Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles
Abstract
:1. Introduction
1.1. Nanomedicine and Blood Brain Barrier
1.2. Endocytosis of Nanomedicine
1.3. Endocytosis Mechanism and Fate of NPs
1.4. Trafficking inside the CNS
1.5. Rab GTPase in Vesicular Transport
1.6. How to Set up NP Trafficking
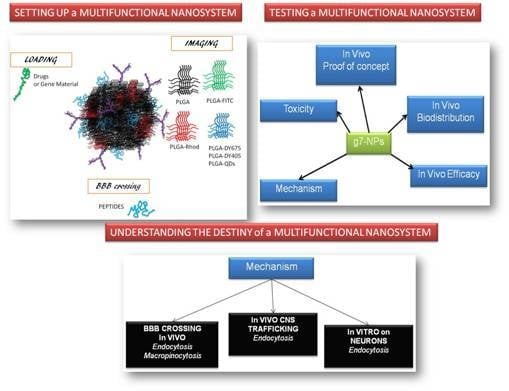
2. Case Analysis of g7-NPs
2.1. Nanoparticle Characterization and Confocal Protocols to Visualized NPs
2.2. Nanoparticle Accumulation in Endocytic Structures: Clathrin and Caveoline Positive Vesicles
2.3. Determination of Positive g7-NP-Early Endosomes

3. Commentary
3.1. Technological Aspect
3.2. In Vivo Experiments
3.3. In Vivo Sample Analysis
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lo, E.H.; Singhal, A.B.; Torchilin, V.P.; Abbott, N.J. Drug delivery to damaged brain. Brain Res. Rev. 2001, 38, 140–148. [Google Scholar] [CrossRef]
- Siegal, T.; Zylber-Katz, E. Strategies for increasing drug delivery to the brain: Focus on brain lymphoma. Clin. Pharmacokinet. 2002, 41, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Vergoni, A.V.; Ruozi, B.; Bondioli, L.; Badiali, L.; Rivasi, F.; Costantino, L.; Forni, F.; Vandelli, M.A. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J. Control. Release 2010, 145, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Costantino, L.; Ruozi, B.; Forni, F.; Vandelli, M.A. Polymeric nanoparticles for the drug delivery to the central nervous system. Exp. Opin. Drug Deliv. 2008, 5, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, A.; Azizi, M.; Thomsen, M.S.; Thomsen, L.B.; Moos, T. Accessing targeted nanoparticles to the brain: The vascular route. Curr. Med. Chem. 2014, 21, 4092–4099. [Google Scholar] [CrossRef] [PubMed]
- van der Meel, R.; Vehmeijer, L.J.; Kok, R.J.; Storm, G.; van Gaal, E.V. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; de Lange, E.C.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Rip, J.; Gaillard, P.J.; Björkman, S.; Hammarlund-Udenaes, M. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: A microdialysis study. Mol. Pharm. 2013, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Fiori, A.; Cardelli, P.; Negri, L.; Savi, M.R.; Strom, R.; Erspamer, V. Deltorphin transport across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1997, 94, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Fine, R.E.; Sandra, A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J. Cell Sci. 1993, 104, 521–532. [Google Scholar] [PubMed]
- Tabernero, A.; Velasco, A.; Granda, B.; Lavado, E.M.; Medina, J.M. Transcytosis of albumin in astrocytes activates the sterol regulatory element-binding protein-1, which promotes the synthesis of the neurotrophic factor oleic acid. J. Biol. Chem. 2002, 277, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.A.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Insulin enhancement of opioid peptide transport across the blood-brain barrier and assessment of analgesic effect. J. Pharmacol. Exp. Ther. 2000, 295, 972–978. [Google Scholar] [PubMed]
- Pang, Z.; Lu, W.; Gao, H.; Hu, K.; Chen, J.; Zhang, C.; Gao, X.; Jiang, X.; Zhu, C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J. Control. Release 2008, 128, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Pardridge, W.M. Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. USA 2000, 97, 7567–7572. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Boado, R.J.; Pardridge, W.M. Receptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomes. Pharm. Res. 2001, 18, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, J.; Wu, D.; Pardridge, W.M. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Reynolds, G.P. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr. Res. 2002, 55, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlachetzki, F.; Pardridge, W.M. Global non-viral gene transfer to the primate brain following intravenous administration. Mol. Ther. 2003, 7, 11–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlachetzki, F.; Zhang, Y.F.; Boado, R.J.; Pardridge, W.M. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 2004, 15, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Egleton, R.D.; Davis, T.P. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx 2005, 2, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.J.; Yeomans, L.; Keyari, C.M.; Davis, P.; Porreca, F.; Knapp, B.I.; Bidlack, J.M.; Bilsky, E.J.; Polt, R. Glycosylation improves the central effects of DAMGO. Chem. Biol. Drug Design 2007, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Gandolfi, F.; Tosi, G.; Rivasi, F.; Vandelli, M.A.; Forni, F. Peptide-derivatized biodegradable nanoparticles able to cross the blood-brain barrier. J. Control. Release 2005, 108, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Polt, R.; Dhanasekaran, M.; Keyari, C.M. Glycosylated neuropeptides: A new vista for neuropsychopharmacology? Med. Res. Rev. 2005, 25, 557–585. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Gandolfi, F.; Bossy-Nobs, L.; Tosi, G.; Gurny, R.; Rivasi, F.; Vandelli, M.A.; Forni, F. Nanoparticulate drug carriers based on hybrid poly(d,l-lactide-co-glycolide)-dendron structures. Biomaterials 2006, 27, 4635–4645. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Rivasi, F.; Gandolfi, F.; Costantino, L.; Vandelli, M.A.; Forni, F. Conjugated poly(d,l-lactide-co-glycolide) for the preparation of in vivo detectable nanoparticles. Biomaterials 2005, 26, 4189–4195. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Costantino, L.; Rivasi, F.; Ruozi, B.; Leo, E.; Vergoni, A.V.; Tacchi, R.; Bertolini, A.; Vandelli, M.A.; Forni, F. Targeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J. Control. Release 2007, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F. Brain-targeted polymeric nanoparticles: In vivo evidence of different routes of administration in rodents. Nanomedicine 2013, 8, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomedicine 2011, 6, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Richardson, S.C. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: Opportunities and challenges. Mol. Pharm. 2012, 9, 2380–2402. [Google Scholar] [CrossRef] [PubMed]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Lossinsky, A.S.; Shivers, R.R. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Rev. Histol. Histopathol. 2004, 19, 535–564. [Google Scholar]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Friedman, A. Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 2012, 53 (Suppl 6), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B.; Branson, M.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, M.; Palian, M.M.; Alves, I.; Yeomans, L.; Keyari, C.M.; Davis, P.; Bilsky, E.J.; Egleton, R.D.; Yamamura, H.I.; Jacobsen, N.E.; et al. Glycopeptides related to beta-endorphin adopt helical amphipathic conformations in the presence of lipid bilayers. J. Am. Chem. Soc. 2005, 127, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Gallop, J. Membrane curvature and mechanisms of dynamic cell membrane remodeling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Zimmemberg, G.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell. Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Nassif, X.; Bourdoulous, S.; Eugene, E.; Couraud, P.O. How do extracellular pathoges cross the blood brain barrier? Trends Microbiol. 2002, 10, 227–232. [Google Scholar] [CrossRef]
- Coureuil, M.; Mikaty, G.; Miller, F.; Lécuyer, H.; Bernard, C.; Bourdoulous, S.; Duménil, G.; Mège, R.M.; Weksler, B.B.; Romero, I.A.; et al. Meningococcal type IV Pili recruit the polarity complex to cross the brain endothelium. Science 2009, 325, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasadaro, N.V.; Wass, C.A.; Weiser, J.N.; Stins, M.F.; Huang, S.H.; Kim, K.S. Outer membrane protein A-promoted of Escherichi coli contributes to invasiton of brain microvascular endothelial cell. Infect. Immun. 1996, 64, 146–153. [Google Scholar]
- Vilella, A.; Tosi, G.; Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Vandelli, M.A.; Boeckers, T.M.; Forni, F.; Zoli, M. Insight on the fate of CNS-targeted nanoparticles. Part I: Rab5-dependent cell-specific uptake and distribution. J. Control. Release 2014, 174, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Vilella, A.; Chhabra, R.; Schmeisser, M.J.; Boeckers, T.M.; Ruozi, B.; Vandelli, M.A.; Forni, F.; Zoli, M.; Grabrucker, A.M. Insight on the fate of CNS-targeted nanoparticles. Part II: Intercellular neuronal cell-to-cell transport. J. Control. Release 2014, 177, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Grabrucker, A.M.; Veratti, P.; Belletti, D.; Boeckers, T.M.; Vandelli, M.A.; Forni, F.; Tosi, G.; Ruozi, B. Characterization of lysosome-destabilizing DOPE/PLGA nanoparticles designed for cytoplasmic drug release. Int. J. Pharm. 2014, 471, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluijs, P.; Hull, M.; Zahraoui, A.; Tavitian, A.; Goud, B.; Mellman, I. The small GTP-binding protein rab4 is associated with early endosomes. Proc. Natl. Acad. Sci. USA 1991, 88, 6313–6317. [Google Scholar]
- Gorvel, J.P.; Chavrier, P.; Zerial, M.; Gruenberg, J. rab5 controls early endosome fusion in vitro. Cell 1991, 64, 915–925. [Google Scholar] [CrossRef]
- Bucci, C.; Parton, R.G.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728. [Google Scholar] [CrossRef]
- Stenmark, H.; Valencia, A.; Martinez, O.; Ullrich, O.; Goud, B.; Zerial, M. Distinct structural elements of rab5 define its functional specificity. EMBO J. 1994, 13, 575–583. [Google Scholar] [PubMed]
- McLauchlan, H.; Newell, J.; Morrice, N.; Osborne, A.; West, M.; Smythe, E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998, 8, 34–45. [Google Scholar] [CrossRef]
- van der Sluijs, P.; Hull, M.; Webster, P.; Mâle, P.; Goud, B.; Mellman, I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992, 70, 729–740. [Google Scholar]
- Ullrich, O.; Reinsch, S.; Urbe, S.; Zerial, M.; Parton, R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996, 135, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, M.; Johannes, L.; Galli, T.; Mayau, V.; Goud, B.; Salamero, J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 2000, 151, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Press, B.; Wandinger-Ness, A. Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Biol. 1995, 131, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, R.; Santillo, M.; Lattero, D.; Chiariello, M.; Bifulco, M.; Bruni, C.B.; Bucci, C. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 1997, 272, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, D.; Soldati, T.; Riederer, M.A.; Goda, Y.; Zerial, M.; Pfeffer, S.R. Rab9 Functions in Transport between Late Endosomes and the Trans Golgi Network. EMBO J. 1993, 12, 677–682. [Google Scholar] [PubMed]
- Pascolo, L.; Bortot, B.; Benseny-Cases, N.; Gianoncelli, A.; Tosi, G.; Ruozi, B.; Rizzardi, C.; De Martino, E.; Vandelli, M.A.; Severini, G.M. Detection of PLGA-based nanoparticles at a single-cell level by synchrotron radiation FTIR spectromicroscopy and correlation with X-ray fluorescence microscopy. Int. J. Nanomed. 2014, 9, 2791–2801. [Google Scholar]
- Tosi, G.; Bondioli, L.; Ruozi, B.; Badiali, L.; Severini, G.M.; Biffi, S.; De Vita, A.; Bortot, B.; Dolcetta, D.; Forni, F.; et al. NIR-labeled nanoparticles engineered for brain targeting: In vivo optical imaging application and fluorescent microscopy evidences. J. Neural Transm. 2011, 118, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bondioli, L.; Costantino, L.; Ballestrazzi, A.; Lucchesi, D.; Boraschi, D.; Pellati, F.; Benvenuti, S.; Tosi, G.; Vandelli, M.A. PLGA nanoparticles surface decorated with the sialic acid, N-acetylneuraminic acid. Biomaterials 2010, 31, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Y.; Markham, K.; Xu, Z.P.; Chen, M.; Max Lu, G.Q.; Bartlett, P.F.; Cooper, H.M. Efficient delivery of siRNA to cortical neurons using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 8770–8779. [Google Scholar] [CrossRef] [PubMed]
- Harush-Frenkel, O.; Rozentur, E.; Benita, S.; Altschuler, Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules 2008, 9, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; de Hoop, M.; Zorzi, N.; Toh, B.H.; Dotti, C.G.; Parton, R.G. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol. Biol. Cell 2000, 11, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilella, A.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Galliani, M.; Semeghini, V.; Forni, F.; Zoli, M.; Vandelli, M.A.; Tosi, G. Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles. Pharmaceutics 2015, 7, 74-89. https://doi.org/10.3390/pharmaceutics7020074
Vilella A, Ruozi B, Belletti D, Pederzoli F, Galliani M, Semeghini V, Forni F, Zoli M, Vandelli MA, Tosi G. Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles. Pharmaceutics. 2015; 7(2):74-89. https://doi.org/10.3390/pharmaceutics7020074
Chicago/Turabian StyleVilella, Antonietta, Barbara Ruozi, Daniela Belletti, Francesca Pederzoli, Marianna Galliani, Valentina Semeghini, Flavio Forni, Michele Zoli, Maria Angela Vandelli, and Giovanni Tosi. 2015. "Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles" Pharmaceutics 7, no. 2: 74-89. https://doi.org/10.3390/pharmaceutics7020074
APA StyleVilella, A., Ruozi, B., Belletti, D., Pederzoli, F., Galliani, M., Semeghini, V., Forni, F., Zoli, M., Vandelli, M. A., & Tosi, G. (2015). Endocytosis of Nanomedicines: The Case of Glycopeptide Engineered PLGA Nanoparticles. Pharmaceutics, 7(2), 74-89. https://doi.org/10.3390/pharmaceutics7020074