The Advancement of In Vitro Lipolysis: Two-Step Flow-Through Method for the Evaluation of Lipid-Based Drug Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SMEDDS Preparation
2.3. Preparation of the Media
2.4. Conventional Dissolution Studies
2.5. Dynamic Light Scattering Analysis
2.6. Determination of Pancreatin Powder Lipolytic Activity
2.7. Pancreatin Solution Preparation
2.8. pH-Stat In Vitro Lipolysis
2.8.1. Pancreatin Activity Effect
2.8.2. Buffer Capacity Effect
2.8.3. Two-Step In Vitro Lipolysis
2.9. Flow-Through In Vitro Lipolysis
Two-Step In Vitro Lipolysis
2.10. In Vitro Lipolysis Sample Manipulation
2.11. Solid-State Characterization
2.12. Buffer Capacity Determination
2.13. Carvedilol Solubility Studies
2.14. HPLC Analysis
3. Results
3.1. Conventional Dissolution Test
3.2. One-Step pH-Stat In Vitro Lipolysis
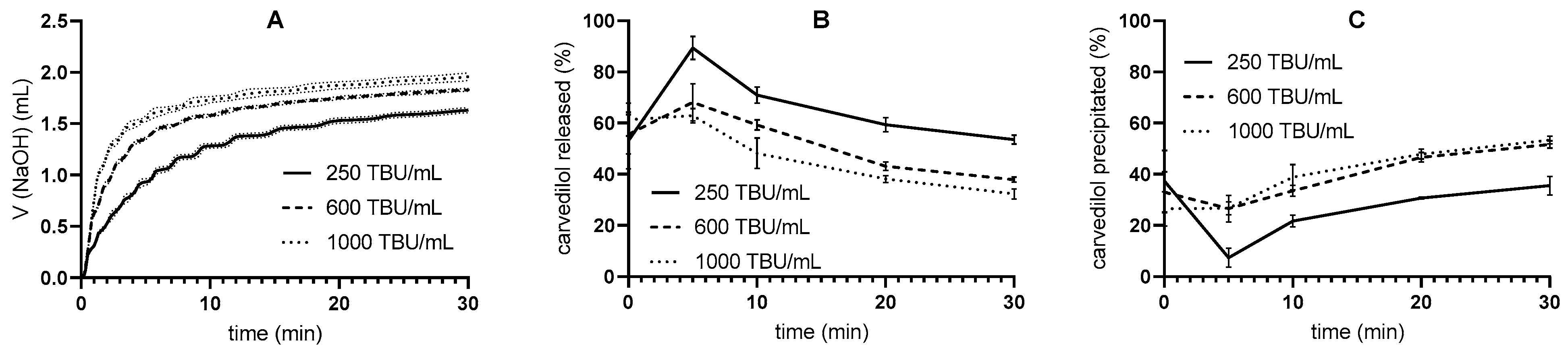
3.2.1. Pancreatin Activity Effect
3.2.2. Buffer Capacity Effect
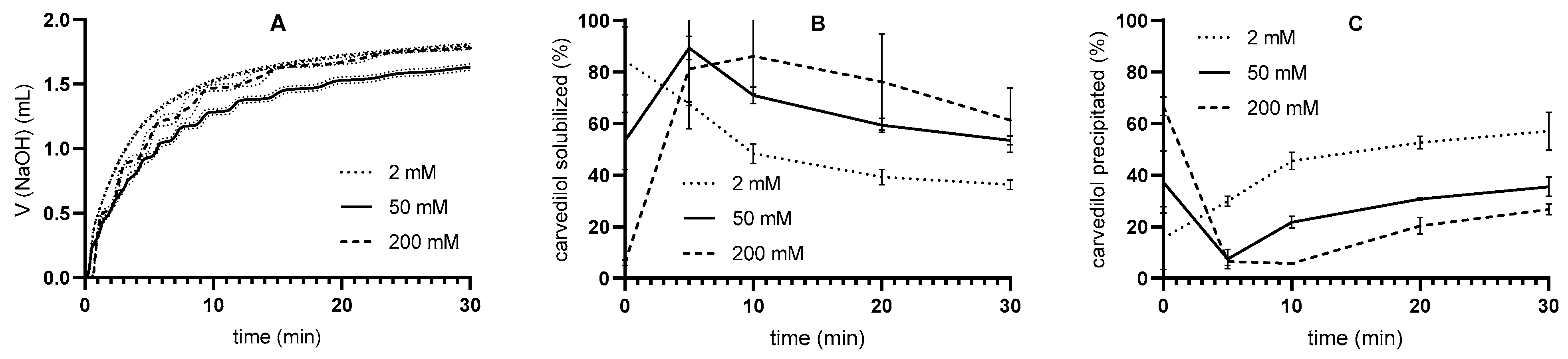
3.3. Two-Step pH-Stat In Vitro Lipolysis
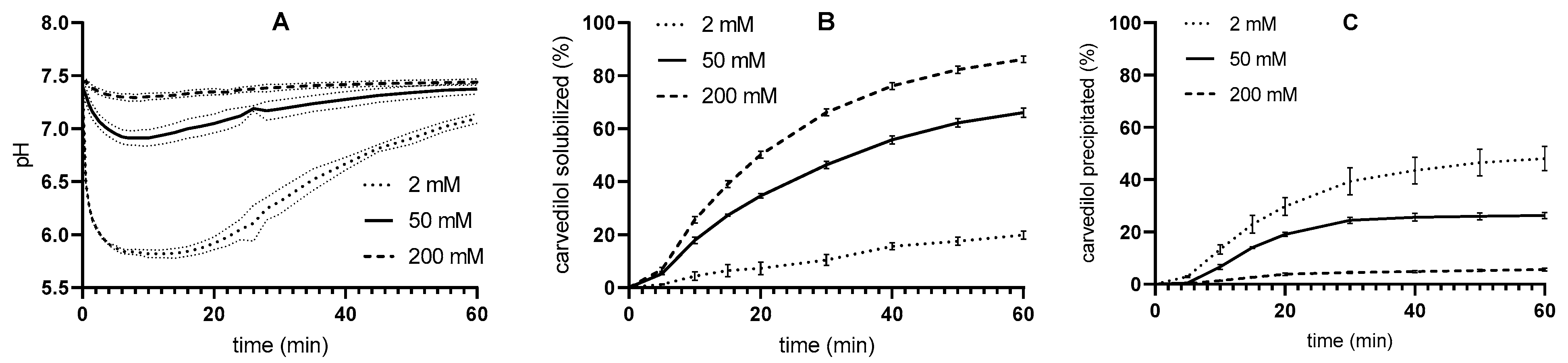
3.4. Two-Step Flow-Through In Vitro Lipolysis
3.5. XPRD Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMEDDS | Self-microemulsifying drug delivery system. |
| API | Active pharmaceutical ingredient. |
| LBDDS | Lipid-based drug delivery system. |
| rDGL | Recombinant dog gastric lipase. |
| TIM | TNO gastrointestinal model. |
| ESIN | Engineered stomach and small intestine. |
| SIMGI | Simulator of the gastrointestinal tract. |
| IVIVC | In vitro in vivo correlation. |
| NaDC | Sodium deoxycholate. |
| PC | L-α-phosphatidylcholine. |
| USP | United States Pharmacopoeia. |
| BBBA | 4-bromophenylboronic acid. |
| FaSSIF | Fasted State Simulated Intestinal Fluid. |
| FeSSIF | Fed State Simulated Intestinal Fluid. |
| FaSSGF | Fasted State Simulated Gastric Fluid. |
| SGFsp | Simulated gastric fluid. |
| SIFsp | Simulated intestinal fluid. |
| HPLC | High-performance liquid chromatography. |
| PDI | Polydispersity index. |
| TBU | Tributyrin unit. |
| XPRD | X-ray powder diffraction. |
References
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In Vitro Digestion Models to Evaluate Lipid Based Drug Delivery Systems; Present Status and Current Trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 Years of Oral Lipid-Based Formulations: Provenance, Progress and Future Perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Holm, R.; Rades, T.; Müllertz, A. Characterising Lipid Lipolysis and Its Implication in Lipid-Based Formulation Development. AAPS J. 2012, 14, 860–871. [Google Scholar] [CrossRef]
- N‘Goma, J.-C.B.; Amara, S.; Dridi, K.; Jannin, V.; Carrière, F. Understanding the Lipid-Digestion Processes in the GI Tract Before Designing Lipid-Based Drug-Delivery Systems. Ther. Deliv. 2012, 3, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.; Whitby, C.P.; Prestidge, C.A. Nanostructuring Biomaterials with Specific Activities towards Digestive Enzymes for Controlled Gastrointestinal Absorption of Lipophilic Bioactive Molecules. Adv. Colloid Interface Sci. 2016, 237, 52–75. [Google Scholar] [CrossRef]
- Siqueira Jørgensen, S.D.; Al Sawaf, M.; Graeser, K.; Mu, H.; Müllertz, A.; Rades, T. The Ability of Two In Vitro Lipolysis Models Reflecting the Human and Rat Gastro-Intestinal Conditions to Predict the In Vivo Performance of SNEDDS Dosing Regimens. Eur. J. Pharm. Biopharm. 2018, 124, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Siqueira Jørgensen, S.; Rades, T.; Mu, H.; Graeser, K.; Müllertz, A. Exploring the Utility of the Chasing Principle: Influence of Drug-Free SNEDDS Composition on Solubilization of Carvedilol, Cinnarizine and R3040 in Aqueous Suspension. Acta Pharm. Sin. B 2019, 9, 194–201. [Google Scholar] [CrossRef]
- Klitgaard, M.; Beilles, S.; Sassene, P.J.; Berthelsen, R.; Müllertz, A. Adding a Gastric Step to the Intestinal In Vitro Digestion Model Improves the Prediction of Pharmacokinetic Data in Beagle Dogs of Two Lipid-Based Drug Delivery Systems. Mol. Pharm. 2020, 17, 3214–3222. [Google Scholar] [CrossRef]
- Klitgaard, M.; Sassene, P.J.; Selen, A.; Müllertz, A.; Berthelsen, R. Studying Furosemide Solubilization Using an in Vitro Model Simulating Gastrointestinal Digestion and Drug Solubilization in Neonates and Young Infants. Eur. J. Pharm. Sci. 2017, 109, 191–199. [Google Scholar] [CrossRef]
- Christophersen, P.C.; Christiansen, M.L.; Holm, R.; Kristensen, J.; Jacobsen, J.; Abrahamsson, B.; Müllertz, A. Fed and Fasted State Gastro-Intestinal In Vitro Lipolysis: In Vitro In Vivo Relations of a Conventional Tablet, a SNEDDS and a Solidified SNEDDS. Eur. J. Pharm. Sci. 2014, 57, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Chevrier, S.; Ritter, N.; Mahler, B.; Demarne, F.; Carrière, F.; Jannin, V. In Vitro Gastrointestinal Lipolysis of Four Formulations of Piroxicam and Cinnarizine with the Self Emulsifying Excipients Labrasol® and Gelucire® 44/14. Pharm. Res. 2009, 26, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Richter, K.; Pedersen, T.B.; Holm, R.; Müllertz, A.; Rades, T. In Vitro Lipolysis Data Does Not Adequately Predict the In Vivo Performance of Lipid-Based Drug Delivery Systems Containing Fenofibrate. AAPS J. 2014, 16, 539–549. [Google Scholar] [CrossRef]
- Keemink, J.; Mårtensson, E.; Bergström, C.A.S. Lipolysis-Permeation Setup for Simultaneous Study of Digestion and Absorption in Vitro. Mol. Pharm. 2019, 16, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Alskär, L.C.; Parrow, A.; Keemink, J.; Johansson, P.; Abrahamsson, B.; Bergström, C.A.S. Effect of Lipids on Absorption of Carvedilol in Dogs: Is Coadministration of Lipids as Efficient as a Lipid-Based Formulation? J. Control. Release 2019, 304, 90–100. [Google Scholar] [CrossRef]
- Bibi, H.A.; Holm, R.; Bauer-Brandl, A. Simultaneous Lipolysis/Permeation in Vitro Model, for the Estimation of Bioavailability of Lipid Based Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2017, 117, 300–307. [Google Scholar] [CrossRef]
- Falavigna, M.; Klitgaard, M.; Berthelsen, R.; Müllertz, A.; Flaten, G.E. Predicting Oral Absorption of Fenofibrate in Lipid-Based Drug Delivery Systems by Combining In Vitro Lipolysis with the Mucus-PVPA Permeability Model. J. Pharm. Sci. 2021, 110, 208–216. [Google Scholar] [CrossRef]
- Keemink, J.; Bergström, C.A.S. Caco-2 Cell Conditions Enabling Studies of Drug Absorption from Digestible Lipid-Based Formulations. Pharm. Res. 2018, 35, 74. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. The Effect of Different Lipid Based Formulations on the Oral Absorption of Lipophilic Drugs: The Ability of in Vitro Lipolysis and Consecutive Ex Vivo Intestinal Permeability Data to Predict in Vivo Bioavailability in Rats. Eur. J. Pharm. Biopharm. 2007, 67, 96–105. [Google Scholar] [CrossRef]
- Klitgaard, M.; Müllertz, A.; Berthelsen, R. Estimating the Oral Absorption from Self-Nanoemulsifying Drug Delivery Systems Using an In Vitro Lipolysis-Permeation Method. Pharmaceutics 2021, 13, 489. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M. The TNO Gastro-Intestinal Model (TIM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Barroso, E.; Cueva, C.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. The Computer-Controlled Multicompartmental Dynamic Model of the Gastrointestinal System SIMGI. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Guerra, A.; Denis, S.; le Goff, O.; Sicardi, V.; François, O.; Yao, A.-F.; Garrait, G.; Manzi, A.P.; Beyssac, E.; Alric, M.; et al. Development and Validation of a New Dynamic Computer-Controlled Model of the Human Stomach and Small Intestine. Biotechnol. Bioeng. 2016, 113, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can Dynamic in Vitro Digestion Systems Mimic the Physiological Reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef]
- O’Farrell, C.; Stamatopoulos, K.; Simmons, M.; Batchelor, H. In Vitro Models to Evaluate Ingestible Devices: Present Status and Current Trends. Adv. Drug Deliv. Rev. 2021, 178, 113924. [Google Scholar] [CrossRef]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used In Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Mosgaard, M.D.; Sassene, P.; Mu, H.; Rades, T.; Müllertz, A. Development of a High-Throughput in Vitro Intestinal Lipolysis Model for Rapid Screening of Lipid-Based Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2015, 94, 493–500. [Google Scholar] [CrossRef]
- Mosgaard, M.D.; Sassene, P.J.; Mu, H.; Rades, T.; Müllertz, A. High-Throughput Lipolysis in 96-Well Plates for Rapid Screening of Lipid-Based Drug Delivery Systems. J. Pharm. Sci. 2017, 106, 1183–1186. [Google Scholar] [CrossRef]
- Rede, K.; Bolko Seljak, K.; Bogataj, M.; Gašperlin, M. Can APIs That Are Poorly Water- and Oil-Soluble Benefit from Incorporation into SMEDDS? The Case of Dipyridamole. Eur. J. Lipid Sci. Technol. 2021, 123, 2000303. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Recommendations on Methods for Dosage Froms Testing; Council of Europe: Strasbourg, France, 2007; pp. 801–803. [Google Scholar]
- Bolko Seljak, K.; Ilić German, I.; Gašperlin, M.; Zvonar Pobirk, A. Self-Microemulsifying Tablets Prepared by Direct Compression for Improved Resveratrol Delivery. Int. J. Pharm. 2018, 548, 263–275. [Google Scholar] [CrossRef]
- Bogataj, M.; Cof, G.; Mrhar, A. Development of a Glass-Bead Device for Dissolution Testing. Dissolution Technol. 2015, 22, 18–24. [Google Scholar] [CrossRef]
- Felicijan, T.; Pišlar, M.; Vene, K.; Bogataj, M. The Influence of Simulated Fasted Gastrointestinal pH Profiles on Diclofenac Sodium Dissolution in a Glass-Bead Flow-Through System. AAPS PharmSciTech 2018, 19, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Merel. Available online: https://merel.si/ (accessed on 10 October 2024).
- Hamed, R.; Awadallah, A.; Sunoqrot, S.; Tarawneh, O.; Nazzal, S.; AlBaraghthi, T.; Al Sayyad, J.; Abbas, A. pH-Dependent Solubility and Dissolution Behavior of Carvedilol—Case Example of a Weakly Basic BCS Class II Drug. AAPS PharmSciTech 2016, 17, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Vogensen, S.B.; Desbos, C.; Jansook, P. Carvedilol: Solubilization and Cyclodextrin Complexation: A Technical Note. AAPS PharmSciTech 2008, 9, 425–430. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Luthman, K.; Artursson, P. Accuracy of Calculated pH-Dependent Aqueous Drug Solubility. Eur. J. Pharm. Sci. 2004, 22, 387–398. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Kaukonen, A.M.; Boyd, B.J.; Edwards, G.A.; Charman, W.N. Susceptibility to Lipase-Mediated Digestion Reduces the Oral Bioavailability of Danazol after Administration as a Medium-Chain Lipid-Based Microemulsion Formulation. Pharm. Res. 2004, 21, 1405–1412. [Google Scholar] [CrossRef]
- Bannow, J.; Yorulmaz, Y.; Löbmann, K.; Müllertz, A.; Rades, T. Improving the Drug Load and in Vitro Performance of Supersaturated Self-Nanoemulsifying Drug Delivery Systems (Super-SNEDDS) Using Polymeric Precipitation Inhibitors. Int. J. Pharm. 2020, 575, 118960. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Siqueira, S.D.V.S.; Amenitsch, H.; Müllertz, A.; Rades, T. In Vitro and in Vivo Performance of Monoacyl Phospholipid-Based Self-Emulsifying Drug Delivery Systems. J. Control. Release 2017, 255, 45–53. [Google Scholar] [CrossRef]
- Tran, T.; Siqueira, S.D.V.S.; Amenitsch, H.; Rades, T.; Müllertz, A. Monoacyl Phosphatidylcholine Inhibits the Formation of Lipid Multilamellar Structures during in Vitro Lipolysis of Self-Emulsifying Drug Delivery Systems. Eur. J. Pharm. Sci. 2017, 108, 62–70. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, T.H.; Feng, W.; Choi, H.G.; Zgair, A.; Shin, S.; Yoo, S.D.; Gershkovich, P.; Shin, B.S. Quantitative Prediction of Oral Bioavailability of a Lipophilic Antineoplastic Drug Bexarotene Administered in Lipidic Formulation Using a Combined In Vitro Lipolysis/Microsomal Metabolism Approach. J. Pharm. Sci. 2019, 108, 1047–1052. [Google Scholar] [CrossRef]
- Alayoubi, A.; Aqueel, M.S.; Cruz, C.N.; Ashraf, M.; Zidan, A.S. Application of in Vitro Lipolysis for the Development of Oral Self-Emulsified Delivery System of Nimodipine. Int. J. Pharm. 2018, 553, 441–453. [Google Scholar] [CrossRef]
- Larsen, A.T.; Ogbonna, A.; Abu-Rmaileh, R.; Abrahamsson, B.; Østergaard, J.; Müllertz, A. SNEDDS Containing Poorly Water Soluble Cinnarizine; Development and in Vitro Characterization of Dispersion, Digestion and Solubilization. Pharmaceutics 2012, 4, 641–665. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.T.; Åkesson, P.; Juréus, A.; Saaby, L.; Abu-Rmaileh, R.; Abrahamsson, B.; Østergaard, J.; Müllertz, A. Bioavailability of Cinnarizine in Dogs: Effect of SNEDDS Loading Level and Correlation with Cinnarizine Solubilization During In Vitro Lipolysis. Pharm. Res. 2013, 30, 3101–3113. [Google Scholar] [CrossRef]
- Hedge, O.J.; Bergström, C.A.S. Suitability of Artificial Membranes in Lipolysis-Permeation Assays of Oral Lipid-Based Formulations. Pharm. Res. 2020, 37, 99. [Google Scholar] [CrossRef]
- Perng, C.-Y.; Kearney, A.S.; Palepu, N.R.; Smith, B.R.; Azzarano, L.M. Assessment of Oral Bioavailability Enhancing Approaches for SB-247083 Using Flow-through Cell Dissolution Testing as One of the Screens. Int. J. Pharm. 2003, 250, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, V.H.; Pedersen, B.L.; Kristensen, H.G.; Müllertz, A. In Vivo in Vitro Correlations for a Poorly Soluble Drug, Danazol, Using the Flow-through Dissolution Method with Biorelevant Dissolution Media. Eur. J. Pharm. Sci. 2005, 24, 305–313. [Google Scholar] [CrossRef]
- Sassene, P.; Kleberg, K.; Williams, H.D.; Bakala-N’Goma, J.-C.; Carrière, F.; Calderone, M.; Jannin, V.; Igonin, A.; Partheil, A.; Marchaud, D.; et al. Toward the Establishment of Standardized In Vitro Tests for Lipid-Based Formulations, Part 6: Effects of Varying Pancreatin and Calcium Levels. AAPS J. 2014, 16, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.; Abrahamse, E.; Almgren, A.; Alminger, M.; Andres, A.; Ariëns, R.M.; Bastiaan-Net, S.; Bourlieu-Lacanal, C.; Brodkorb, A.; Bronze, M.R.; et al. INFOGEST Inter-Laboratory Recommendations for Assaying Gastric and Pancreatic Lipases Activities Prior to in Vitro Digestion Studies. J. Funct. Foods 2021, 82, 104497. [Google Scholar] [CrossRef]
- Armand, M.; Borel, P.; Pasquier, B.; Dubois, C.; Senft, M.; Andre, M.; Peyrot, J.; Salducci, J.; Lairon, D. Physicochemical Characteristics of Emulsions during Fat Digestion in Human Stomach and Duodenum. Am. J. Physiol. 1996, 271, G172–G183. [Google Scholar] [CrossRef]
- Armand, M.; Pasquier, B.; André, M.; Borel, P.; Senft, M.; Peyrot, J.; Salducci, J.; Portugal, H.; Jaussan, V.; Lairon, D. Digestion and Absorption of 2 Fat Emulsions with Different Droplet Sizes in the Human Digestive Tract2. Am. J. Clin. Nutr. 1999, 70, 1096–1106. [Google Scholar] [CrossRef]
- Bozkurt, T.; Adler, G.; Leferink, S.; Arnold, R. Volume and Enzyme Kinetics of Human Pancreatic Secretion after Endogenous Stimulation with the Lundh Test Meal. Int. J. Pancreatol. 1990, 6, 281–293. [Google Scholar] [CrossRef]
- Ulleberg, E.K.; Comi, I.; Holm, H.; Herud, E.B.; Jacobsen, M.; Vegarud, G.E. Human Gastrointestinal Juices Intended for Use in In Vitro Digestion Models. Food Dig. 2011, 2, 52–61. [Google Scholar] [CrossRef]
- Michaelsen, M.H.; Wasan, K.M.; Sivak, O.; Müllertz, A.; Rades, T. The Effect of Digestion and Drug Load on Halofantrine Absorption from Self-Nanoemulsifying Drug Delivery System (SNEDDS). AAPS J. 2016, 18, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Layer, P. Human Pancreatic Exocrine Response to Nutrients in Health and Disease. Gut 2005, 54, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Fadda, H.M.; Sousa, T.; Carlsson, A.S.; Abrahamsson, B.; Williams, J.G.; Kumar, D.; Basit, A.W. Drug Solubility in Luminal Fluids from Different Regions of the Small and Large Intestine of Humans. Mol. Pharm. 2010, 7, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Hens, B.; Tsume, Y.; Bermejo, M.; Paixao, P.; Koenigsknecht, M.J.; Baker, J.R.; Hasler, W.L.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low Buffer Capacity and Alternating Motility along the Human Gastrointestinal Tract: Implications for in Vivo Dissolution and Absorption of Ionizable Drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the Human Upper Gastrointestinal Contents Under Conditions Simulating Bioavailability/Bioequivalence Studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef]
- Perez de la Cruz Moreno, M.; Oth, M.; Deferme, S.; Lammert, F.; Tack, J.; Dressman, J.; Augustijns, P. Characterization of Fasted-State Human Intestinal Fluids Collected from Duodenum and Jejunum. J. Pharm. Pharmacol. 2006, 58, 1079–1089. [Google Scholar] [CrossRef]
- Persson, E.M.; Gustafsson, A.-S.; Carlsson, A.S.; Nilsson, R.G.; Knutson, L.; Forsell, P.; Hanisch, G.; Lennernäs, H.; Abrahamsson, B. The Effects of Food on the Dissolution of Poorly Soluble Drugs in Human and in Model Small Intestinal Fluids. Pharm. Res. 2005, 22, 2141–2151. [Google Scholar] [CrossRef]
- Borgström, B.; Erlanson, C. Pancreatic Lipase and Co-Lipase. Eur. J. Biochem. 1973, 37, 60–68. [Google Scholar] [CrossRef]
- Brogström, B.; Hildebrand, H. Lipase and Co-Lipase Activities of Human Small Intestinal Contents after a Liquid Test Meal. Scand. J. Gastroenterol. 1975, 10, 585–591. [Google Scholar] [CrossRef]
- McClements, D.J.; Dungan, S.R. Factors That Affect the Rate of Oil Exchange between Oil-in-Water Emulsion Droplets Stabilized by a Nonionic Surfactant: Droplet Size, Surfactant Concentration, and Ionic Strength. J. Phys. Chem. 1993, 97, 7304–7308. [Google Scholar] [CrossRef]
- Malaki Nik, A.; Wright, A.J.; Corredig, M. Micellization of Beta-Carotene from Soy-Protein Stabilized Oil-in-Water Emulsions under In Vitro Conditions of Lipolysis. J. Am. Oil Chem. Soc. 2011, 88, 1397–1407. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of Chitosan Coating on Curcumin Loaded Nano-Emulsion: Study on Stability and In Vitro Digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Maestre, A.; Guardado, P.; Moyá, M.L. Thermodynamic Study of Bile Salts Micellization. J. Chem. Eng. Data 2014, 59, 433–438. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Wunderlich, M.; Brauns, U.; Becker, R.; Bock, T.; Dressman, J.B. Predicting the Precipitation of Poorly Soluble Weak Bases upon Entry in the Small Intestine. J. Pharm. Pharmacol. 2004, 56, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yeap, Y.Y.; Trevaskis, N.L.; Porter, C.J.H. The Potential for Drug Supersaturation during Intestinal Processing of Lipid-Based Formulations May Be Enhanced for Basic Drugs. Mol. Pharm. 2013, 10, 2601–2615. [Google Scholar] [CrossRef]
- Gu, C.H.; Rao, D.; Gandhi, R.B.; Hilden, J.; Raghavan, K. Using a Novel Multicompartment Dissolution System to Predict the Effect of Gastric pH on the Oral Absorption of Weak Bases with Poor Intrinsic Solubility. J. Pharm. Sci. 2005, 94, 199–208. [Google Scholar] [CrossRef]
- Stillhart, C.; Dürr, D.; Kuentz, M. Toward an Improved Understanding of the Precipitation Behavior of Weakly Basic Drugs from Oral Lipid-Based Formulations. J. Pharm. Sci. 2014, 103, 1194–1203. [Google Scholar] [CrossRef]






| Tris-Maleate Concentration (mM) | Carvedilol Solubility at 37 °C (mg/L) | pH |
|---|---|---|
| 2 | 18.1 ± 0.9 | 7.53 ± 0.06 |
| 50 | 15.8 ± 1.7 | 7.52 ± 0.04 |
| 200 | 15.3 ± 1.0 | 7.48 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rede, K.; Gašperlin, M.; Bogataj, M.; Bolko Seljak, K. The Advancement of In Vitro Lipolysis: Two-Step Flow-Through Method for the Evaluation of Lipid-Based Drug Delivery Systems. Pharmaceutics 2025, 17, 545. https://doi.org/10.3390/pharmaceutics17050545
Rede K, Gašperlin M, Bogataj M, Bolko Seljak K. The Advancement of In Vitro Lipolysis: Two-Step Flow-Through Method for the Evaluation of Lipid-Based Drug Delivery Systems. Pharmaceutics. 2025; 17(5):545. https://doi.org/10.3390/pharmaceutics17050545
Chicago/Turabian StyleRede, Katarina, Mirjana Gašperlin, Marija Bogataj, and Katarina Bolko Seljak. 2025. "The Advancement of In Vitro Lipolysis: Two-Step Flow-Through Method for the Evaluation of Lipid-Based Drug Delivery Systems" Pharmaceutics 17, no. 5: 545. https://doi.org/10.3390/pharmaceutics17050545
APA StyleRede, K., Gašperlin, M., Bogataj, M., & Bolko Seljak, K. (2025). The Advancement of In Vitro Lipolysis: Two-Step Flow-Through Method for the Evaluation of Lipid-Based Drug Delivery Systems. Pharmaceutics, 17(5), 545. https://doi.org/10.3390/pharmaceutics17050545





