. Res. 2025, 15, 1299–1316. [Google Scholar] [CrossRef]
- Yin, S.; Huai, J.; Chen, X.; Yang, Y.; Zhang, X.; Gan, Y.; Wang, G.; Gu, X.; Li, J. Intracellular delivery and antitumor effects of a redox-responsive polymeric paclitaxel conjugate based on hyaluronic acid. Acta Biomater. 2015, 26, 274–285. [Google Scholar] [CrossRef]
- Xu, C.; He, W.; Lv, Y.; Qin, C.; Shen, L.; Yin, L. Self-assembled nanoparticles from hyaluronic acid-paclitaxel prodrugs for direct cytosolic delivery and enhanced antitumor activity. Int. J. Pharm. 2015, 493, 172–181. [Google Scholar] [CrossRef]
- Wang, J.; Abbas, M.; Huang, Y.; Wang, J.; Li, Y. Redox-responsive peptide-based complex coacervates as delivery vehicles with controlled release of proteinous drugs. Commun. Chem. 2023, 6, 243. [Google Scholar] [CrossRef]
- Yadav, S.; Ramesh, K.; Reddy, O.S.; Karthika, V.; Kumar, P.; Jo, S.H.; Yoo, S.I.; Park, S.H.; Lim, K.T. Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application. Pharmaceutics 2023, 15, 1159. [Google Scholar] [CrossRef]
- Kang, X.; Bu, F.; Feng, W.; Liu, F.; Yang, X.; Li, H.; Yu, Y.; Li, G.; Xiao, H.; Wang, X. Dual-Cascade Responsive Nanoparticles Enhance Pancreatic Cancer Therapy by Eliminating Tumor-Resident Intracellular Bacteria. Adv. Mater. 2022, 34, e2206765. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Reed, E.H.; Parthasarathy, R.; Jahnke, C.N.; Caldwell, R.M.; Bermudez, J.G.; Ramage, H.; Good, M.C.; Hammer, D.A. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 2018, 9, 2985. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef]
- Zheng, H.; Tao, H.; Wan, J.; Lee, K.Y.; Zheng, Z.; Leung, S.S.Y. Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers. Pharmaceutics 2022, 14, 1223. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Q.; Yu, G.; Yi, W.; Gu, M.; Xu, Q.; Ye, Z.; Zhang, M.; Tang, S. Development of GSH-Stimuli-Responsive Micelles Using a Targeted Paclitaxel Prodrug for Enhanced Anticancer Effect. Pharmaceutics 2025, 17, 538. https://doi.org/10.3390/pharmaceutics17040538
Ning Q, Yu G, Yi W, Gu M, Xu Q, Ye Z, Zhang M, Tang S. Development of GSH-Stimuli-Responsive Micelles Using a Targeted Paclitaxel Prodrug for Enhanced Anticancer Effect. Pharmaceutics. 2025; 17(4):538. https://doi.org/10.3390/pharmaceutics17040538
Chicago/Turabian StyleNing, Qian, Guangping Yu, Wenkai Yi, Minhui Gu, Qianqian Xu, Zhiting Ye, Mengxia Zhang, and Shengsong Tang. 2025. "Development of GSH-Stimuli-Responsive Micelles Using a Targeted Paclitaxel Prodrug for Enhanced Anticancer Effect" Pharmaceutics 17, no. 4: 538. https://doi.org/10.3390/pharmaceutics17040538
APA StyleNing, Q., Yu, G., Yi, W., Gu, M., Xu, Q., Ye, Z., Zhang, M., & Tang, S. (2025). Development of GSH-Stimuli-Responsive Micelles Using a Targeted Paclitaxel Prodrug for Enhanced Anticancer Effect. Pharmaceutics, 17(4), 538. https://doi.org/10.3390/pharmaceutics17040538





