Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review
Abstract
1. Introduction
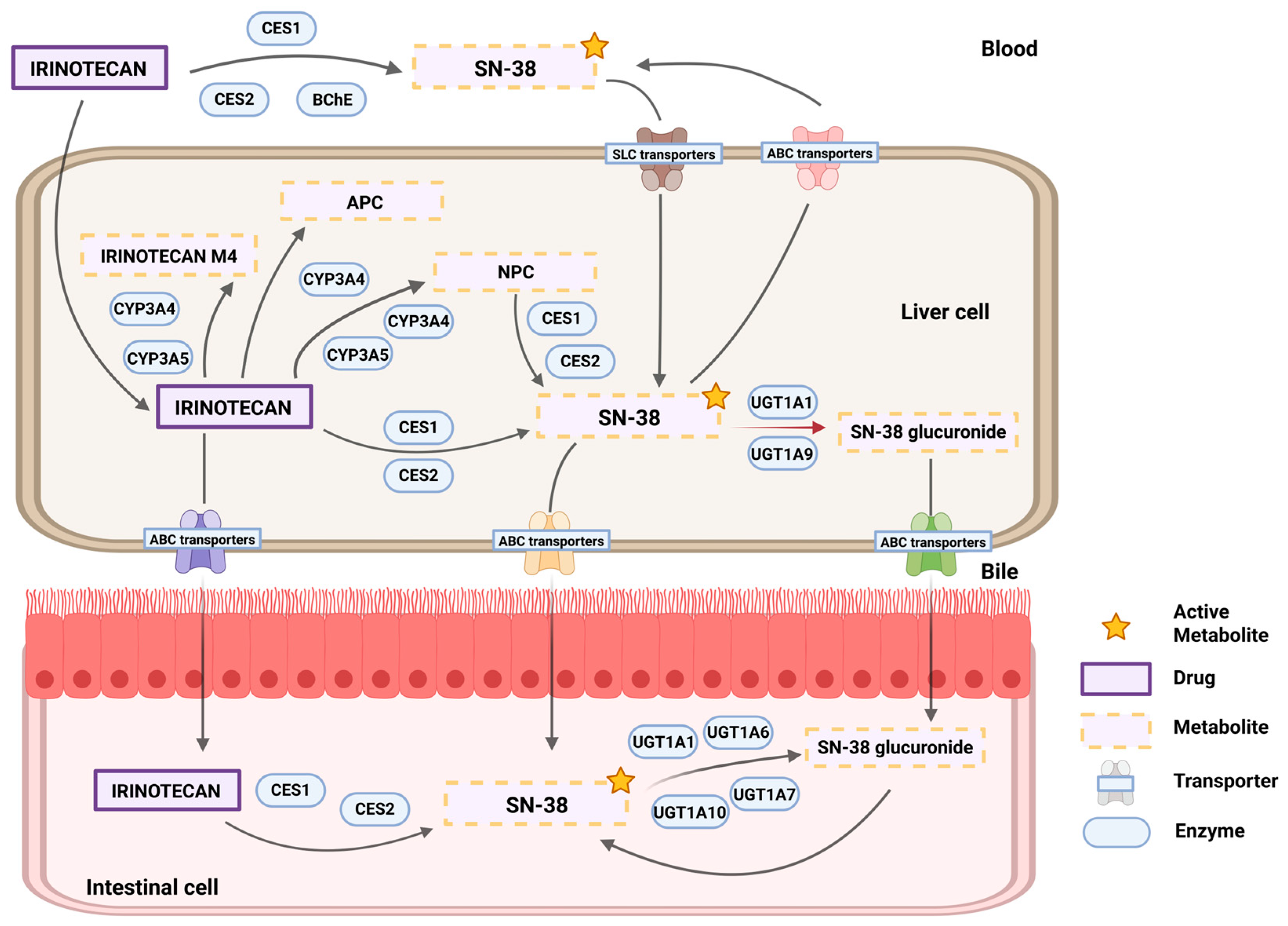
1.1. UGT1A1: Uridine Diphosphate (UDP) Glucuronosyltransferase Family 1 Member A1
1.2. Pharmacogenetics of the UGT1A1–Irinotecan Interaction
2. Materials and Methods
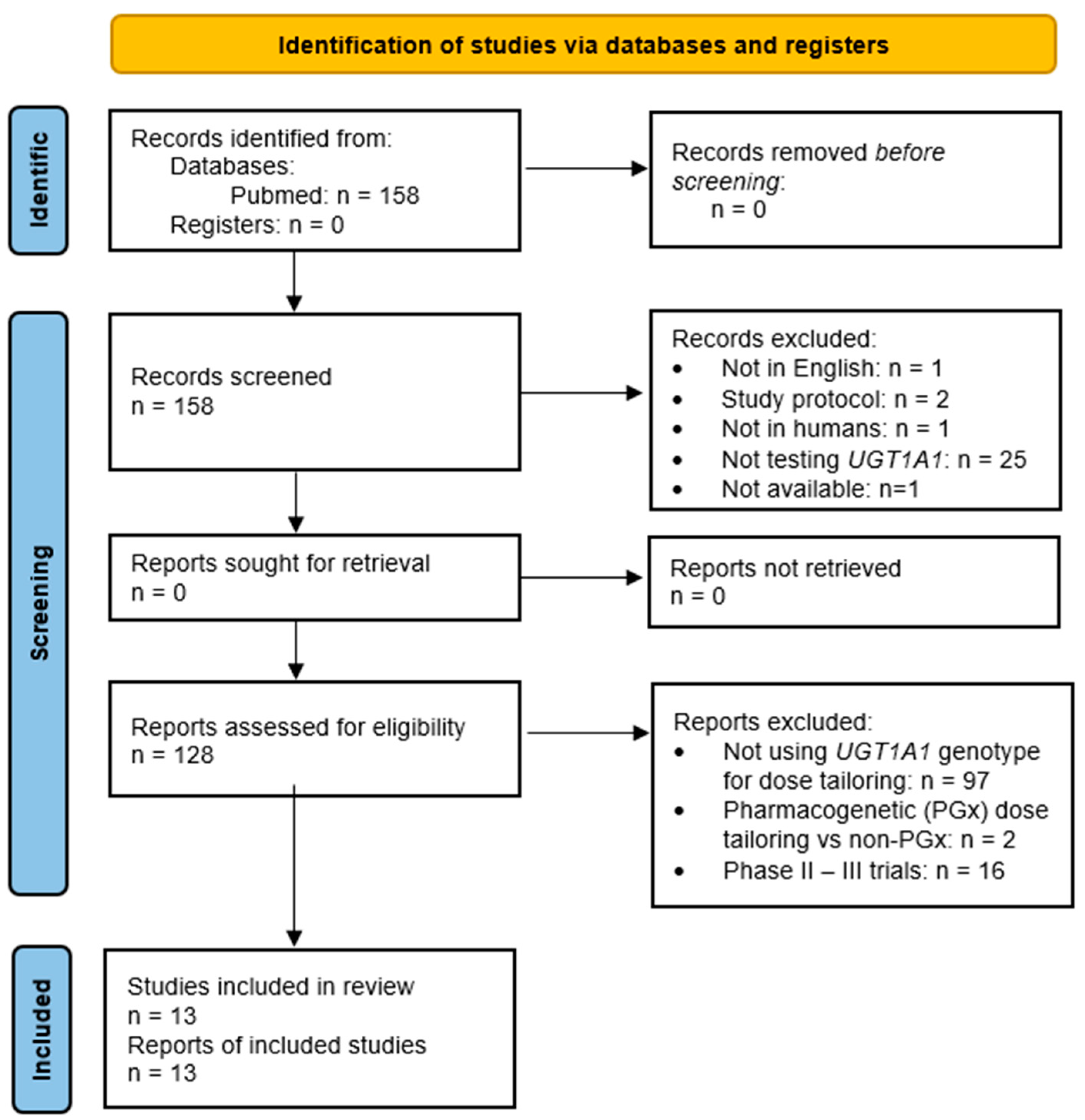
2.1. Search Strategy and Inclusion/Exclusion Criteria
2.2. Data Extraction and Quality Assessment
3. Results
3.1. Dose-Limiting Toxicities and Maximum-Tolerated Doses
3.2. Pharmacokinetics
4. Discussion
4.1. Limitations
4.2. Safety of Irinotecan-Containing Schemes
4.3. Usefulness of UGT1A1 Genotyping for Cancer Treatment Using Irinotecan
4.4. Insights Related to Pharmacogenetic Dosing Guidelines and Irinotecan SmPC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | Adverse drug event |
| ALFA | Allele frequency aggregator |
| APC | 7-Ethyl-10-[4-N-(5-aminopentanoic ac-id)-1-piperidino]carbonyloxycamptothecin |
| AUC | Area under the curve |
| CAPIRINOX | Capecitabine, irinotecan, and oxaliplatin |
| CES1-2 | Carboxylesterases type 1 and 2 |
| Cmax | Maximum concentration |
| CPIC | Clinical Pharmacogenenetic Implementation Consortium |
| CPNDS | Canadian Pharmacogenomics Network for Drug Safety |
| DLT | Dose-limiting toxicity |
| DPWG | Dutch Pharmacogenetics Working Group |
| EMA | European Medicines Agency |
| EPAR | European Public Assessment Report |
| ESMO | European Society for Medical Oncology |
| FDA | Food and Drug Administration |
| G1-4 | Grade 1-4 |
| GI | Gastrointestinal |
| IBS | Iberian Peninsula |
| IHL-305 | A pegylated liposome containing irinotecan |
| IM | Intermediate metabolizer |
| JCR | Journal Citation Report |
| MAF | Minor allele frequency |
| Nal-IRI | A irinotecan liposome injection |
| NCI | National Cancer Institute |
| NK012 | An SN-38 (irinotecan active metabolite) incorporating macromolecular polymeric micelle |
| NM | Normal metabolizer |
| NOS | Newcastle–Ottawa quality assessment Scale |
| NPC | 7-Ethyl-10-[4-(1-piperidino)-1-amino]carbonyloxycamptothecin |
| mCRC | Metastatic colorectal cancer |
| MTD | Maximum tolerated dose |
| FOLFIRABRAX | 5-Fluourouracil (5-FU), irinotecan, and nab-paclitaxel |
| FOLFIRI | Leucovorin (folinic acid), 5-FU, and irinotecan |
| FOLFIRINOX | Leucovorin (folinic acid), 5-FU, irinotecan, and oxaliplatin |
| OXIRI | Oxaliplatin (O), chronomodulated capecitabine (X), irinotecan (IRI) |
| PGx | Pharmqacogenetic |
| PK | Pharmacokinetic |
| PM | Poor metabolizer |
| RNPGx | French National Network of Pharmacogenetics |
| rsID | Reference SNP ID |
| SEFF | Sociedad Española de Farmacogenética y Farmacogenómica (Spanish Society of Pharmacogenetics and Pharmacogenomics) |
| SmPC | Drug’s summary of product characteristics |
| SNP | Single nucleotide polymorphism |
| UGT1A1 | Uridine diphosphate (UDP) glucuronosyltransferase family 1 member A1 |
| XELIRI | Capecitabine and irinotecan (also known as CAPIRI) |
References
- Marsh, S.; Hoskins, J.M. Irinotecan Pharmacogenomics. Pharmacogenomics 2010, 11, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Clinical Pharmacology and Biopharmaceutics Review. 2003. Available online: https://www.fda.gov/files/drugs/published/N20-571S023-Irinotecan-Clinpharm-BPCA.pdf (accessed on 15 January 2025).
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, É.; Bélanger, A.-S.; Harvey, M.; Couture, F.; Jonker, D.; Innocenti, F.; Cecchin, E.; Toffoli, G.; Guillemette, C. Refining the UGT1A Haplotype Associated with Irinotecan-Induced Hematological Toxicity in Metastatic Colorectal Cancer Patients Treated with 5-Fluorouracil/Irinotecan-Based Regimens. J. Pharmacol. Exp. Ther. 2013, 345, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rouits, E.; Charasson, V.; Pétain, A.; Boisdron-Celle, M.; Delord, J.-P.; Fonck, M.; Laurand, A.; Poirier, A.-L.; Morel, A.; Chatelut, E.; et al. Pharmacokinetic and pharmacogenetic determinants of the activity and toxicity of irinotecan in metastatic colorectal cancer patients. Br. J. Cancer 2008, 99, 1239–1245. [Google Scholar] [CrossRef]
- Takasuna, K.; Hagiwara, T.; Hirohashi, M.; Kato, M.; Nomura, M.; Nagai, E.; Yokoi, T.; Kamataki, T. Involvement of be-ta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydro-chloride (CPT-11) in rats. Cancer Res. 1996, 56, 3752–3757. [Google Scholar] [PubMed]
- Brandi, G.; Dabard, J.; Raibaud, P.; Di Battista, M.; Bridonneau, C.; Pisi, A.M.; Morselli Labate, A.M.; Pantaleo, M.A.; De Vivo, A.; Biasco, G. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin. Cancer Res. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Brandi, G.; de Rosa, F.; Biasco, G. Irinotecan toxicity: Genes or intestinal microflora? Br. J. Cancer 2009, 100, 1017. [Google Scholar] [CrossRef]
- Han, J.-Y.; Lim, H.-S.; Park, Y.H.; Lee, S.Y.; Lee, J.S. Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 2009, 63, 115–120. [Google Scholar] [CrossRef]
- van der Bol, J.M.; Mathijssen, R.H.; Creemers, G.-J.M.; Planting, A.S.; Loos, W.J.; Wiemer, E.A.; Friberg, L.E.; Verweij, J.; Sparreboom, A.; de Jong, F.A. A CYP3A4 Phenotype–Based Dosing Algorithm for Individualized Treatment of Irinotecan. Clin. Cancer Res. 2010, 16, 736–742. [Google Scholar] [CrossRef]
- Dodds, H.M.; Rivory, L.P. The Mechanism for the Inhibition of Acetylcholinesterases by Irinotecan (CPT-11). Mol. Pharmacol. 1999, 56, 1346–1353. [Google Scholar] [CrossRef]
- Blandizzi, C.; Danesi, R.; De Paolis, B.; Di Paolo, A.; Colucci, R.; Falcone, A.; Del Tacca, M. Cholinergic toxic syndrome by the anticancer drug irinotecan: Acetylcholinesterase does not play a major role. Clin. Pharmacol. Ther. 2002, 71, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Athanasou, N.; Quinn, J.; Horton, M.; McGee, J. New sites of cellular vitronectin receptor immunoreactivity detected with osteoclast-reacting monoclonal antibodies 13C2 and 23C6. Bone Miner. 1990, 8, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Gagné, J.-F.; Montminy, V.; Belanger, P.; Journault, K.; Gaucher, G.; Guillemette, C. Common Human UGT1A Polymorphisms and the Altered Metabolism of Irinotecan Active Metabolite 7-Ethyl-10-hydroxycamptothecin (SN-38). Mol. Pharmacol. 2002, 62, 608–617. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). UGT1A1 UDP Glucuronosyltransferase Family 1 Member A1 [Homo sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/54658 (accessed on 15 January 2025).
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; E Klein, T. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1,092 human genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef]
- Phan, L.; Jin, Y.; Zhan, H.; Qian, W.; Shekhtman, E.; Shao, D.; Revoe, D.; Villamarin, R.; Ivanchenko, E.; Kimura, M.; et al. ALFA: Allele Frequency Aggregator; National Center for Biotechnology Information, U.S. National Library of Medicine: Bethesda, MD, USA, 2020. Available online: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 15 January 2025).
- Hart, R.K.; Fokkema, I.F.A.C.; DiStefano, M.; Hastings, R.; Laros, J.F.J.; Taylor, R.; Wagner, A.H.; Dunnen, J.T.D. HGVS Nomenclature 2024: Improvements to community engagement, usability, and computability. Genome Med. 2024, 16, 149. [Google Scholar] [CrossRef]
- Vukovic, M.; Radlovic, N.; Lekovic, Z.; Vucicevic, K.; Maric, N.; Kotur, N.; Gasic, V.; Ugrin, M.; Stojiljkovic, M.; Dokmanovic, L.; et al. UGT1A1 (TA)n promoter genotype: Diagnostic and population pharmacogenetic marker in Serbia. Balk. J. Med. Genet. 2018, 21, 59–68. [Google Scholar] [CrossRef]
- Beutler, E.; Gelbart, T.; Demina, A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. USA 1998, 95, 8170–8174. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, D.; Kuang, Q.; Liu, G.; Xu, W. Association between UGT1A1*28 Polymorphisms and Clinical Outcomes of Irinotecan-Based Chemotherapies in Colorectal Cancer: A Meta-Analysis in Caucasians. PLoS ONE 2013, 8, e58489. [Google Scholar] [CrossRef]
- Sugatania, J.; Yamakawaa, K.; Yoshinaria, K.; Machidab, T.; Takagib, H.; Morib, M.; Kakizakic, S.; Sueyoshic, T.; Negishic, M.; Miwa, M. Identification of a Defect in the UGT1A1 Gene Promoter and Its Association with Hyperbilirubinemia. Biochem. Biophys. Res. Commun. 2002, 292, 492–497. [Google Scholar] [CrossRef]
- Inoue, K.; Sonobe, M.; Kawamura, Y.; Etoh, T.; Takagi, M.; Matsumura, T.; Kikuyama, M.; Kimura, M.; Minami, S.; Utsuki, H.; et al. Polymorphisms of the UDP-Glucuronosyl Transferase 1A Genes Are Associated with Adverse Events in Cancer Patients Receiving Irinotecan-Based Chemotherapy. Tohoku J. Exp. Med. 2013, 229, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, L.; Xu, N.; Wang, J.W.; Jiao, S.C.; Liu, Z.Y.; Xu, J.M. UGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracil. World J. Gastroenterol. 2012, 18, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, E.C.; Deenen, M.J.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.-M.; Houwink, E.J.F.; Risselada, A.; Rongen, G.A.P.J.M.; van Schaik, R.H.N.; et al. Dutch pharmacogenetics working group (DPWG) guideline for the gene–drug interaction between UGT1A1 and irinotecan. Eur. J. Hum. Genet. 2023, 31, 982–987, Corrected in: Eur. J. Hum. Genet. 2023, 31, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Ybazeta, G.; Destro-Bisol, G.; Petzl-Erler, M.L.; Di Rienzo, A. Variability at the uridine diphosphate glucuronosyl-transferase 1A1 promoter in human populations and primates. Pharmacogenetics 1999, 9, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Genomes Aggregation Database (gnomAD). Available online: https://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=2:233759734-233760748;v=rs3064744;vdb=variation;vf=89581836 (accessed on 15 January 2025).
- Harrison, P.W.; Amode, M.R.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2024. Nucleic Acids Res. 2023, 52, D891–D899. [Google Scholar] [CrossRef]
- Food and Drugs Administration. ONIVYDE® (Irinotecan Liposome Injection). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207793lbl.pdf (accessed on 15 January 2025).
- European Medicine Agency. ONIVYDE 4.3 mg/mL Concentrate for Solution for Infusion. Available online: https://www.ema.europa.eu/en/documents/product-information/onivyde-pegylated-liposomal-epar-product-information_en.pdf (accessed on 15 January 2025).
- Ministerio de Sanidad, Consumo y Bienestar Social. Catálogo Común de Pruebas Genéticas del Sistema Nacional de Salud (pp. 1–100). 2023. Available online: https://www.sanidad.gob.es/organizacion/consejoInterterri/docs/1553.pdf (accessed on 15 January 2025).
- Sociedad Española de Farmacogenética y Farmacogenómica. Recomendaciones Irinotecan SEFF. Available online: https://www.Seff.Es/Irinotecan/ (accessed on 15 January 2025).
- Schirripa, M.; Procaccio, L.; Lonardi, S.; Loupakis, F. The Role of Pharmacogenetics in the New ESMO Colorectal Cancer Guidelines. Pharmacogenomics 2017, 18, 197–200. [Google Scholar] [CrossRef]
- Caudle, K.E.; Klein, T.E.; Hoffman, J.M.; Muller, D.J.; Whirl-Carrillo, M.; Gong, L.; McDonagh, E.M.; Sangkuhl, K.; Thorn, C.F.; Schwab, M.; et al. Incorporation of Pharmacogenomics into Routine Clinical Practice: The Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline Development Process. Curr. Drug Metab. 2014, 15, 209–217. [Google Scholar] [CrossRef]
- Etienne-Grimaldi, M.; Boyer, J.; Thomas, F.; Quaranta, S.; Picard, N.; Loriot, M.; Narjoz, C.; Poncet, D.; Gagnieu, M.; Ged, C.; et al. UGT1A1 genotype and irinotecan therapy: General review and implementation in routine practice. Fundam. Clin. Pharmacol. 2015, 29, 219–237. [Google Scholar] [CrossRef]
- Gruppo di Lavoro di Associazione Italiana di Oncologia Medica—Società Italiana di Farmacologia. Raccomandazioni per Analisi Farmacogenetiche. Available online: https://www.aiom.it/2024-aiom-sif-raccomandazioni-per-analisi-farmacogenetiche/ (accessed on 15 January 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 October 2024).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; McKean, H.A.; Reid, J.M.; Mandrekar, S.J.; Tan, A.D.; Kuffel, M.A.; Safgren, S.L.; McGovern, R.M.; Goldberg, R.M.; Grothey, A.A.; et al. UGT1A1 genotype-guided phase I study of irinotecan, oxaliplatin, and capecitabine. Investig. New Drugs 2013, 31, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Ura, T.; Yamada, Y.; Yamazaki, K.; Tsujinaka, T.; Munakata, M.; Nishina, T.; Okamura, S.; Esaki, T.; Sasaki, Y.; et al. Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer Sci. 2011, 102, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Schilsky, R.L.; Ramírez, J.; Janisch, L.; Undevia, S.; House, L.K.; Das, S.; Wu, K.; Turcich, M.; Marsh, R.; et al. Dose-Finding and Pharmacokinetic Study to Optimize the Dosing of Irinotecan According to the UGT1A1 Genotype of Patients With Cancer. J. Clin. Oncol. 2014, 32, 2328–2334. [Google Scholar] [CrossRef]
- Joshi, S.S.; Catenacci, D.V.; Karrison, T.G.; Peterson, J.D.; Zalupski, M.M.; Sehdev, A.; Wade, J.; Sadiq, A.; Picozzi, V.J.; Amico, A.; et al. Clinical Assessment of 5-Fluorouracil/Leucovorin, Nab-Paclitaxel, and Irinotecan (FOLFIRABRAX) in Untreated Patients with Gastrointestinal Cancer Using UGT1A1 Genotype–Guided Dosing. Clin. Cancer Res. 2020, 26, 18–24. [Google Scholar] [CrossRef]
- Ng, M.; Chen, S.; Ong, W.S.; Balachander, A.; Seet, A.; Yeong, J.; Sutiman, N.; Lim, T.K.H.; Lee, B.; Guo, Y.A.; et al. A phase 1b study of OXIRI in pancreatic adenocarcinoma patients and its immunomodulatory effects. Int. J. Cancer 2022, 151, 435–449. [Google Scholar] [CrossRef]
- Kim, K.-P.; Kim, H.-S.; Sym, S.J.; Bae, K.S.; Hong, Y.S.; Chang, H.-M.; Lee, J.L.; Kang, Y.-K.; Lee, J.S.; Shin, J.-G.; et al. A UGT1A1*28 and *6 genotype-directed phase I dose-escalation trial of irinotecan with fixed-dose capecitabine in Korean patients with metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2013, 71, 1609–1617. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Shen, Y.; Guan, Y.; Gu, W.; Lian, P.; Sheng, W.; Cai, S.; Zhang, Z. Genotype-driven phase I study of weekly irinotecan in combination with capecitabine-based neoadjuvant chemoradiation for locally advanced rectal cancer. Radiother. Oncol. 2018, 129, 143–148. [Google Scholar] [CrossRef]
- Toffoli, G.; Sharma, M.R.; Marangon, E.; Posocco, B.; Gray, E.; Mai, Q.; Buonadonna, A.; Polite, B.N.; Miolo, G.; Tabaro, G.; et al. Genotype-Guided Dosing Study of FOLFIRI plus Bevacizumab in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2017, 23, 918–924. [Google Scholar] [CrossRef]
- Kim, K.-P.; Hong, Y.S.; Lee, J.-L.; Bae, K.S.; Kim, H.-S.; Shin, J.-G.; Lee, J.S.; Kim, T.W. A Phase I Study of UGT1A1 *28/*6 Genotype-Directed Dosing of Irinotecan (CPT-11) in Korean Patients with Metastatic Colorectal Cancer Receiving FOLFIRI. Oncology 2014, 88, 164–172. [Google Scholar] [CrossRef]
- Toffoli, G.; Cecchin, E.; Gasparini, G.; D’Andrea, M.; Azzarello, G.; Basso, U.; Mini, E.; Pessa, S.; De Mattia, E.; Re, G.L.; et al. Genotype-Driven Phase I Study of Irinotecan Administered in Combination With Fluorouracil/Leucovorin in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2010, 28, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Keedy, V.L.; Jones, S.F.; Zamboni, W.C.; Chan, E.; Bendell, J.C.; Lee, W.; Wu, H.; Ikeda, S.; Kodaira, H.; et al. Phase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Infante, J.R.; Greco, F.A.; Thompson, D.S.; Barton, J.H.; Bendell, J.C.; Nambu, Y.; Watanabe, N.; Jones, S.F. A phase I dose escalation study of NK012, an SN-38 incorporating macromolecular polymeric micelle. Cancer Chemother. Pharmacol. 2016, 77, 1079–1086. [Google Scholar] [CrossRef]
- Clarke, J.L.; Molinaro, A.M.; Cabrera, J.R.; DeSilva, A.A.; Rabbitt, J.E.; Prey, J.; Drummond, D.C.; Kim, J.; Noble, C.; Fitzgerald, J.B.; et al. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer Chemother. Pharmacol. 2017, 79, 603–610. [Google Scholar] [CrossRef]
- Han, J.-Y.; Lim, H.-S.; Shin, E.S.; Yoo, Y.-K.; Park, Y.H.; Lee, J.-E.; Jang, I.-J.; Lee, D.H.; Lee, J.S. Comprehensive Analysis ofUGT1A Polymorphisms Predictive for Pharmacokinetics and Treatment Outcome in Patients With Non–Small-Cell Lung Cancer Treated With Irinotecan and Cisplatin. J. Clin. Oncol. 2006, 24, 2237–2244. [Google Scholar] [CrossRef]
- Innocenti, F.; Undevia, S.D.; Iyer, L.; Chen, P.X.; Das, S.; Kocherginsky, M.; Karrison, T.; Janisch, L.; Ramírez, J.; Rudin, C.M.; et al. Genetic Variants in the UDP-glucuronosyltransferase 1A1 Gene Predict the Risk of Severe Neutropenia of Irinotecan. J. Clin. Oncol. 2004, 22, 1382–1388. [Google Scholar] [CrossRef]
- Minami, H.; Sai, K.; Saeki, M.; Saito, Y.; Ozawa, S.; Suzuki, K.; Kaniwa, N.; Sawada, J.-I.; Hamaguchi, T.; Yamamoto, N.; et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: Roles of UGT1A1*6 and *28. Pharmacogenetics Genom. 2007, 17, 497–504. [Google Scholar] [CrossRef]
- Ramchandani, R.P.; Wang, Y.; Booth, B.P.; Ibrahim, A.; Johnson, J.R.; Rahman, A.; Mehta, M.; Innocenti, F.; Ratain, M.J.; Gobburu, J.V.S. The Role of SN-38 Exposure, UGT1A1*28 Polymorphism, and Baseline Bilirubin Level in Predicting Severe Irinotecan Toxicity. J. Clin. Pharmacol. 2007, 47, 78–86. [Google Scholar] [CrossRef]
| UGT1A1 Allele | rsID | Enzyme Function ^ | |||
|---|---|---|---|---|---|
| rs887829 | rs3064744 | rs4148323 | rs35350960 | ||
| *1 | C | A(TA)7A | G | C | normal |
| *6 | A | decreased | |||
| *27 | A | decreased | |||
| *28 | A(TA)8A | decreased | |||
| *36 | A(TA)6A | increased | |||
| *37 | A(TA)9A | decreased | |||
| *80 | T | unknown | |||
| *80 + *28 | T | A(TA)8A | decreased | ||
| *80 + *37 | T | A(TA)9A | decreased | ||
| Allele Frequency | |||||
| IBS (1000 genomes) | 28.04 (T) | - | 0.00 (A) | 0.00 (A) | |
| Europe (1000 genomes) | 29.82 (T) | - | 0.70 (A) | 0.00 (A) | |
| Global (1000 Genomes) | 35.40 (T) | - | 3.43 (A) | 0.28 (A) | |
| All populations (ALFA) | 32.79 (T) | 17.01 | 0.67 (A) | 0.09 (A) | |
| Ref. | Phase | Population (Diagnosis) | Chemotherapy Scheme | Follow-up Cycles. (in DLT Study) | Primary Endpoint | Ethnicity 1 | N | rs | Allele | MAF% | Genotype MM/Mm/mm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [42] | I | Multiple | CAPIRINOX | All (1) | Safety, efficacy, PK | White | 50 | rs3064744 (TA8) | *28 | 40.0 | 21/18/11 |
| [43] | I | Metastatic GI cancer | Monotherapy | 2 (2) | Safety, PK | Japan | 82 | rs3064744 (TA8) | *28 | 12.2 | 41/20/21 2 |
| rs4148323 | *6 | 25.6 | |||||||||
| [44] | I | Multiple | Monotherapy | All (1) | Safety, efficacy, PK | White | 68 | rs3064744 (TA8) | *28 | 33.8 | 31/28/9 |
| [45] | I–II | Locally advanced/metastatic GI cancer | FOLFIRABRAX | All (1) | Safety, efficacy | White | 50 | rs3064744 (TA8) | *28 | 34.0 | 23/20/7 |
| [46] | I | Pancreatic adenocarcinoma | OXIRI | All (1) | Safety, efficacy, PK/PD | China | 36 | rs3064744 (TA8) | *28 | 9.7 | 18/17/1 4 |
| rs4148323 | *6 | 16.7 | |||||||||
| [47] | I | Metastatic CRC | XELIRI | 3y (1) | Safety, efficacy, PK | Korea | 50 | rs3064744 (TA8) | *28 | 12.0 | 23/20/7 3 |
| rs4148323 | *6 | 22.0 | |||||||||
| [48] | I | Locally advanced rectal cancer | XELIRI | All (1) | Safety, efficacy | Asian | 26 | rs3064744 (TA8) | *28 | - | 15/11/exc |
| [49] | I | Metastatic CRC | FOLFIRI + Bevacizumab | All (1) | Safety, efficacy, PK | White | 48 | rs3064744 (TA8) | *28 | - | 25/23/exc |
| [50] | I | Metastatic CRC | FOLFIRI | All (1) | Safety, PK | Korea | 43 | rs3064744 (TA8) | *28 | 10.5 | 19/20/4 5 |
| rs4148323 | *6 | 22.1 | |||||||||
| [51] | I | Metastatic CRC | FOLFIRI | All (1) | Safety, efficacy, PK | white | 63 | rs3064744 (TA8) | *28 | 25.4 | 35/24/4 |
| [52] | I | Multiple | IHL-305 | All (1) | Safety, efficacy, PK | White | 37 | rs3064744 (TA8) | *28 | 16.2 | 25/12/0 |
| [53] | I | Multiple | NK012 | All (1) | Safety, efficacy, PK | White | 38 | rs3064744 (TA8) | *28 | 34.2 | 19/12/7 |
| [54] | I | Recurrent malignant glioma | nal-IRI | 1 (1) | Safety, efficacy, PK | NS | 34 | rs3064744 (TA8) | *28 | - | 16/18/exc |
| Ref. | Scheme | UGT1A1 Genotype: n | Irinotecan Dose mg/m2 (n) | DLT n (%) | MTD mg/m2 | DLT Definition (Summarized) |
|---|---|---|---|---|---|---|
| [43] | Monotherapy | Wt: 40 | 150 (40) | 1 (2.5) | >150 | G4 neutropenia, G4 thrombocytopenia, febrile neutropenia (neutrophil count < 1000/mm3 and fever ≥ 38.5 °C), or G3 diarrhea. |
| Het: 20 | 150 (16) | 0 (0) | >150 | |||
| Hom: 19 | 150 (16) | 6 (37.5) | 150 | |||
| [42] | CAPIRINOX | Wt: 21 | 175 (3) | 2 (66.7) | 150 ^1 | G4 absolute neutrophil count > 5 days, G4 hemoglobin, platelet < 25,000/μL, serum creatinine ≥ 2× baseline, treatment delay >14 days, and sensory neuropathy ≥ G3. G3-4 non-hematologic toxicity. Inability to complete 10 days of prescribed dose of capecitabine during cycle one. |
| 150 ^1 (12) | 5 (41.7) | |||||
| 150 ^2 (6) | 0 (0) | |||||
| Het: 18 | 150 ^3 (6) | 2 (33.3) | 150 ^4 | |||
| 150 ^4 (12) | 0 (0) | |||||
| Hom: 11 | 150 (2) | 1 (50) | 75 ^4 | |||
| 100 (5) | 2 (40) | |||||
| 75 (6) | 0 (0) | |||||
| [45] | FOLFIRABRAX | Wt: 23 | 180 (23) | 5 (21.7) | - | Non-hematologic: ≥grade 3 events. Hematologic: G4 neutropenia lasting ≥ 5 days; G3-4 neutropenia with fever ≥ 38.5 °C and/or infection requiring antibiotics; G4 thrombocytopenia; and G3 thrombocytopenia accompanied by ≥ G2 hemorrhage. 14 days delay of cycle 1. |
| Het: 19 | 135 (19) | 1 (5.3) | ||||
| Hom: 7 | 90 (7) | 0 (0) | ||||
| [47] | XELIRI | Wt: 23 | <350 (6) | 0 (0) | 380 | G4 neutropenia > 5 days, febrile neutropenia, G4 thrombocytopenia or any other G3-4 non-hematological toxicity that did not improve after the institution of appropriate therapy, other toxicities that prevented completion of the prescribed dose of capecitabine during the first cycle, ADEs causing a delay > 2 weeks of the second cycle. |
| 350 (11) | 2 (18.2) | |||||
| 380 (6) | 2 (33.3) | |||||
| Het: 20 | <350 (10) | 0 (0) | 380 | |||
| 350 (8) | 1 (12.5) | |||||
| 380 (2) | 2 (100) | |||||
| Hom: 7 | 200 (4) | 0 (0) | 240 | |||
| 240 (3) | 2 (66.7) | |||||
| [46] | OXIRI | Wt: 18 | - | - | 75 | Hematological: ≥ G3 neutropenia with infection and ≥3 thrombocytopenia with bleeding > 7 days. Non-hematological: ≥3 nonhematological toxicity other than untreated G3 diarrhea and nausea/vomiting, elevated alkaline phosphatase, and gamma glutamyl transferase levels. |
| Het: 17 | 75 | |||||
| Hom: 1 | 50 | |||||
| [44] | Monotherapy | Wt: 31 | 700 (9) | 1 (11.1) | 850 | G4 neutropenia ≥ 4 days, ≥G3 neutropenia/day, ≥G3 febrile neutropenia, G4 anemia/thrombocytopenia, ≥G3 diarrhea, ≥G3 nonhematologic toxicity, or G4 nausea/vomiting. |
| 850 (16) | 4 (25) | |||||
| 1000 (6) | 2 (33.3) | |||||
| Het: 28 | 700 (22) | 5 (22.7) | 700 | |||
| 850 (6) | 4 (66.6) | |||||
| Hom: 9 | 700 (6) | 3 (50) | 400 | |||
| 850 (3) | 3 (100) | |||||
| [49] | FOLFIRI $1 | Wt: 24 | 260 (10) | 1 (10) | 310 | ≥G4 hematologic toxicity or ≥G3 non-hematologic toxicity despite maximal supportive measures (such as anti-diarrheal and anti-emetics) according to the NCI Common Terminology Criteria for Adverse Events (version 3.0). |
| 310 (10) | 2 (20) | |||||
| 370 (4) | 2 (50) | |||||
| Het: 23 | 260 (10) | 2 (20) | 260 | |||
| 310 (10) | 4 (40) | |||||
| 370 (3) | 2 (66.7) | |||||
| [50] | FOLFIRI | Wt: 19 | ≤300 (13) | 0 (0) | >330 | G4 neutropenia > 5 days, febrile neutropenia; G4 thrombocytopenia, and any other ≥ G3 nonhematologic toxicities that did not improve to despite management therapies. |
| 330 (6) | 1 (16.7) | |||||
| Het: 20 | <300 (17) | 0 (0) | 300 | |||
| 300 (3) | 2 (66.7) | |||||
| Hom: 4 | 150 (4) | - | 150 $2 | |||
| [52] | IHL-305 | Wt + het: 53 | ≤105 A (12) | 3 (25) | 160 B | G4 hematologic toxicity ≥5 days; G3-4 febrile neutropenia; G4 thrombocytopenia; ≥G3 non-hematologic toxicities; prolonged QTc > 500 ms; or any toxicity resulting in a treatment delay > 1 week. |
| 140 A (5) | 2 (40) | 80 A | ||||
| <160 B (28) | 1 (3.6) | |||||
| 160 B (6) | 1 (16.7) | |||||
| Hom: 7 | 210 B (2) | 2 (100) | - | |||
| NS (7) | 1 (14.3) | |||||
| [53] | NK012 | Wt + het: 29 | ≤21 (12) | 0 (0) | 28 | G4 hematologic toxicity ≥ 5 days; G3-4 febrile neutropenia or G4 thrombocytopenia; ≥G3 non-hematologic toxicities; prolonged QTc > 500 ms (G3); any ADE resulting in a treatment delay beyond 1 week. |
| 28 (12) | 1 (8.3) | |||||
| Hom: 7 | 37 (5) | 2 (40) | ||||
| 18.5 (7) | 0 (0) | - | ||||
| [48] | XELIRI | Wt: 15 | ≤80 (12) | 1 (8.3) | 80 | Hematologic G4 toxicities and non-hematologic G3 toxicities, with the exception of skin reactions and hand–foot syndromes. |
| 95 (3) | 2 (66.7) | |||||
| Het: 11 | ≤65 (9) | 1 (11.1) | 65 | |||
| 80 (2) | 2 (100) | |||||
| [51] | FOLFIRI | Wt: 35 | ≤370 (32) | 2 (6.3) | 370 | Hematologic G4 toxicity or nonhematologic G3-4 toxicity that developed or persisted despite supportive measures. |
| 420 (3) | 2 (66.7) | |||||
| Het: 24 | ≤310 (20) | 1 (5) | 310 | |||
| 370 (4) | 2 (50) | |||||
| Hom: 4 | exc | - | - | |||
| [54] | nal-IRI | Wt: 16 | ≤180 (13) | 2 (15.4) | 120 | Hematological: ≥G3 thrombocytopenia > 5 days. G4 neutropenia > 5 days. G4 anemia of any duration. Non-hematological: ≥G3 toxicity except for G3 alopecia. Other: failure to recover from toxicities to be eligible for re-treatment within 35 days. |
| 240 (3) | 2 (66.7) | |||||
| Het: 18 | ≤120 (12) | 1 (8.3) | 150 | |||
| 150 (6) | 1 (16.7) |
| Reference | Genotype: n (Irinotecan Dose) | AUC (Mean ± sd) | AUC Ratio | |
|---|---|---|---|---|
| SN-38 | SN-38G | |||
| [43] AUC 0–24 h (ng × h/mL) | Wt: 46 | 264 ± 114 | 1266.8 ± 667.5 | 5.03 ± 2.25 |
| Het:16 | 279.6 ± 152.0 | 820.7 ± 378.7 | 3.25 ± 1.32 | |
| Hom:16 | 509.8 ± 261.8 | 849.0 ± 561.9 | 1.85 ± 1.13 | |
| [42] AUC 0–48 h (ng × h/mL) | Wt: 9 (150) | 291± 146 | 1124 ± 543 | 3.86 ± 3.72 * |
| Het: 9 (150) | 226 ± 100 | 876 ± 543 | 3.88 ± 5.43 * | |
| Hom: 3 (75) | 135 ± 64 | 413 ± 84 | 3.06 ± 1.31 * | |
| Hom: 3 (100) | 454 ± 81 | 865 ±433 | 1.91 ± 5.35 * | |
| [47] AUC last/dose ng × h/mL/mg | Wt: 23 | 0.32 ± 0.23 | 2.76 ± 1.13 | 7.72 ± 3.90 |
| Het: 20 | 0.55 ± 0.37 | 3.77 ± 1.85 | 5.71 ± 3.00 | |
| Hom: 7 | 0.97 ± 0.42 | 3.24 ± 1.19 | 2.72 ± 1.56 | |
| [46] (median range) AUC 0-inf ^ | Wt: 14 | 0.9 (0.4−2.0) | 5.2 (1.5−18.4) | 6.3 (2.5−15.4) |
| Het: 15 | 1.6 (0.6−4.0) | 6.7 (2.4−22.2) | 3.9 (2.14−11.9) | |
| [44] AUC 0-Inf (mg × h/L) | Wt: 6 (400) | 0.867 ± 0.708 | 1.37 ± 0.59 | 2.63 ± 1.95 |
| Wt: 3 (500) | 1.196 ± 0.662 | 3.41 ± 2.19 | 2.65 ± 0.61 | |
| Het/hom: 30 (700) | 0.808 ± 0.655 | 2.76 ± 1.96 | 5.17 ± 5.06 | |
| Het/hom: 22 (850) | 0.868 ± 0.761 | 3.63 ± 2.15 | 5.43 ± 3.24 | |
| Hom: 6 (1000) | 0.665 ± 0.290 | 2.87 ± 0.81 | 4.81 ± 1.63 | |
| [50] AUC last/dose h × nmol/l/mg | Wt: 19 | 1.07 ± 0.44 | 6.63 ± 3.96 | 6.46 ± 2.73 |
| Het: 20 | 1.55 ± 0.61 | 6.83 ± 3.46 | 4.69 ± 1.82 | |
| Hom: 4 | 1.67 ± 0.43 | 5.68 ± 1.55 | 3.49 ± 1.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Villamarín, X.; Nieto-Sánchez, M.T.; Martínez-Pérez, M.; Novo-González, P.; Fernández-Varón, E.; Torres-García, A.; González Astorga, B.; Blancas, I.; Cabeza-Barrera, J.; Morón, R. Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review. Pharmaceutics 2025, 17, 542. https://doi.org/10.3390/pharmaceutics17050542
Díaz-Villamarín X, Nieto-Sánchez MT, Martínez-Pérez M, Novo-González P, Fernández-Varón E, Torres-García A, González Astorga B, Blancas I, Cabeza-Barrera J, Morón R. Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review. Pharmaceutics. 2025; 17(5):542. https://doi.org/10.3390/pharmaceutics17050542
Chicago/Turabian StyleDíaz-Villamarín, Xando, María Teresa Nieto-Sánchez, María Martínez-Pérez, Paula Novo-González, Emilio Fernández-Varón, Alicia Torres-García, Beatriz González Astorga, Isabel Blancas, José Cabeza-Barrera, and Rocío Morón. 2025. "Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review" Pharmaceutics 17, no. 5: 542. https://doi.org/10.3390/pharmaceutics17050542
APA StyleDíaz-Villamarín, X., Nieto-Sánchez, M. T., Martínez-Pérez, M., Novo-González, P., Fernández-Varón, E., Torres-García, A., González Astorga, B., Blancas, I., Cabeza-Barrera, J., & Morón, R. (2025). Dose-Limiting Toxicities and the Maximum Tolerated Dose of Irinotecan Based on UGT1A1 Genotypes: A Systematic Review. Pharmaceutics, 17(5), 542. https://doi.org/10.3390/pharmaceutics17050542






