Drug Delivery Approaches for the Treatment of Cervical Cancer
Abstract
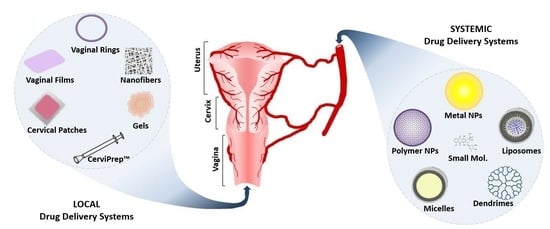
:1. Introduction
2. Systemic Drug Delivery Systems
2.1. Inorganic Nanocarriers
2.2. Polymeric Nanoparticles
2.3. Micelles
2.4. Liposomes
2.5. Dendrimers
2.6. Self-Emulsifying Drug Delivery Systems
2.7. Antibody–Drug Conjugates
3. Localized Drug Delivery Systems
3.1. Intra-Vaginal Rings
3.2. Nanofibers
3.3. Vaginal Films
3.4. Gels
3.5. Cervical Patches
3.6. CerviPrep™
4. Conclusion and Future Direction
Acknowledgments
Conflicts of Interest
References
- Shazly, S.A.M.; Murad, M.H.; Dowdy, S.C.; Gostout, B.S.; Famuyide, A.O. Robotic radical hysterectomy in early stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2015, 138, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Wang, X.; Yang, Y.; Wu, W.; Li, H.; Ma, Y.; Lin, W.; Sun, T.; Huang, Y.; Xie, Z.; et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur. J. Pharm. Biopharm. 2015, 93, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P. Cervical cancer: Can it be prevented? World. J. Clin. Oncol. 2014, 5, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Petrelli, F.; Coinu, A.; Raspagliesi, F.; Barni, S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014, 133, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; O’Connell, D.L.; Tan, J.; Lew, J.-B.; Demers, A.; Lotocki, R.; Kliewer, E.V.; Hacker, N.F.; Jackson, M.; Delaney, G.P.; et al. Optimal uptake rates for initial treatments for cervical cancer in concordance with guidelines in australia and canada: Results from two large cancer facilities. Cancer. Epidemiol. 2015, 39, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. New Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Pfaendler, K.S.; Tewari, K.S. Changing paradigms in the systemic treatment of advanced cervical cancer. Am. J. Obstet. Gynecol. 2016, 214, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Leisching, G.R.; Loos, B.; Botha, M.H.; Engelbrecht, A.M. The role of mtor during cisplatin treatment in an in vitro and ex vivo model of cervical cancer. Toxicol. 2015, 335, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Katanyoo, K.; Tangjitgamol, S.; Chongthanakorn, M.; Tantivatana, T.; Manusirivithaya, S.; Rongsriyam, K.; Cholpaisal, A. Treatment outcomes of concurrent weekly carboplatin with radiation therapy in locally advanced cervical cancer patients. Gynecol. Oncol. 2011, 123, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Zhao, Y.; Li, Z.; Gou, H.; Cao, D.; Yang, Y.; Qiu, M.; Li, Q.; Liu, J.; et al. Adjuvant intensity-modulated radiotherapy (IMRT) with concurrent paclitaxel and cisplatin in cervical cancer patients with high risk factors: A phase ii trial. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Downs, L.S., Jr.; Chura, J.C.; Argenta, P.A.; Judson, P.L.; Ghebre, R.; Geller, M.A.; Carson, L.F. Ifosfamide, paclitaxel, and carboplatin, a novel triplet regimen for advanced, recurrent, or persistent carcinoma of the cervix: A phase ii trial. Gynecol. Oncol. 2011, 120, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Muderspach, L.I.; Blessing, J.A.; Levenback, C.; Moore, J.L., Jr. A phase ii study of topotecan in patients with squamous cell carcinoma of the cervix: A gynecologic oncology group study. Gynecol. Oncol. 2001, 81, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Nurullahoglu Atalik, K.E.; Yerlikaya, F.H.; Demir, E.A. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol. Learn. Mem. 2015, 123, 43–49. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Soares, C.P.; Fontana, C.R. Synergistic effect of photodynamic therapy and cisplatin: A novel approach for cervical cancer. J. Photochem. Photobiol. B 2014, 140, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Bhattacharya, K.; Samanta, S.K.; Pal, B.C.; Mandal, C. Improved chemosensitivity in cervical cancer to cisplatin: Synergistic activity of mahanine through STAT3 inhibition. Cancer Lett. 2014, 351, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yohe, S.T.; Herrera, V.L.M.; Colson, Y.L.; Grinstaff, M.W. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J. Control. Release 2012, 162, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- McConville, C. The use of localised vaginal drug delivery as part of a neoadjuvant chemotherapy strategy in the treatment of cervical cancer. Gynecol. Obstet. Res. Open. J. 2015, 2, 26–28. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; en Henegouwen, P.M.V.B. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Karim, A.A.; Loh, X.J. Current treatment options and drug delivery systems as potential therapeutic agents for ovarian cancer: A review. Mater. Sci. Eng. 2014, 45, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedecine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, L.; Song, C.; Zeng, X.; Liu, G.; Mei, L. Nanoparticle formulation of poly(ɛ-caprolactone-co-lactide)-d-α-tocopheryl polyethylene glycol 1000 succinate random copolymer for cervical cancer treatment. Polymer 2010, 51, 5952–5959. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Q.; Peng, F.; Liu, L.; Gong, C. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B 2015, 135, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, A.R., Rathbone, J.M., Eds.; Springer US: Boston, MA, USA, 2012; pp. 19–43. [Google Scholar]
- Lucey, B.P.; Nelson-Rees, W.A.; Hutchins, G.M. Henrietta lacks, hela cells, and cell culture contamination. Archiv. Pathol. Lab. Med. 2009, 133, 1463–1467. [Google Scholar]
- Gillet, J.-P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. JNCI J. Nat. Cancer Inst. 2013, 105, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Kim, Y.; Zustiak, S.P. The role of biomaterials on cancer stem cell enrichment and behavior. JOM 2015, 67, 2543–2549. [Google Scholar] [CrossRef]
- de la Puente, P.; Muz, B.; Gilson, R.C.; Azab, F.; Luderer, M.; King, J.; Achilefu, S.; Vij, R.; Azab, A.K. 3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma. Biomate. 2015, 73, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mazinder Boruah, B.; Liang, X.-J. Gold nanoparticles: Promising nanomaterials for the diagnosis of cancer and hiv/aids. J. Nanomater. 2011, 2011, 17. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release 2011, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K.; et al. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid a431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ghosh, S.; Das, D.K.; Chakraborty, P.; Choudhury, S.; Gupta, P.; Adhikary, A.; Dey, S.; Chattopadhyay, S. Gold-conjugated green tea nanoparticles for enhanced anti-tumor activities and hepatoprotection—Synthesis, characterization and in vitro evaluation. J. Nutr. Biochem. 2015, 26, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B 2013, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver. I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Casañas Pimentel, R.; San Martín Martínez, E.; Monroy García, A.; Gómez-García, C.; Alvarado Palacios, Q.G. Silver nanoparticles nanocarriers, synthesis and toxic effect on cervical cancer cell lines. BioNanoSci. 2013, 3, 198–207. [Google Scholar] [CrossRef]
- Rajasekharreddy, P.; Rani, P.U. Biofabrication of ag nanoparticles using sterculia foetida l. Seed extract and their toxic potential against mosquito vectors and HeLa cancer cells. Mater. Sci. Eng. 2014, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Arun, R.; Sathishkumar, G.; MubarakAli, D.; Rajesh, M.; Sivanandhan, G.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Ganapathi, A. An evidence on G2/M arrest, DNA damage and caspase mediated apoptotic effect of biosynthesized gold nanoparticles on human cervical carcinoma cells (HeLa). Mater. Res. Bull. 2014, 52, 15–24. [Google Scholar] [CrossRef]
- Daduang, J.; Palasap, A.; Daduang, S.; Boonsiri, P.; Suwannalert, P.; Limpaiboon, T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tomoaia, G.; Horovitz, O.; Mocanu, A.; Nita, A.; Avram, A.; Racz, C.P.; Soritau, O.; Cenariu, M.; Tomoaia-Cotisel, M. Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf. B 2015, 135, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Firer, M.A.; Gellerman, G. Targeted drug delivery for cancer therapy: The other side of antibodies. J. Hematol. Oncol. 2012, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Wang, J.; Wang, L.; Tan, X.; Tu, K.; Tong, X.; Qi, L. Aptamer functionalized cisplatin-albumin nanoparticles for targeted delivery to epidermal growth factor receptor positive cervical cancer. J. Biomed. Nanotechnol. 2016, 12, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Vives, E.; Schmidt, J.; Pelegrin, A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta (BBA) Rev. Cancer 2008, 1786, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Siahaan, T.J. Peptide-mediated targeted drug delivery. Med. Res. Rev. 2012, 32, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Kamkaew, A.; Burgess, K.; Kiew, L.V.; Chung, L.Y.; Lee, H.B. Small molecules for active targeting in cancer. Med. Res. Rev. 2016, 36, 494–575. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Song, Q. Promising potency of retinoic acid-poly(ethylene glycol)-thiol gold nanoparticle conjugates for cervical cancer treatment. Int. J. Clin. Exp. Med. 2015, 8, 10501–10507. [Google Scholar] [PubMed]
- Alshatwi, A.A.; Athinarayanan, J.; Vaiyapuri Subbarayan, P. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. 2014, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frías González, S.E.; Angeles Anguiano, E.; Mendoza Herrera, A.; Escutia Calzada, D.; Ordaz Pichardo, C. Cytotoxic, pro-apoptotic, pro-oxidant, and non-genotoxic activities of a novel copper(ii) complex against human cervical cancer. Toxicol. 2013, 314, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Vaibhav, K.; Pandey, R.S.; Jain, U.K.; Katare, O.P.; Katyal, A.; Madan, J. Improved cisplatin delivery in cervical cancer cells by utilizing folate-grafted non-aggregated gelatin nanoparticles. Biomed. Pharmacother. 2015, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tao, W.; Mei, L.; Huang, L.; Tan, C.; Feng, S.-S. Cholic acid-functionalized nanoparticles of star-shaped P-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomater. 2013, 34, 6058–6067. [Google Scholar] [CrossRef] [PubMed]
- JOSHI, J.R.; PATEL, R.P. Role of biodegradable polymers in drug delivery. Int. J. Curr. Pharm. Res. 2012, 4, 74–81. [Google Scholar]
- Yang, H.; Li, K.; Liu, Y.; Liu, Z.; Miyoshi, H. Poly(d,l-lactide-co-glycolide) nanoparticles encapsulated fluorescent isothiocyanate and paclitaxol: Preparation, release kinetics and anticancer effect. J. Nanosci. Nanotechnol. 2009, 9, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Ji, M.; Song, X.; Zhu, Y.; Wang, Z.; Zhang, X.; Wu, S.; Chen, H.; Mei, L.; Zheng, Y. Co-delivery of docetaxel and endostatin by a biodegradable nanoparticle for the synergistic treatment of cervical cancer. Nanoscale Res. Lett. 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, Y.; Liu, K.; Tian, G.; Tian, Y.; Xu, L.; Yan, F.; Huang, L.; Mei, L. Nanoparticles of poly(lactide-co-glycolide)-d-a-tocopheryl polyethylene glycol 1000 succinate random copolymer for cancer treatment. Nanoscale Res. Lett. 2010, 5, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; RajendraPrasad, N.; Karthikeyan, S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Zeng, X.; Wu, Y.; Yang, C.; Mei, L.; Wang, Z.; Huang, L. Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. Int. J. Nanomed. 2015, 10, 2461–2473. [Google Scholar]
- Zhang, C.; Zhang, Z.; Zhao, L. Folate-decorated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting delivery: Optimization and in vivo antitumor activity. Drug Deliv. 2016, 23, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.J.; Shah, K.N.; Robishaw, N.K.; Panzner, M.J.; Youngs, W.J.; Yun, Y.H. The interactions between l-tyrosine based nanoparticles decorated with folic acid and cervical cancer cells under physiological flow. Mol. Pharm. 2012, 9, 3089–3098. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, H.; Zhang, J.; Zheng, W.; Chen, T. Rational design and fabrication of a cancer-targeted chitosan nanocarrier to enhance selective cellular uptake and anticancer efficacy of selenocystine. J. Mater. Chem. B 2015, 3, 2497–2504. [Google Scholar] [CrossRef]
- Ji, J.; Zuo, P.; Wang, Y.L. Enhanced antiproliferative effect of carboplatin in cervical cancer cells utilizing folate-grafted polymeric nanoparticles. Nanoscale Res. Lett. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Feng, X.; Zhang, T.; Dai, Y.; Zhou, Z.; Chen, H.; Liu, L.; Li, X.; Zhuang, T.; Liu, X.; et al. Stability, pharmacokinetics, biodistribution and safety assessment of folate-conjugated pullulan acetate nanoparticles as cervical cancer targeted drug carriers. J. Nanosci. Nanotechnol. 2015, 15, 6405–6412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; van Gaal, E.V.B.; Brundel, P.; Ippel, H.; Hackeng, T.; Rijcken, C.J.F.; Storm, G.; Hennink, W.E.; Prakash, J. A novel approach for the intravenous delivery of leuprolide using core-cross-linked polymeric micelles. J. Control. Release 2015, 205, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dong, D.; Fu, F.; Zheng, Y.H.; Liu, S.; Chang, M.X.; Jing, X.B. Anti-tumor activity of biodegradable polymer-paclitaxel conjugated micelle against mice U14 cervical cancers. Chem. Res. Chin. Univ. 2012, 28, 656–661. [Google Scholar]
- Guo, Q.; Guan, D.; Dong, B.; Nan, F.; Zhang, Y. Charge-conversional binary drug delivery polymeric micelles for combined chemotherapy of cervical cancer. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 978–987. [Google Scholar] [CrossRef]
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedecine 2015, 11, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Bansal, V.; Chandra, A.; Madan, J.; Jain, U.K.; Chandra, R.; Jain, S.M. Bleomycin sulphate loaded nanostructured lipid particles augment oral bioavailability, cytotoxicity and apoptosis in cervical cancer cells. Colloids Surf. B 2014, 118, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, N.; De Paoli, M.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical evaluation of a new liposomal formulation of cisplatin, lipoplatin, to treat cisplatin-resistant cervical cancer. Gynecol. Oncol. 2013, 131, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf. B 2014, 114, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; Liu, J.; Yang, Z.; Rong, X.; Li, M.; Liang, X.; Wu, Y. Transferrin conjugated poly(γ-glutamic acid-maleimide-co-l-lactide)-1,2-dipalmitoylsn-glycero-3-phosphoethanolamine copolymer nanoparticles for targeting drug delivery. Colloids Surf. B 2014, 123, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Mohd Amin, M.C.I.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Pamam dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Hussein, W.M.; Jia, Z.; Ziora, Z.M.; McMillan, N.A.J.; Monteiro, M.J.; Toth, I.; Skwarczynski, M. Self-adjuvanting polymer–peptide conjugates as therapeutic vaccine candidates against cervical cancer. Biomacromol. 2013, 14, 2798–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekuria, S.L.; Debele, T.A.; Chou, H.-Y.; Tsai, H.-C. Il-6 antibody and RGD peptide conjugated poly(amidoamine) dendrimer for targeted drug delivery of HeLa cells. J. Phys. Chem. B 2016, 120, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Neslihan Gursoy, R.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Holm, R.; Rades, T.; Müllertz, A. Characterising lipid lipolysis and its implication in lipid-based formulation development. AAPS J. 2012, 14, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Vecsernyés, M.; Bácskay, I. Formulation and characetization of self-microemulsifying drug delivery systems according to their cytotoxic attributes. Acta. Pharm. Hung. 2014, 84, 69–76. [Google Scholar] [PubMed]
- Nigade, P.M.; Patil, S.L.; Tiwari, S.S. Self emulsifying drug delivery system (SEDDS): A review. Int. J. of Pharm. Biol. Sci. 2012, 2, 42–52. [Google Scholar]
- Kumar, A.; Sharma, S.; Kamble, R. Self emulsifying drug delivery system (SEDDS): Future aspects. Int. J. Pharm. Pharm. Sci. 2010, 2, 7–13. [Google Scholar]
- Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Róka, E.; Fenyvesi, F.; Póka, R.; Kozma, B.; Bácskay, I. The enhanced inhibitory effect of different antitumor agents in self-microemulsifying drug delivery systems on human cervical cancer hela cells. Molecules 2015, 20, 13226–13239. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, D.M.; Kothari, P.R.; Kalhapure, R.S.; Akamanchi, K.G. Self-microemulsifying drug delivery system of curcumin with enhanced solubility and bioavailability using a new semi-synthetic bicephalous heterolipid: In vitro and in vivo evaluation. RSC Adv. 2015, 5, 90295–90306. [Google Scholar] [CrossRef]
- Bouchard, H.; Viskov, C.; Garcia-Echeverria, C. Antibody–drug conjugates—A new wave of cancer drugs. Bioorganic Med. Chem. Lett. 2014, 24, 5357–5363. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, I.; Blanc, V. Antibody–drug conjugate (ADC) clinical pipeline: A review. In Antibody-drug conjugates; Ducry, L., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 1–27. [Google Scholar]
- Peters, C.; Brown, S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.W.; de Goeij, B.E.C.G.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody–drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Starodub, A.; Moroose, R.L.; Mayer, I.A.; Diamond, J.R.; Chuang, E.; Govindan, S.V.; Sharkey, R.M.; Maliakal, P.; Wegener, W.A.; et al. Abstract p5-19-27: Immu-132, a new antibody-drug conjugate (ADC) against Trop-2, as a novel therapeutic for patients with relapsed/refractory, metastatic, triple-negative breast cancer (TNBC): Results from phase I/II clinical trial (nct01631552). Cancer Res. 2015. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (immu-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.L.; Werner, T.L.; Jarboe, E.A.; Gaffney, D.K. Adenocarcinoma of the cervix: Should we treat it differently? Curr. Oncol. Rep. 2015, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keskar, V.; Mohanty, P.S.; Gemeinhart, E.J.; Gemeinhart, R.A. Cervical cancer treatment with a locally insertable controlled release delivery system. J. Control. Release 2006, 115, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ball, C.; Krogstad, E.A.; Woodrow, K.A. Electrospun fibers for vaginal anti-HIV drug delivery. Antivir. Res. 2013, 100 (Suppl.), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.; Major, I.; Wang, W.; McConville, C. Development of disulfiram-loaded vaginal rings for the localised treatment of cervical cancer. Eur. J. Pharm. Biopharm. 2014, 88, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Liu, D.X.; Zheng, Y.H.; Yue, Y.; Huang, Y.B.; Jing, X.B. Inhibitory effects of paclitaxel-loaded PLA nanofibers against mice cervical cancers. Acta Polym. Sin. 2012, 1029–1034. [Google Scholar] [CrossRef]
- Hani, U.; Shivakumar, H.G.; Anjum, H.; Pasha, M.Y. Preparation and optimization of curcumin-hydroxy propyl β cyclodextrin bioadhesive vaginal films for human papilloma virus-induced cervical cancer. J. Biomater. Tissue Eng. 2014, 4, 796–803. [Google Scholar] [CrossRef]
- Bilensoy, E.; Çırpanlı, Y.; Şen, M.; Doğan, A.L.; Çalış, S. Thermosensitive mucoadhesive gel formulation loaded with 5-fu: Cyclodextrin complex for HPV-induced cervical cancer. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 363–370. [Google Scholar] [CrossRef]
- Woolfson, A.D.; McCafferty, D.F.; McCarron, P.A.; Price, J.H. A bioadhesive patch cervical drug delivery system for the administration of 5-fluorouracil to cervical tissue. J. Control. Release 1995, 35, 49–58. [Google Scholar] [CrossRef]
- McCarron, P.A.; Woolfson, A.D.; McCafferty, D.F.; Price, J.H.; Sidhu, H.; Hickey, G.I. Cytotoxicity of 5-fluorouracil released from a bioadhesive patch into uterine cervical tissue. Int. J. Pharm. 1997, 151, 69–74. [Google Scholar] [CrossRef]
- Hodge, L.S.; Downs, L.S., Jr.; Chura, J.C.; Thomas, S.G.; Callery, P.S.; Soisson, A.P.; Kramer, P.; Wolfe, S.S.; Tracy, T.S. Localized delivery of chemotherapy to the cervix for radiosensitization. Gynecol. Oncol. 2012, 127, 121–125. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordikhani, F.; Erdem Arslan, M.; Marcelo, R.; Sahin, I.; Grigsby, P.; Schwarz, J.K.; Azab, A.K. Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics 2016, 8, 23. https://doi.org/10.3390/pharmaceutics8030023
Ordikhani F, Erdem Arslan M, Marcelo R, Sahin I, Grigsby P, Schwarz JK, Azab AK. Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics. 2016; 8(3):23. https://doi.org/10.3390/pharmaceutics8030023
Chicago/Turabian StyleOrdikhani, Farideh, Mustafa Erdem Arslan, Raymundo Marcelo, Ilyas Sahin, Perry Grigsby, Julie K. Schwarz, and Abdel Kareem Azab. 2016. "Drug Delivery Approaches for the Treatment of Cervical Cancer" Pharmaceutics 8, no. 3: 23. https://doi.org/10.3390/pharmaceutics8030023
APA StyleOrdikhani, F., Erdem Arslan, M., Marcelo, R., Sahin, I., Grigsby, P., Schwarz, J. K., & Azab, A. K. (2016). Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics, 8(3), 23. https://doi.org/10.3390/pharmaceutics8030023