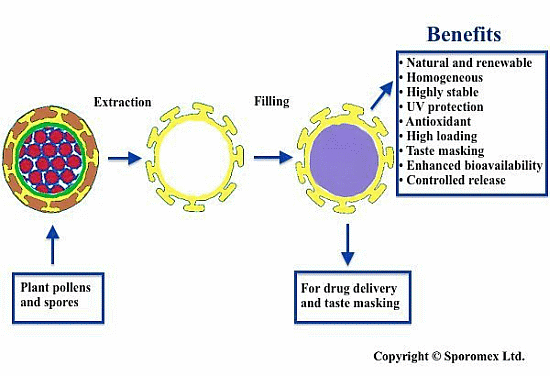
Hollow Pollen Shells to Enhance Drug Delivery
Abstract
:1. Introduction


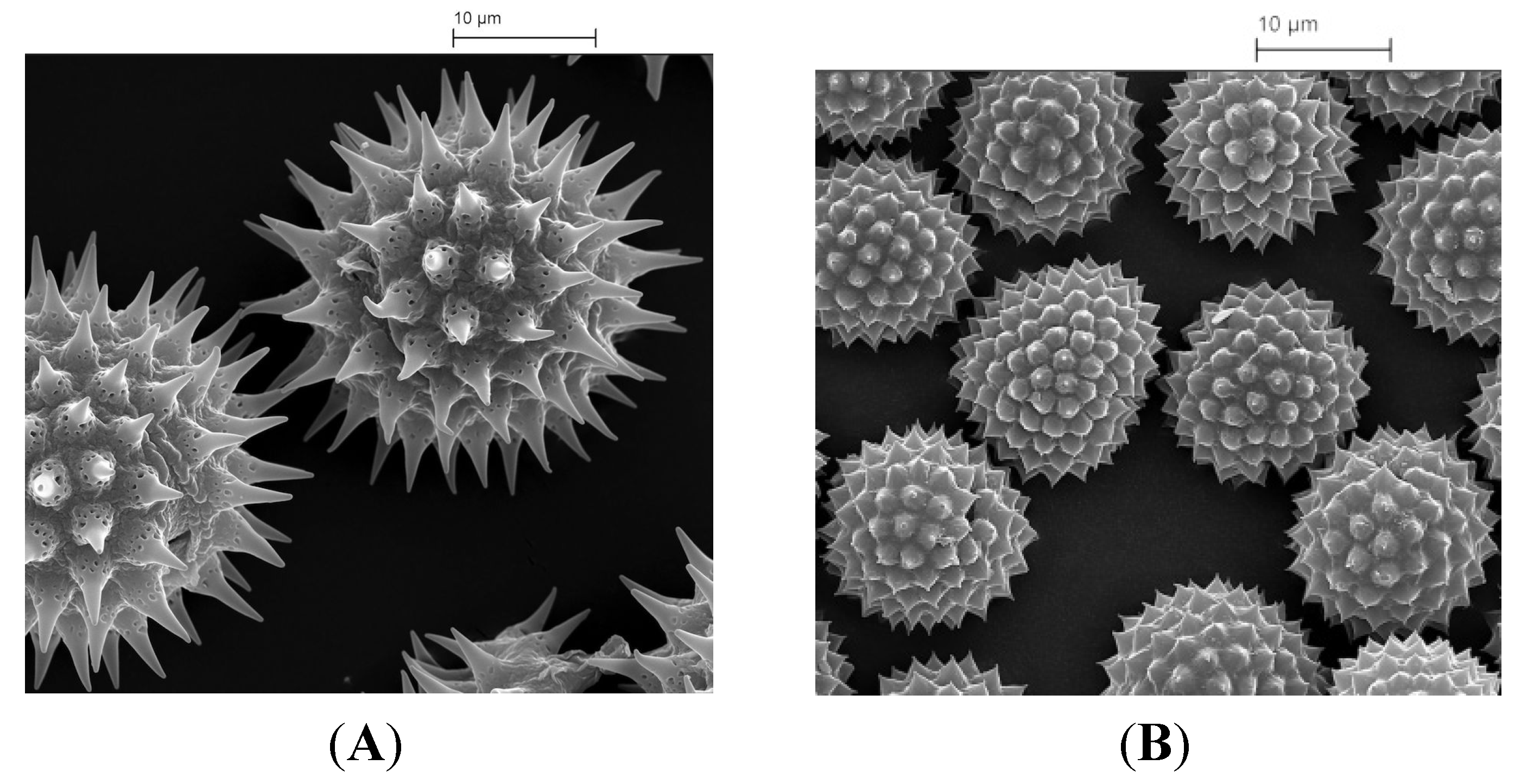
2. Extraction of Pollen Shells
2.1. Chemical Extraction
2.2. Enzymatic Extraction
3. Chemical Structure of Pollen Shells (Sporopollenin)
4. Physical and Chemical Properties of Pollen Shells
5. Encapsulation and Release of an Active
5.1. Filling the Shell

5.2. Release of the Active Material

6. Enhance Bioavailability
7. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, F.; Li, D.X. Morphology-controlled synthesis of SiO2 hollow microspheres using pollen grain as a biotemplate. Biomed. Mater. 2009. [Google Scholar] [CrossRef]
- Guan, Z.-S.; Zhang, Y.; Lu, C.-H.; Xu, Z.-Z. Morphology-controlled synthesis of SiO2 hierarchical structures using pollen grains as templates. Chin. J. Chem. 2008, 26, 467–470. [Google Scholar] [CrossRef]
- Hall, S.R.; Bolger, H.; Mann, S. Morphosynthesis of complex inorganic forms using pollen grain templates. Chem. Commun. 2003, 2784–2785. [Google Scholar] [CrossRef]
- Brandon Goodwin, W.; Gomez, I.J.; Fang, Y.; Meredith, J.C.; Sandhage, K.H. Conversion of pollen particles into three-dimensional ceramic replicas tailored for multimodal adhesion. Chem. Mater. 2013, 25, 4529–4536. [Google Scholar] [CrossRef]
- Adamson, R.; Gregson, S.; Shaw, G. New applications of sporopollenin as a solid-phase support for peptide-synthesis and the use of sonic agitation. Int. J. Pept. Protein Res. 1983, 22, 560–564. [Google Scholar] [CrossRef]
- Mackenzie, G.; Shaw, G. Sporopollenin. A novel, naturally occuring support for solid phase peptide synthesis. Int. J. Pept. Protein Res. 1980, 15, 298–300. [Google Scholar] [CrossRef]
- Shaw, G.; Sykes, M.; Humble, R.W.; Mackenzie, G.; Marsden, D.; Pehlivan, E. The use of modified sporopollenin from lycopodium clavatum as a novel ion- or ligand-exchange medium. React. Polym. Ion Exch. Sorbents 1988, 9, 211–217. [Google Scholar] [CrossRef]
- Paunov, V.N.; Mackenzie, G.; Stoyanov, S.D. Sporopollenin micro-reactors for in situ preparation, encapsulation and targeted delivery of active components. J. Mater. Chem. 2007, 17, 609–612. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Sporopollenin in the biological context. In Sporopollenin; Brooks, J., Ed.; London & New York Academic Press: London, UK, 1971; pp. 1–30. [Google Scholar]
- Brooks, J.; Shaw, G. Chemical structure of the exine of pollen walls and a new function for carotenoids in nature. Nature 1968, 219, 532–533. [Google Scholar] [CrossRef]
- Brooks, J.; Shaw, G. Geochemistry of sporopollenin. Chem. Geol. 1972, 10, 69–87. [Google Scholar] [CrossRef]
- Shaw, G. Sporopollenin. In Phytochemical Phylogeny; Harborne, J.B., Ed.; Academic Press: New York, NY, USA, 1970; pp. 31–58. [Google Scholar]
- Shaw, G. The chemistry of sporopollenin. In Sporopollenin; Brooks, J., Grant, P.R., Muir, M., van Gijzel, P., Shaw, G., Eds.; Academic Press: New York, NY, USA, 1971; pp. 305–348. [Google Scholar]
- Dominguez, E.; Mercado, J.A.; Quesada, M.A.; Heredia, A. Pollen sporopollenin: Degradation and structural elucidation. Sex. Plant Reprod. 1999, 12, 171–178. [Google Scholar] [CrossRef]
- Hemsley, A.R.; Scott, A.C.; Barrie, P.J.; Chaloner, W.G. Studies of fossil and modern spore wall biomacromolecules using 13C solid state NMR. Ann. Bot. 1996, 78, 83–94. [Google Scholar] [CrossRef]
- Watson, J.S.; Fraser, W.T.; Sephton, M.A. Formation of a polyalkyl macromolecule from the hydrolysable component within sporopollenin during heating/pyrolysis experiments with lycopodium spores. J. Anal. Appl. Pyrolysis 2012, 95, 138–144. [Google Scholar] [CrossRef]
- Thomasson, M.J.; Baldwin, D.J.; Diego-Taboada, A.; Atkin, S.L.; Mackenzie, G.; Wadhawan, J.D. Electrochemistry and charge transport in sporopollenin particle arrays. Electrochem. Commun. 2010, 12, 1428–1431. [Google Scholar] [CrossRef]
- Pettitt, J.M. A route for the passage of substances through the developing pteridophyte exine. Protoplasma 1976, 88, 117–131. [Google Scholar] [CrossRef]
- Rowley, J.R.; Skvarla, J.J.; El-Ghazaly, G. Transfer of material through the microspore exine—From the loculus into the cytoplasm. Can. J. Bot. 2003, 81, 1070–1082. [Google Scholar] [CrossRef]
- Diego-Taboada, A.; Maillet, L.; Banoub, J.H.; Lorch, M.; Rigby, A.S.; Boa, A.N.; Atkin, S.L.; Mackenzie, G. Protein free microcapsules obtained from plant spores as a model for drug delivery: Ibuprofen encapsulation, release and taste masking. J. Mater. Chem. 2013, 1, 707–713. [Google Scholar]
- Wolken, W.A.M.; Tramper, J.; van der Werf, M.J. What can spores do for us? Trends Biotechnol. 2003, 21, 338–345. [Google Scholar]
- Elist, J. Effects of pollen extract preparation prostat/poltit on lower urinary tract symptoms in patients with chronic nonbacterial prostatitis/chronic pelvic pain syndrome: A randomized, double-blind, placebo-controlled study. Urology 2006, 67, 60–63. [Google Scholar] [CrossRef]
- Winther, K.; Rein, E.; Hedman, C. Femal, a herbal remedy made from pollen extracts, reduces hot flushes and improves quality of life in menopausal women: A randomized, placebo-controlled, parallel study. Climacteric 2005, 8, 162–170. [Google Scholar] [CrossRef]
- Zetzsche, F.; Huggler, K. Untersuchungen über die membran des sporen und pollen I. 1. Lycopodium clavatum L. Justus Liebigs Annalen der Chemie 1928, 461, 89–108. [Google Scholar] [CrossRef]
- Zetzsche, F.; Kälin, O. Untersuchungen über die membran der sporen und pollen V. 4. Zur autoxydation der sporopollenine. Helv. Chim. Acta 1931, 14, 517–519. [Google Scholar] [CrossRef]
- Zetzsche, F.; Kalt, P.; Lietchi, J.; Ziegler, E. Zur konstitution des lycopodium-sporonins, des tasmanins und des lange-sporonins. J. Für Prakt. Chem. 1937, 148, 267–286. [Google Scholar] [CrossRef]
- Zetzsche, F.; Vicari, H. Untersuchungen über die membran der sporen und pollen. III. 2. Picea orientalis, Pinus sylvestris L., Corylus avellana L. Helv. Chim. Acta 1931, 14, 62–67. [Google Scholar] [CrossRef]
- Guilford, W.J.; Schneider, D.M.; Labovitz, J.; Opella, S.J. High-resolution solid-state 13C NMR spectroscopy of sporopollenins from different plant taxa. Plant Physiol. 1988, 86, 134–136. [Google Scholar] [CrossRef]
- Shaw, G.; Apperley, D.C. 13C NMR spectra of Lycopodium clavatum sporopollenin and oxidatively polymerised β-carotene. Grana 1996, 35, 125–127. [Google Scholar] [CrossRef]
- Boasman, A.J. Investigation into the amination and thiolation of sporopollenin. Ph.D. Thesis, The University of Hull, Hull, UK, 30 September 2003. [Google Scholar]
- Kettley, S.J. Novel derivatives of sporopollenin for potential applications in solid phase organic synthesis and drug delivery. Ph.D. Thesis, The University of Hull, Hull, UK, 28 September 2001. [Google Scholar]
- Shaw, G.; Yeadon, A. Chemical studies on the constitution of some pollen and spore membranes. Grana Palynol. 1964, 5, 247–252. [Google Scholar] [CrossRef]
- Shaw, G.; Yeadon, A. Chemical studies on the constitution of some pollen and spore membranes. J. Chem. Soc. 1966, 16–22. [Google Scholar]
- Green, D. A radiochemical study of spores and sporopollenin. Ph.D. Thesis, The University of Bradford, Bradford, UK, 1 August 1973. [Google Scholar]
- Atkin, S.L.; Beckett, S.T.; Mackenzie, G. Dosage Form Comprising an Exine Coating of Sporopollenin or Derivatized Sporopollenin. WO Patent 2005000280, 6 January 2005. [Google Scholar]
- Amer, M.S.; Tawashi, R. Modified Pollen Grains for Delivering Biologically Active Substances to Plants and Animals. U.S. Patent Patent 5,013,552A, 7 May 1991. [Google Scholar]
- Amer, M.S.; Tawashi, R. Drug Loaded Pollen Grains with an Outer Coating for Pulsed Delivery. U.S. Patent 5,275,819A, 4 January 1994. [Google Scholar]
- Tawashi, R. Drug Delivery of Nitric Oxide. U.S. Patent 5,648,101A, 15 July 1997. [Google Scholar]
- Hesse, M.; Waha, M. A new look at the acetolysis method. Plant Syst. Evol. 1989, 163, 147–152. [Google Scholar]
- Erdtman, G. The acetolysis method. A revised description. Svensk Botanisk Tidskrift 1960, 54, 561–564. [Google Scholar]
- Domínguez, E.; Mercado, J.A.; Quesada, M.A.; Heredia, A. Isolation of intact pollen exine using anhydrous hydrogen fluoride. Grana 1998, 37, 93–96. [Google Scholar] [CrossRef]
- Baldi, B.G.; Franceschi, V.R.; Loewus, F.A. Preparation and properties of pollen sporoplasts. Protoplasma 1987, 141, 47–55. [Google Scholar] [CrossRef]
- Gubatz, S.; Rittscher, M.; Meuter, A.; Nagler, A.; Wiermann, R. Tracer experiments on sporopollenin biosynthesis—An overview. Grana 1993, 32, 12–17. [Google Scholar]
- Jungfermann, C.; Ahlers, F.; Grote, M.; Gubatz, S.; Steuernagel, S.; Thom, I.; Wetzels, G.; Wiermann, R. Solution of sporopollenin and reaggregation of a sporopollenin-like material: A new approach in the sporopollenin research. J. Plant Physiol. 1997, 151, 513–519. [Google Scholar] [CrossRef]
- Ahlers, F.; Lambert, J.; Wiermann, R. Structural elements of sporopollenin from the pollen of torreya Californica torr. (gymnospermae): Using the 1H NMR technique. Zeitschrift Für Naturforschung 1999, 54, 492–495. [Google Scholar]
- Herminghaus, S.; Gubatz, S.; Arendt, S.; Wiermann, R. The occurrence of phenols as degradation products of natural sporopollenin—A comparison with synthetic sporopollenin. Zeitschrift Für Naturforschung 1988, 43, 491–500. [Google Scholar]
- Schulze Osthoff, K.; Wiermann, R. Phenols as integrated compounds of sporopollenin from pinus pollen. J. Plant Physiol. 1987, 131, 5–15. [Google Scholar] [CrossRef]
- Couderchet, M.; Schmalfuß, J.; Böger, P. Incorporation of oleic acid into sporopollenin and its inhibition by the chloroacetamide herbicide metazachlor. Pestic. Biochem. Physiol. 1996, 55, 189–199. [Google Scholar] [CrossRef]
- Brooks, J.; Shaw, G. Recent advances in the chemistry and geochemistry of pollen and spore walls. Trans. Bose Res. Inst. 1977, 40, 19–38. [Google Scholar]
- Brooks, J.; Shaw, G. Sporopollenin a review of its chemistry paleochemistry and geochemistry. Grana 1978, 17, 91–98. [Google Scholar] [CrossRef]
- Espelie, K.E.; Loewus, F.A.; Pugmire, R.J.; Woolfenden, W.R.; Baldi, B.G.; Given, P.H. Structural-analysis of lilium-longiflorum sporopollenin by 13C NMR spectroscopy. Phytochemistry 1989, 28, 751–753. [Google Scholar]
- Kawase, M.; Takahashi, M. Chemical-composition of sporopollenin in Magnolia grandiflora (magnoliaceae) and Hibiscus syriacus (malvaceae). Grana 1995, 34, 242–245. [Google Scholar] [CrossRef]
- Hayatsu, R.; Botto, R.E.; McBeth, R.L.; Scott, R.G.; Winans, R.E. Chemical alteration of a biological polymer “sporopollenin” during coalification: Origin, formation, and transformation of the coal maceral sporinite. Energy Fuels 1988, 2, 843–847. [Google Scholar] [CrossRef]
- Bubert, H.; Lambert, J.; Steuernagel, S.; Ahlers, F.; Wiermann, R. Continuous decomposition of sporopollenin from pollen of Typha angustifolia L. by acidic methanolysis. Zeitschrift Für Naturforschung 2002, 57, 1035–1041. [Google Scholar]
- Meuter-Gerhards, A.; Schwerdtfeger, C.; Steuernagel, S.; Wilmesmeier, S.; Wiermann, R. Studies on sporopollenin structure during pollen development. Zeitschrift Für Naturforschung 1995, 50, 487–492. [Google Scholar]
- Rittscher, M.; Wiermann, R. Studies on sporopollenin biosynthesis in Tulipa anthers. I. The development of an appropriate application method for radiolabeled precursors. Sex. Plant Reprod. 1988, 1, 125–131. [Google Scholar] [CrossRef]
- Rittscher, M.; Wiermann, R. Studies on sporopollenin biosynthesis in Tulipa anthers. Sex. Plant Reprod. 1988, 1, 132–139. [Google Scholar] [CrossRef]
- Wilmesmeier, S.; Steuernagel, S.; Wiermann, R. Comparative ftir and 13C cp/mas NMR spectroscopic investigations on sporopollenin of different systematic origins. Zeitschrift Fur Naturforschung 1993, 48, 697–701. [Google Scholar]
- De Leeuw, J.W.; Versteegh, G.J.M.; van Bergen, P.F. Biomacromolecules of algae and plants and their fossil analogues. Plant Ecol. 2006, 182, 209–233. [Google Scholar]
- Van Bergen, P.F.; Blokker, P.; Collinson, M.E.; Sinninghe Damsté, J.S.; de Leeuw, J.W. Structural biomacromolecules in plants: What can be learnt from the fossil record? In The Evolution of Plant Physiology. From Whole Plants to Ecosystems; Hemsley, A.R., Poole, I., Eds.; Elsevier Academic Press: London, UK, 2004; pp. 133–154. [Google Scholar]
- Ahlers, F.; Bubert, H.; Steuernagel, S.; Wiermann, R. The nature of oxygen in sporopollenin from the pollen of Typha angustifolia L. Zeitschrift Fur Naturforschung 2000, 55, 129–136. [Google Scholar]
- Barrier, S.; Diego-Taboada, A.; Thomasson, M.J.; Madden, L.; Pointon, J.C.; Wadhawan, J.D.; Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Viability of plant spore exine capsules for microencapsulation. J. Mater. Chem. 2011, 21, 975–981. [Google Scholar]
- Diego-Taboada, A.; Cousson, P.; Raynaud, E.; Huang, Y.; Lorch, M.; Binks, B.P.; Queneau, Y.; Boa, A.N.; Atkin, S.L.; Beckett, S.T.; et al. Sequestration of edible oil from emulsions using new single and double layered microcapsules from plant spores. J. Mater. Chem. 2012, 22, 9767–9773. [Google Scholar]
- Binks, B.P.; Boa, A.N.; Kibble, M.A.; Mackenzie, G.; Rocher, A. Sporopollenin capsules at fluid interfaces: Particle-stabilised emulsions and liquid marbles. Soft Matter 2011, 7, 4017–4024. [Google Scholar] [CrossRef]
- Potonié, R.; Rehnelt, K. Aspects of sporin. In Sporopollenin; Brooks, J., Grant, P.R., Muir, M., van Gijzel, P., Shaw, G., Eds.; Academic Press: New York, NY, USA, 1971; pp. 295–304. [Google Scholar]
- Wehling, K.; Niester, C.; Boon, J.J.; Willemse, M.T.M.; Wiermann, R. P-coumaric acid—A monomer in the sporopollenin skeleton. Planta 1989, 179, 376–380. [Google Scholar] [CrossRef]
- Gubatz, S.; Wiermann, R. Studies on sporopollenin biosynthesis in tulipa anthers. III. Incorporation of specifically labeled 14C-phenylalanine in comparison to other precursors. Bot. Acta 1992, 105, 407–413. [Google Scholar] [CrossRef]
- Ahlers, F.; Lambert, J.; Wiermann, R. Acetylation and silylation of piperidine solubilized sporopollenin from pollen of Typha angustifolia L. Zeitschrift Für Naturforschung 2003, 58, 807–811. [Google Scholar]
- Watson, J.S.; Sephton, M.A.; Sephton, S.V.; Self, S.; Fraser, W.T.; Lomax, B.H.; Gilmour, I.; Wellman, C.H.; Beerling, D.J. Rapid determination of spore chemistry using thermochemolysis gas chromatography-mass spectrometry and micro-fourier transform infrared spectroscopy. Photochem. Photobiol. Sci. 2007, 6, 689–694. [Google Scholar]
- Wiermann, R.; Gubatz, S. Pollen wall and sporopollenin. Int. Rev. Cytol. 1992, 140, 35–72. [Google Scholar] [CrossRef]
- Barrier, S.; Lobbert, A.; Boasman, A.J.; Boa, A.N.; Lorch, M.; Atkin, S.L.; Mackenzie, G. Access to a primary aminosporopollenin solid support from plant spores. Green Chem. 2010, 12, 234–240. [Google Scholar] [CrossRef]
- Fawcett, P.; Green, D.; Holleyhead, R.; Shaw, G. Application of radiochemical techniques to the determination of the hydroxyl content of some sporopollenins. Grana 1970, 10, 246–247. [Google Scholar] [CrossRef]
- Bohne, G.; Richter, E.; Woehlecke, H.; Ehwald, R. Diffusion barriers of tripartite sporopollenin microcapsules prepared from pine pollen. Ann. Bot. 2003, 92, 289–297. [Google Scholar] [CrossRef]
- Rozema, J.; Broekman, R.A.; Blokker, P.; Meijkamp, B.B.; de Bakker, N.; van de Staaij, J.; van Beem, A.; Ariese, F.; Kars, S.M. UV-B absorbance and UV-B absorbing compounds (p-coumaric acid) in pollen and sporopollenin: The perspective to track historic UV-B levels. J. Photochem. Photobiol. 2001, 62, 108–117. [Google Scholar]
- Atkin, S.L.; Barrier, S.; Cui, Z.; Fletcher, P.D.I.; Mackenzie, G.; Panel, V.; Sol, V.; Zhang, X. UV and visible light screening by individual sporopollenin exines derived from Lycopodium clavatum (club moss) and Ambrosia trifida (giant ragweed). J. Photochem. Photobiol. 2011, 102, 209–217. [Google Scholar]
- Atkin, S.L.; Beckett, S.T.; Mackenzie, G. Uses of Sporopollenin. U.S. Patent 7,846,654, 7 December 2010. [Google Scholar]
- Arrieta-Baez, D.; Dorantes-Alvarez, L.; Martinez-Torres, R.; Zepeda-Vallejo, G.; Jaramillo-Flores, M.E.; Ortiz-Moreno, A.; Aparicio-Ozores, G. Effect of thermal sterilization on ferulic, coumaric and cinnamic acids: Dimerization and antioxidant activity. J. Sci. Food Agric. 2012, 92, 2715–2720. [Google Scholar] [CrossRef]
- Atkin, S.L.; Beckett, S.T.; Mackenzie, G. Method of Obtaining Exine Shells of Naturally Occurring Spores. GB Patent 2,453,931, 2009. [Google Scholar]
- Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Dosage Form. U.S. Patent 7,608,270, 27 October 2009. [Google Scholar]
- Atkin, S.L.; Beckett, S.T.; Diego-Taboada, A.; Mackenzie, G. Formulations Comprising Exine Shells. U.S. Patent 20,110,002,984, 6 January 2011. [Google Scholar]
- Barrier, S.; Rigby, A.S.; Diego-Taboada, A.; Thomasson, M.J.; Mackenzie, G.; Atkin, S.L. Sporopollenin exines: A novel natural taste masking material. LWT Food Sci. Technol. 2010, 43, 73–76. [Google Scholar] [CrossRef]
- Atkin, S.L.; Barrier, S.; Beckett, S.T.; Brown, T.; Mackenzie, G.; Madden, L. Towards the use of sporopollenin in drug delivery: Efficient encapsulation of oligonucleotides. In Proceeding of Chemistry of Nucleic Acid Components, XIIIth Symposium, Špindlerův Mlýn, Czech Republic, 3–9 September 2005; Volume 7, pp. 307–311.
- Atkin, S.L.; Beckett, S.T.; Mackenzie, G. Topical Formulations Containing Sporopollenin. WO Patent 2007012857, 26 April 2007. [Google Scholar]
- Lorch, M.; Thomasson, M.J.; Diego-Taboada, A.; Barrier, S.; Atkin, S.L.; Mackenzie, G.; Archibald, S.J. MRI contrast agent delivery using spore capsules: Controlled release in blood plasma. Chem. Commun. 2009, 6442–6444. [Google Scholar]
- Wakil, A.; Mackenzie, G.; Diego-Taboada, A.; Bell, J.G.; Atkin, S.L. Enhanced bioavailability of eicosapentaenoic acid from fish oil after encapsulation within plant spore exines as microcapsules. Lipids 2010, 45, 645–649. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Diego-Taboada, A.; Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Hollow Pollen Shells to Enhance Drug Delivery. Pharmaceutics 2014, 6, 80-96. https://doi.org/10.3390/pharmaceutics6010080
Diego-Taboada A, Beckett ST, Atkin SL, Mackenzie G. Hollow Pollen Shells to Enhance Drug Delivery. Pharmaceutics. 2014; 6(1):80-96. https://doi.org/10.3390/pharmaceutics6010080
Chicago/Turabian StyleDiego-Taboada, Alberto, Stephen T. Beckett, Stephen L. Atkin, and Grahame Mackenzie. 2014. "Hollow Pollen Shells to Enhance Drug Delivery" Pharmaceutics 6, no. 1: 80-96. https://doi.org/10.3390/pharmaceutics6010080
APA StyleDiego-Taboada, A., Beckett, S. T., Atkin, S. L., & Mackenzie, G. (2014). Hollow Pollen Shells to Enhance Drug Delivery. Pharmaceutics, 6(1), 80-96. https://doi.org/10.3390/pharmaceutics6010080