Membrane-Mediated Action of Phosphodiesterase 5 Inhibitors
Abstract
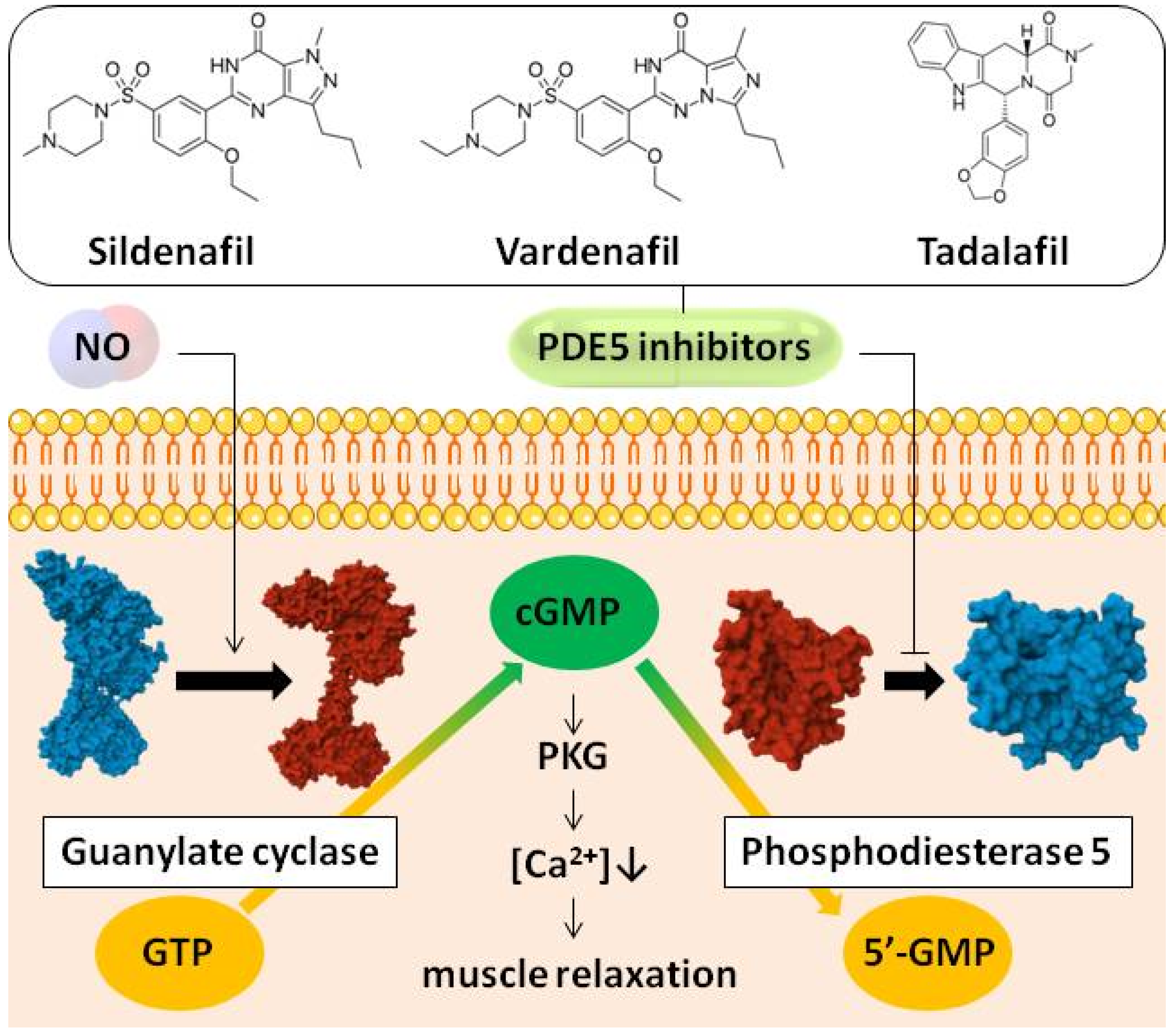
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Differential Scanning Microcalorimetry (DSC)
2.3. Molecular Dynamics Simulation (MD)
3. Results and Discussion
3.1. PDE Inhibitors Change Elastic Properties of Model Membranes
3.2. PDE Inhibitors Affect Elastic Properties of Lipid Rafts and NO Diffusion Coefficient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDE5 | Phosphodiesterase 5 |
| cGMP | Guanosine monophosphate |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DAPC | 1,2-diarachidoyl-sn-glycero-3-phosphocholine |
| POPI | 1-palmitoyl-2-oleoyl-inositol |
| POPS | 3-palmitoyl-2-oleoyl- D-glycero-1-phosphatidylserine |
| POPC | 3-palmitoyl-2-oleoyl-D-glycero-1-phosphatidylcholine |
| POPE | 3-palmitoyl-2-oleoyl-D-glycero-1-phosphatidylethanolamine |
| SSM | sphingomyelin |
References
- Andersson, K.E. PDE5 inhibitors—Pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 2018, 175, 2554–2565. [Google Scholar] [CrossRef]
- Samidurai, A.; Xi, L.; Das, A.; Kukreja, R.C. Beyond Erectile Dysfunction: CGMP-Specific Phosphodiesterase 5 Inhibitors for Other Clinical Disorders. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 585–615. [Google Scholar] [CrossRef]
- Luo, W.; Liu, R.; Cai, X.; Zhou, Q.; Zhang, C. Molecular Dynamics-Assisted Discovery of Novel Phosphodiesterase-5 Inhibitors Targeting a Unique Allosteric Pocket. Molecules 2025, 30, 588. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.A.; Hari Priya, V.M.; Baby, K.; Bindhu, S.; Jayan, R.; Krishnamoorthi, R.; Somappa, S.B.; Nayak, Y.; Kumaran, A. Flavone-C-glycosides from Cassia auriculata L. as possible inhibitors of phosphodiesterase-5 (PDE5): In vitro, molecular docking and molecular dynamics studies. J. Biomol. Struct. Dyn. 2024, 26, 1–23. [Google Scholar] [CrossRef]
- Dash, P.; Bala Divya, M.; Guruprasad, L.; Guruprasad, K. Three-dimensional models of Mycobacterium tuberculosis proteins Rv1555, Rv1554 and their docking analyses with sildenafil, tadalafil, vardenafil drugs, suggest interference with quinol binding likely to affect protein’s function. BMC Struct. Biol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Yin, D.; Ong, J.M.; Hu, J.; Konda, B.M.; Wang, X.; Ko, M.K.; Bayan, J.A.; Sacapano, M.R.; Espinoza, A.; et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008, 1230, 290–302. [Google Scholar] [CrossRef]
- Li, Q.; Shu, Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm. Res. 2014, 31, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, W.; Zhang, Q.; Liu, Y.; Qiao, X.; Meng, K.; Mao, Y. Phosphodiesterase type 5 inhibitor Tadalafil increases Rituximab treatment efficacy in a mouse brain lymphoma model. J. Neurooncol. 2015, 122, 35–42. [Google Scholar] [CrossRef]
- Hu, J.; Ljubimova, J.Y.; Inoue, S.; Konda, B.; Patil, R.; Ding, H.; Espinoza, A.; Wawrowsky, K.A.; Patil, C.; Ljubimov, A.V.; et al. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS ONE 2010, 5, e10108. [Google Scholar] [CrossRef]
- Sinha, B.; Köster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef]
- Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Phosphodiesterase Type 5 Inhibitors Greatly Affect Physicochemical Properties of Model Lipid Membranes. Membranes 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Available online: https://go.drugbank.com (accessed on 10 December 2024).
- Vanommeslaeghe, K.; MacKerell, A.D. Automation of the CHARMM General Force Field (CGenFF) I: Bond perception and atom typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Mishra, S.; Meuwly, M. Nitric oxide dynamics in truncated hemoglobin: Docking sites, migration pathways, and vibrational spectroscopy from molecular dynamics simulations. Biophys. J. 2009, 96, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef]
- Baron, C.B.; Coburn, R.F. Smooth muscle raft-like membranes. J. Lipid Res. 2004, 45, 41–53. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Bernetti, M.; Bussi, G. Pressure control using stochastic cell rescaling. J. Chem. Phys. 2020, 153, 114107. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Guixà-González, R.; Rodriguez-Espigares, I.; Ramírez-Anguita, J.M.; Carrió-Gaspar, P.; Martinez-Seara, H.; Giorgino, T.; Selent, J. MEMBPLUGIN: Studying membrane complexity in VMD. Bioinformatics 2014, 30, 1478–1480. [Google Scholar] [CrossRef]
- Yuan, H.; Jameson, C.J.; Murad, S. Exploring gas permeability of lipid membranes using coarse-grained molecular dynamics. Mol. Simul. 2009, 35, 953–961. [Google Scholar] [CrossRef]
- Slater, J.L.; Huang, C.H. Interdigitated bilayer membranes. Prog. Lipid Res. 1988, 27, 325–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Guo, T. Phase Behavior of Lipid Bilayers: A Dissipative Particle Dynamics Simulation Study. Adv. Theory Simul. 2018, 1, 1800013. [Google Scholar] [CrossRef]
- Mavromoustakos, T.; Chatzigeorgiou, P.; Koukoulitsa, C.; Durdagi, S. Partial interdigitation of lipid bilayers. Int. J. Quantum Chem. 2010, 111, 1172–1183. [Google Scholar] [CrossRef]
- Smith, E.A.; Dea, P.K. Differential Scanning Calorimetry Studies of Phospholipid Membranes: The Interdigitated Gel Phase. In Applications of Calorimetry in a Wide Context—Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Chen, J.J.; Sun, Y.L.; Tiwari, A.K.; Xiao, Z.J.; Sodani, K.; Yang, D.H.; Vispute, S.G.; Jiang, W.Q.; Chen, S.D.; Chen, Z.S. PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C10) transporter. Cancer Sci. 2012, 103, 1531–1537. [Google Scholar] [CrossRef]
- Ding, P.R.; Tiwari, A.K.; Ohnuma, S.; Lee, J.W.; An, X.; Dai, C.L.; Lu, Q.S.; Singh, S.; Yang, D.H.; Talele, T.T.; et al. The phosphodiesterase-5 inhibitor vardenafil is a potent inhibitor of ABCB1/P-glycoprotein transporter. PLoS ONE 2011, 6, e19329. [Google Scholar] [CrossRef]
- Möller, M.N.; Denicola, A. Diffusion of nitric oxide and oxygen in lipoproteins and membranes studied by pyrene fluorescence quenching. Free Radic. Biol. Med. 2018, 128, 137–143. [Google Scholar] [CrossRef]
- Denicola, A.; Souza, J.M.; Radi, R.; Lissi, E. Nitric oxide diffusion in membranes determined by fluorescence quenching. Arch. Biochem. Biophys. 1996, 328, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Lomnicka, M.; Hyde, J.S. Permeability of nitric oxide through lipid bilayer membranes. Free Radic. Res. 1996, 24, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mamonov, A.A.; Stefanov, V.E.; Shchegolev, B.F. Molecular dynamics investigation of nitric oxide (II) interaction with a model biological membrane. Biochem. Moscow Suppl. Ser. A 2009, 3, 231–238. [Google Scholar] [CrossRef]



| PDE Inhibitors | Ratio | ΔTm, °C | ΔΔTb, °C | ΔΔTh, °C | ΔΔH, kcal/mol |
|---|---|---|---|---|---|
| vardenafil | 100:1 | −0.1 | 0.4 | 0 | −0.1 |
| 50:1 | −0.2 | 0.5 | 0 | −0.2 | |
| 25:1 | −0.3 | 0.7 | 0.1 | −0.6 | |
| 10:1 | −0.3 | 0.8 | 0.2 | −0.7 | |
| 5:1 | −0.1 | 0.9 | 0.3 | −0.9 | |
| sildenafil | 100:1 | −0.1 | 0.1 | 0 | 0 |
| 50:1 | −0.2 | 0.2 | 0 | −0.1 | |
| 25:1 | −0.3 | 0.2 | 0 | −0.2 | |
| 10:1 | −0.3 | 0.3 | 0 | −0.2 | |
| 5:1 | −0.2 | 0.3 | 0.1 | −0.3 | |
| tadalafil | 100:1 | −0.1 | 0.2 | 0 | −0.1 |
| 50:1 | −0.1 | 0.3 | 0.1 | −0.2 | |
| 25:1 | −0.2 | 0.3 | 0.1 | −0.3 | |
| 10:1 | −0.2 | 0.3 | 0.2 | −0.3 | |
| 5:1 | −0.2 | 0.4 | 0.2 | −0.4 |
| Area per Lipid, Å2 | Thickness, Å | |
|---|---|---|
| control | 42.42 ± 0.39 | 46.42 ± 0.40 |
| vardenafil | 46.64 ± 0.83 | 44.07 ± 0.55 |
| sildenafil | 48.48 ± 0.75 | 44.22 ± 0.51 |
| tadalafil | 44.72 ± 0.69 | 45.01 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malykhina, A.I.; Efimova, S.S.; Ostroumova, O.S. Membrane-Mediated Action of Phosphodiesterase 5 Inhibitors. Pharmaceutics 2025, 17, 563. https://doi.org/10.3390/pharmaceutics17050563
Malykhina AI, Efimova SS, Ostroumova OS. Membrane-Mediated Action of Phosphodiesterase 5 Inhibitors. Pharmaceutics. 2025; 17(5):563. https://doi.org/10.3390/pharmaceutics17050563
Chicago/Turabian StyleMalykhina, Anna I., Svetlana S. Efimova, and Olga S. Ostroumova. 2025. "Membrane-Mediated Action of Phosphodiesterase 5 Inhibitors" Pharmaceutics 17, no. 5: 563. https://doi.org/10.3390/pharmaceutics17050563
APA StyleMalykhina, A. I., Efimova, S. S., & Ostroumova, O. S. (2025). Membrane-Mediated Action of Phosphodiesterase 5 Inhibitors. Pharmaceutics, 17(5), 563. https://doi.org/10.3390/pharmaceutics17050563






