Nanostructured Lipid Carrier-Filled Hydrogel Beads for the Delivery of Curcumin: Digestion, Intestinal Permeation, and Antioxidant Bioactivity After Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Curcumin-Loaded NLCs
2.3. Fabrication of Curcumin-Loaded NLC-Hydrogel Beads
2.4. Determination of Encapsulation Efficiency
2.5. Determination of Particle Size
2.6. Scanning Electron Microscope (SEM)
2.7. X-Ray Diffraction (XRD)
2.8. Rheological Analysis
2.9. Stability Study
2.10. DPPH Free Radical Scavenging Assay
2.11. Swelling and Dissolution Study
2.12. Dissolution Study
2.13. In Vitro Simulated Digestion Study
2.14. Ex Vivo Intestinal Permeation Assessment
2.15. Antioxidant Bioactivity Study
2.16. Statistical Analysis
3. Results and Discussion
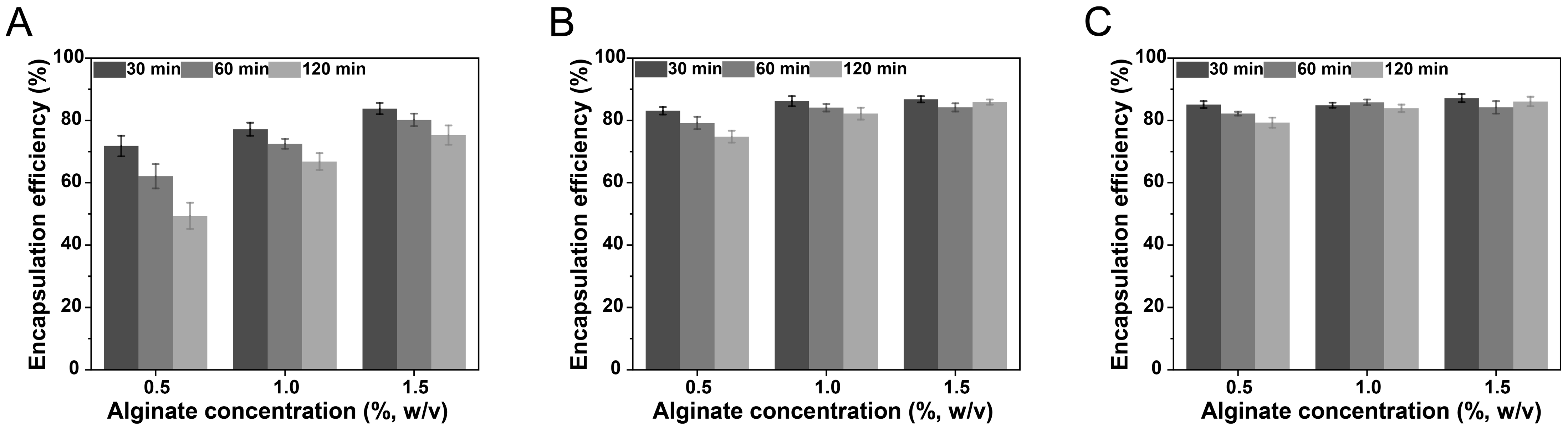
3.1. Encapsulation Efficiency of Curcumin-Loaded NLC-Hydrogel Beads
3.2. Physicochemical Characteristics
3.3. Storage Stability
3.4. In Vitro Antioxidant Study
3.5. Swelling and Dissolution Characteristics
3.6. In Vitro Digestion Study
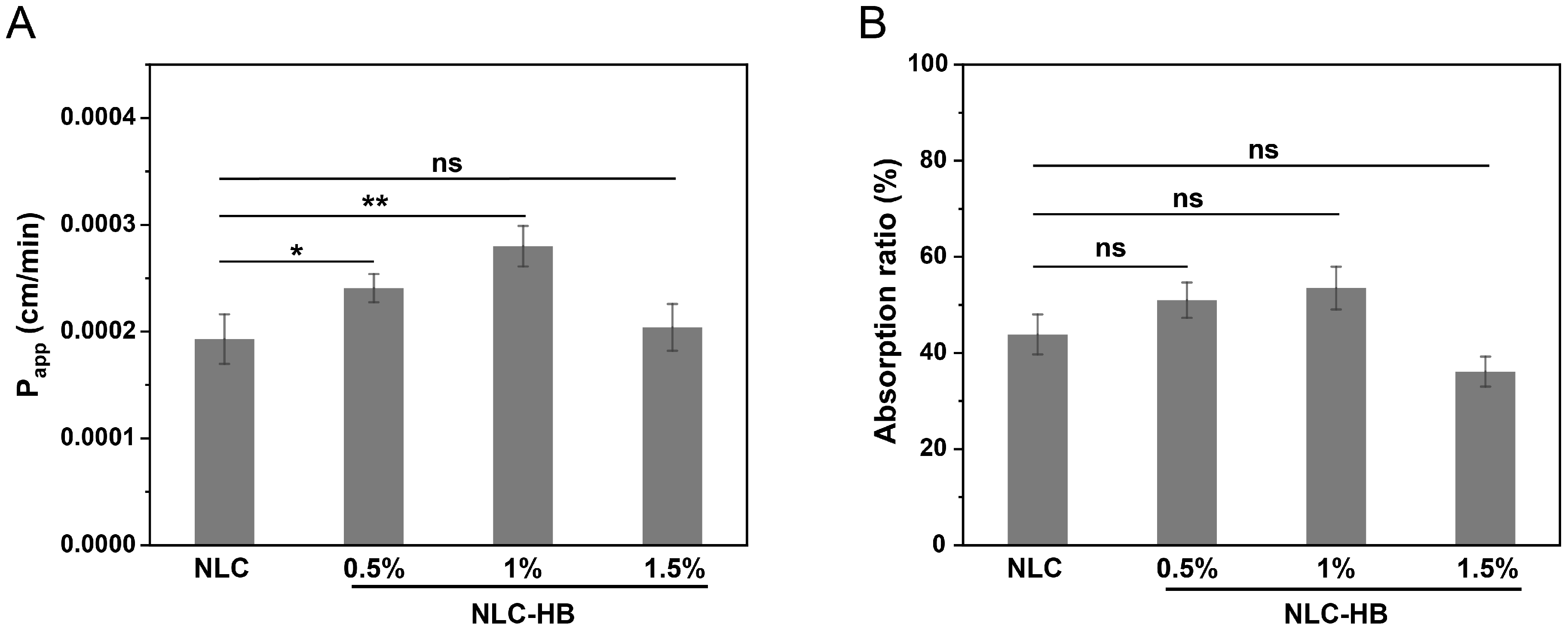
3.7. Ex Vivo Permeation and Absorption of Curcumin
3.8. Antioxidant Bioactivity of Digestion Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kan, G.; Chen, L.; Zhang, W.; Bian, Q.; Wang, X.; Zhong, J. Recent advances in the development and application of curcumin-loaded micro/nanocarriers in food research. Adv. Colloid Interface Sci. 2025, 335, 103333. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Assadpour, E.; Jafari, S.M. Curcumin-protein complexes: Technological and biological functionalities. Trends Food Sci. Technol. 2024, 145, 104372. [Google Scholar] [CrossRef]
- Han, D.; Ji, L.; Lu, M.; Li, D.; Sheng, X.; Zhang, J.; Wang, C. Pickering emulsion stabilized by egg derived reconstituted lipid nanoparticles for encapsulation and oral delivery of curcumin. Food Chem. 2025, 472, 142912. [Google Scholar] [CrossRef]
- Goncalves, R.F.S.; Fernandes, J.M.; Martins, J.T.; Vieira, J.M.; Abreu, C.S.; Pinheiro, A.C. Incorporation of curcumin-loaded solid lipid nanoparticles into yogurt: Tribo-rheological properties and dynamic in vitro digestion. Food Res. Int. 2024, 181, 114112. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Toledo, J.; Herrera, J.; Morales, J.; Robert, P.; Oyarzun-Ampuero, F.; Gimenez, B. Bioaccessibility of chlorogenic acid and curcumin co-encapsulated in double emulsions with the inner interface stabilized by functionalized silica nanoparticles. Food Chem. 2024, 445, 138828. [Google Scholar] [CrossRef]
- Zhu, Y.-a.; Sun, P.; Duan, C.; Cao, Y.; Kong, B.; Wang, H.; Chen, Q. Improving stability and bioavailability of curcumin by quaternized chitosan coated nanoemulsion. Food Res. Int. 2023, 174, 113634. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, M.; Song, Z.; Jia, Y.; Li, Y.; Liu, Q.; Wang, F.; Zhai, G. pH-responsive hydrogel containing curcumin-loaded lipid nanocapsules for oral curcumin’s stability, bioaccessibility and intestinal absorption improvement. Food Hydrocoll. 2025, 158, 110488. [Google Scholar] [CrossRef]
- Chaves, M.A.; Dacanal, G.C.; Pinho, S.C. High-shear wet agglomeration process for enriching cornstarch with curcumin and vitamin D3 co-loaded lyophilized liposomes. Food Res. Int. 2023, 169, 112809. [Google Scholar] [CrossRef]
- Mehnert, W.; Maeder, K. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, L. Nanoparticles fabricated from bulk solid lipids: Preparation, properties, and potential food applications. Adv. Colloid Interface Sci. 2019, 273, 102033. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Corona, A., III; Schubert, B.; Reeder, R.; Henson, M.A. The effect of oil type on the aggregation stability of nanostructured lipid carriers. J. Colloid Interface Sci. 2014, 418, 261–272. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T. Observation of chitosan coated lipid nanoparticles with different lipid compositions under simulated in vitro digestion system. Food Hydrocoll. 2018, 84, 146–153. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing β-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y.; Zhang, M.; Xiao, H.; Peng, Y.; Song, M.; Cao, Y.; Xiao, J. Engineering mucus-penetrating and enzyme-responsive nanostructured carriers for precision targeting of curcumin’s pharmacokinetics and colitis-alleviating pathways. Nano Today 2025, 61, 102602. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Morhard, R.; Mauda-Havakuk, M.; Kassin, M.; Arrichiello, A.; Wood, B.J. Hydrogel drug delivery systems for minimally invasive local immunotherapy of cancer. Adv. Drug Deliv. Rev. 2023, 202, 115083. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Composite hydrogels assembled from food-grade biopolymers: Fabrication, properties, and applications. Adv. Colloid Interface Sci. 2024, 332, 103278. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel beads for designing future foods: Structures, mechanisms, applications, and challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Li, Y.; McClements, D.J. Construction of biopolymer-based hydrogel beads for encapsulation, retention, and colonic delivery of tributyrin: Development of functional beverages (fortified bubble tea). Food Res. Int. 2024, 197, 115165. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Zhang, H.; Hossen, M.A.; Sameen, D.E.; Dai, J.; Qin, W.; Liu, Y.; Li, S. In vitro digestion of sodium alginate/pectin co-encapsulated Lactobacillus bulgaricus and its application in yogurt bilayer beads. Int. J. Biol. Macromol. 2021, 193, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Microgels—An alternative colloidal ingredient for stabilization of food emulsions. Trends Food Sci. Technol. 2015, 43, 178–188. [Google Scholar] [CrossRef]
- Paswan, M.; Chandel, A.K.S.; Malek, N.I.; Dholakiya, B.Z. Preparation of sodium alginate/Cur-PLA hydrogel beads for curcumin encapsulation. Int. J. Biol. Macromol. 2024, 254, 128005. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Smaldone, G.; Cimmino, L.; Braile, M.; Orlandella, F.M.; Luciano, N.; Accardo, A.; Salvatore, G. Fmoc-FF Nanogel-Mediated Delivery of Doxorubicin and Curcumin in Thyroid Cancer Cells. Pharmaceutics 2025, 17, 263. [Google Scholar] [CrossRef]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-loaded alginate hydrogels for cancer therapy and wound healing applications: A review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical aspects of lipase immobilization at polysaccharides for biotechnology. Adv. Colloid Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Li, J.; Gao, Y.; Mao, L. Modification of the interface of oleogel-hydrogel bigel beads for enhanced stability and prolonged release of bioactives. Food Chem. 2025, 468, 142448. [Google Scholar] [CrossRef]
- Kour, P.; Afzal, S.; Gani, A.; Zargar, M.I.; Tak, U.N.; Rashid, S.; Dar, A.A. Effect of nanoemulsion-loaded hybrid biopolymeric hydrogel beads on the release kinetics, antioxidant potential and antibacterial activity of encapsulated curcumin. Food Chem. 2022, 376, 131925. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef]
- Le, T.T.N.; Nguyen, T.K.N.; Nguyen, V.M.; Dao, T.C.M.; Nguyen, H.B.C.; Dang, C.T.; Le, T.B.C.; Nguyen, T.K.L.; Nguyen, P.T.T.; Dang, L.H.N.; et al. Development and Characterization of a Hydrogel Containing Curcumin-Loaded Nanoemulsion for Enhanced In Vitro Antibacteria and In Vivo Wound Healing. Molecules 2023, 28, 6433. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, F.L.; Stahl, M.A.; Grimaldi, R.; Forte, M.B.S.; Gigante, M.L.; Ribeiro, A.P.B. Optimization of high pressure homogenization conditions to produce nanostructured lipid carriers using natural and synthetic emulsifiers. Food Res. Int. 2022, 160, 111746. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Shi, S.; He, J.; Liu, W.; Li, Y.; Zeng, X.; Pang, J.; Wu, C. Preparation and characterization of pH-sensitive calcium alginate hydrogel beads as delivery carriers for the controlled release of fucoxanthin. Food Hydrocoll. 2025, 163, 111106. [Google Scholar] [CrossRef]
- Leonida, M.; Ispas-Szabo, P.; Mateescu, M.A. Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery. Bioact. Mater. 2018, 3, 334–340. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, C.; Li, Y.; Pan, Y.; Huang, Q. Pickering emulsions immobilized within hydrogel matrix with enhanced resistance against harsh processing conditions and sequential digestion. Food Hydrocoll. 2017, 62, 35–42. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Z.; Chen, X.D.; Wu, P. Unlocking the release, digestion and absorption kinetics of DHA from different fish oil delivery systems: An in vitro and ex vivo study. Food Hydrocoll. 2025, 162, 110966. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, Y.; Luo, G.; Zeng, Y.; McClements, D.J.; Hu, K. Curcumin encapsulated zein/caseinate-alginate nanoparticles: Release and antioxidant activity under in vitro simulated gastrointestinal digestion. Curr. Res. Food Sci. 2023, 6, 100463. [Google Scholar] [CrossRef]
- Lin, S.; Cai, X.; Chen, H.; Xu, Y.; Wu, J.; Wang, S. Development of fish gelatin-chitooligosaccharide conjugates through the Maillard reaction for the encapsulation of curcumin. Curr. Res. Food Sci. 2022, 5, 1625–1639. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015, 50, 27–36. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured lipid carriers (NLCs) stabilized by natural or synthetic emulsifiers for lutein delivery: Improved physicochemical stability, antioxidant activity, and bioaccessibility. Food Chem. 2023, 403, 134465. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, Y.; Li, Y.; Li, H.; Li, L.; Xia, Q. Encapsulation of resveratrol-loaded Pickering emulsions in alginate/pectin hydrogel beads: Improved stability and modification of digestive behavior in the gastrointestinal tract. Int. J. Biol. Macromol. 2022, 222, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Peng, S.; Zhou, L.; McClements, D.J.; Fang, S.; Liu, W. Colonic delivery and controlled release of curcumin encapsulated within plant-based extracellular vesicles loaded into hydrogel beads. Food Res. Int. 2025, 202, 115540. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pilawa, B.; Ramos, P.; Glinka, R. Effect of UV Radiation and Temperature on Radical Scavenging Activity of Hippophaë rhamnoides L. and Vaccinium oxycoccos L. Fruit Extracts. Int. J. Mol. Sci. 2024, 25, 9810. [Google Scholar] [CrossRef]
- Zupanc, O.; Kushwah, V.; Paudel, A. Pancreatic lipase digestion: The forgotten barrier in oral administration of lipid-based delivery systems? J. Control. Release 2023, 362, 381–395. [Google Scholar] [CrossRef]
- Deng, B.; Kamperman, T.; Rangel, V.; Zoetebier-Liszka, B.; Schroen, K.; Corstens, M. Controlled digestion of lipids from oil-laden core-shell beads with tunable core and shell design. Food Hydrocoll. 2025, 163, 111024. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Yan, X.; Huang, J.; Huang, L.; Luo, C.; Li, Z.; Xu, P.; Tan, K.; Cheong, K.-L.; Tan, K. Effects of dietary lipids on bioaccessibility and bioavailability of natural carotenoids. LWT-Food Sci. Technol. 2024, 200, 116171. [Google Scholar] [CrossRef]
- Zuo, T.-T.; Liu, J.; Zan, K.; Liu, L.-N.; Wang, Q.; Wang, Z.; Xu, W.-Y.; Liu, Y.-X.; Guo, Y.-S.; Kang, S.; et al. Bioaccessibility and bioavailability of exogenous and endogenous toxic substances in traditional Chinese medicine and their significance in risk assessment. Pharmacol. Res. 2024, 208, 107388. [Google Scholar] [CrossRef]
- Jin, H.; Shang, L.; Xue, Y.; Wan, Y.; Liu, C.; Fan, Z.; Xu, J.; Zhao, Q. Lipolytic behavior and bioaccessibility of curcumin nanoemulsions stabilized by rice bran protein hydrolysate. LWT-Food Sci. Technol. 2023, 179, 114616. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, P.; Chen, Y.; Wang, L.; Jin, X.; Chen, X.D. Intestinal absorption of DHA microcapsules with different formulations based on ex vivo rat intestine and in vitro dialysis models. Food Funct. 2023, 14, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Pereira, C.; Ribeiro, A.; Castro, P.; Freixo, R.; Martins, E.; Tavares-Valente, D.; Fernandes, J.; Pintado, M.; Ramos, O. Biological Potential and Bioaccessibility of Encapsulated Curcumin into Cetyltrimethylammonium Bromide Modified Cellulose Nanocrystals. Pharmaceuticals 2023, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Chuesiang, P.; Zhang, J.; Choi, E.; Yoon, I.-S.; Kim, J.T.; Shin, G.H. Observation of curcumin-loaded hydroxypropyl methylcellulose (HPMC) oleogels under in vitro lipid digestion and in situ intestinal absorption in rats. Int. J. Biol. Macromol. 2022, 208, 520–529. [Google Scholar] [CrossRef]
- Ma, L.; Long, W.; Liu, Y. The fabrication of gastrointestinal pH-responsive sodium alginate surface-modified protein vehicles to improve limonin digestive stability, bioaccessibility and trans-intestinal mucus barrier capacity. Food Hydrocoll. 2025, 160, 110719. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Guo, J.-J.; Ma, L.-L.; Shi, H.-T.; Zhu, J.-B.; Wu, J.; Ding, Z.-W.; An, Y.; Zou, Y.-Z.; Ge, J.-B. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Wei, C.; Tang, X.; Sun, Y.; Ji, J. Nanostructured Lipid Carrier-Filled Hydrogel Beads for the Delivery of Curcumin: Digestion, Intestinal Permeation, and Antioxidant Bioactivity After Gastrointestinal Digestion. Pharmaceutics 2025, 17, 541. https://doi.org/10.3390/pharmaceutics17050541
Sun R, Wei C, Tang X, Sun Y, Ji J. Nanostructured Lipid Carrier-Filled Hydrogel Beads for the Delivery of Curcumin: Digestion, Intestinal Permeation, and Antioxidant Bioactivity After Gastrointestinal Digestion. Pharmaceutics. 2025; 17(5):541. https://doi.org/10.3390/pharmaceutics17050541
Chicago/Turabian StyleSun, Rui, Chenyu Wei, Xiaoyan Tang, Yufeng Sun, and Juling Ji. 2025. "Nanostructured Lipid Carrier-Filled Hydrogel Beads for the Delivery of Curcumin: Digestion, Intestinal Permeation, and Antioxidant Bioactivity After Gastrointestinal Digestion" Pharmaceutics 17, no. 5: 541. https://doi.org/10.3390/pharmaceutics17050541
APA StyleSun, R., Wei, C., Tang, X., Sun, Y., & Ji, J. (2025). Nanostructured Lipid Carrier-Filled Hydrogel Beads for the Delivery of Curcumin: Digestion, Intestinal Permeation, and Antioxidant Bioactivity After Gastrointestinal Digestion. Pharmaceutics, 17(5), 541. https://doi.org/10.3390/pharmaceutics17050541







